The Role of BMP Signaling in Osteoclast Regulation
Abstract
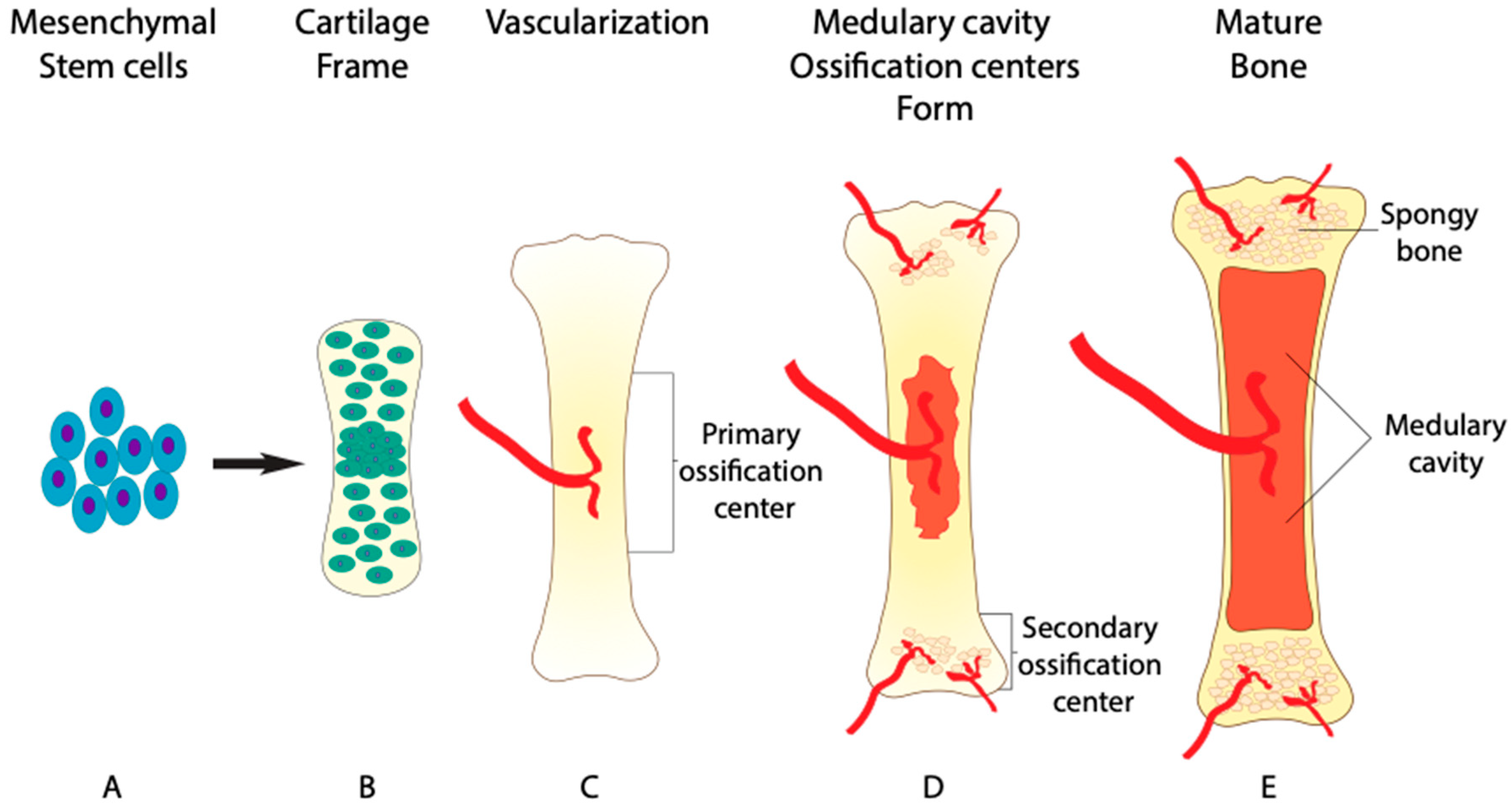
1. Role of BMP in Bone Formation
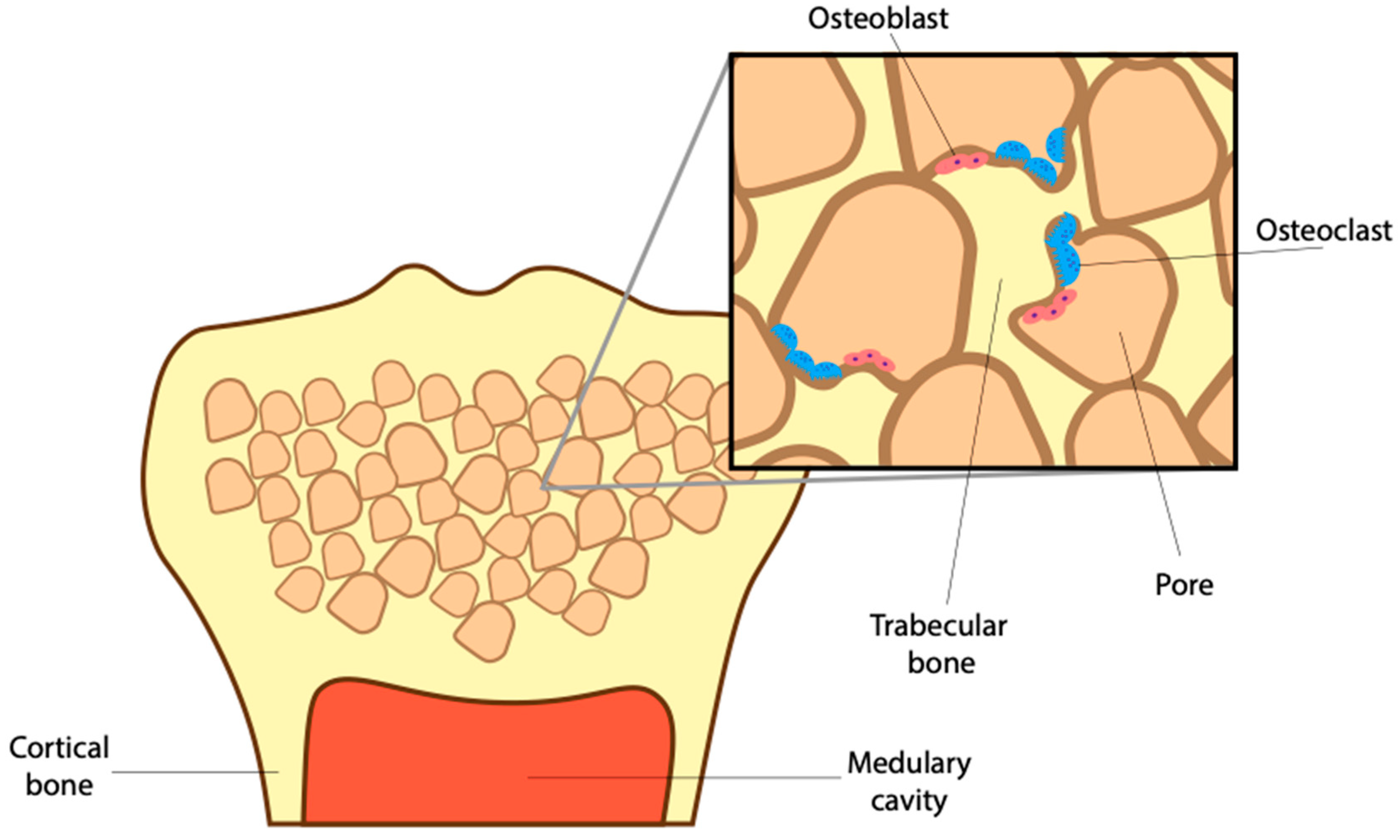
2. The Role of BMP and Osteoclasts in Maintaining Bone Microstructure
3. The Role of BMP in Stages of Osteoclast Differentiation
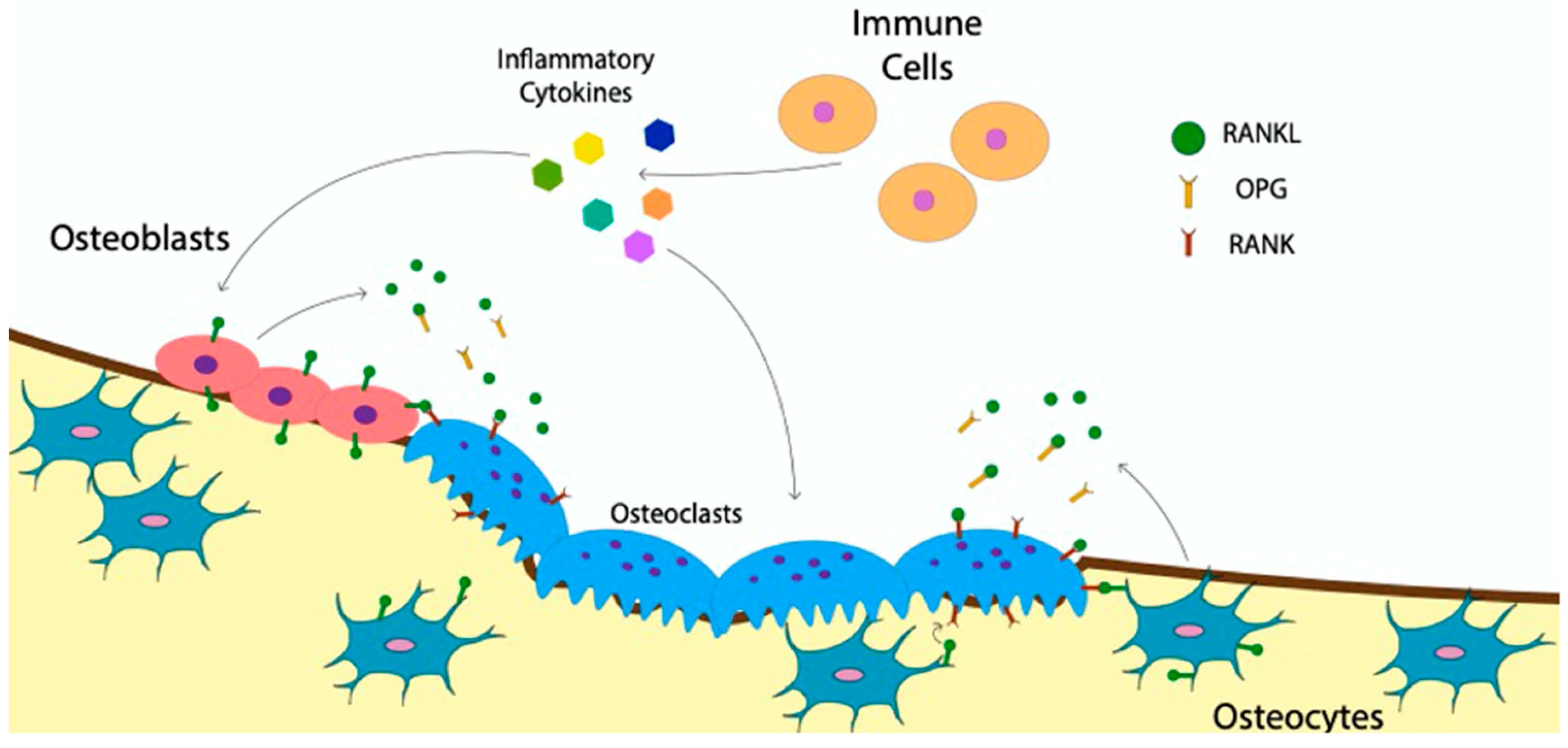
4. The Communication of Osteoclasts with Bone and Immune Cells
Indirect Effect of BMP-Signaling on Osteoclast Communication
5. Direct Effect of BMP-Signaling in Osteoclasts
6. BMP-Signaling Pathway in Osteoclasts
7. The Role of Osteoclasts in Bone Disease
8. Treatments for Osteoporosis
9. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef]
- Breeland, G.; Sinkler, M.A.; Menezes, R.G. Embryology, Bone Ossification. In StatPearls; StatPearls Publishing Copyright ©: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Fuentes-Boquete, I.; Díaz-Prado, S.; Blanco, F.J. Alternative protocols to induce chondrogenic differentiation: Transforming growth factor-β superfamily. Cell Tissue Bank. 2015, 16, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Jones, E.; Giannoudis, P.V. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J. Orthop. Trauma 2010, 24, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Yukata, K.; Yin, G.; Sheu, T.; Maruyama, T.; Jonason, J.H.; Hsu, W.; Zhang, X.; Xiao, G.; Konttinen, Y.T.; et al. BMP-2 induces ATF4 phosphorylation in chondrocytes through a COX-2/PGE2 dependent signaling pathway. Osteoarthr. Cartil. 2014, 22, 481–489. [Google Scholar] [CrossRef]
- Lim, J.; Tu, X.; Choi, K.; Akiyama, H.; Mishina, Y.; Long, F. BMP-Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Dev. Biol. 2015, 400, 132–138. [Google Scholar] [CrossRef]
- Grimsrud, C.D.; Romano, P.R.; D’Souza, M.; Puzas, J.E.; Schwarz, E.M.; Reynolds, P.R.; Roiser, R.N.; O’Keefe, R.J. BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog. J. Orthop. Res. 2001, 19, 18–25. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef]
- Wang, W.; Lian, N.; Li, L.; Moss, H.E.; Wang, W.; Perrien, D.S.; Elefteriou, F.; Yang, X. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development 2009, 136, 4143. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Pogue, R.; Ovchinnikov, D.A.; Yoshii, I.; Mishina, Y.; Behringer, R.R.; Lyons, K.M. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 2006, 133, 4667. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W.F. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; Mark, K.V.D.; Sakane, C.; Kaneko, H.; et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wang, Z.; Li, H.; Ma, C.; Feng, J. Chondrogenesis Defines Future Skeletal Patterns Via Cell Transdifferentiation from Chondrocytes to Bone Cells. Curr. Osteoporos. Rep. 2020, 18, 199–209. [Google Scholar] [CrossRef]
- Gross, P.M.; Marcus, M.L.; Heistad, D.D. Measurement of blood flow to bone and marrow in experimental animals by means of the microsphere technique. J. Bone Jt. Surg. Am. 1981, 63, 1028–1031. [Google Scholar] [CrossRef]
- Ray, R.D.; Kawabata, M.; Galante, J. Experimental study of peripheral circulation and bone growth. An experimental method for the quantitative determination of bone blood flow. 3. Clin. Orthop. Relat. Res. 1967, 54, 175–185. [Google Scholar] [CrossRef]
- Mishbak, H.; Vyas, C.; Cooper, G.; Peach, C.; Pereira, R.F.; Bártolo, P.J. Engineering Natural-Based Photocrosslinkable Hydrogels for Cartilage Applications. In Bio-Materials and Prototyping Applications in Medicine; Bártolo, P.J., Bidanda, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 111–138. [Google Scholar]
- Turner, C.H. Biomechanical Aspects of Bone Formation. In Bone Formation; Bronner, F., Farach-Carson, M.C., Eds.; Springer: London, UK, 2004; pp. 79–105. [Google Scholar]
- Chow, J.; Tobias, J.H.; Colston, K.W.; Chambers, T.J. Estrogen maintains trabecular bone volume in rats not only by suppression of bone resorption but also by stimulation of bone formation. J. Clin. Investig. 1992, 89, 74–78. [Google Scholar] [CrossRef]
- Ueyama, H.; Ohta, Y.; Imai, Y.; Suzuki, A.; Sugama, R.; Minoda, Y.; Takaoka, K.; Nakamura, H. Topical co--administration of zoledronate with recombinant human bone morphogenetic protein-2 can induce and maintain bone formation in the bone marrow environment. BMC Musculoskelet. Disord. 2021, 22, 94. [Google Scholar] [CrossRef]
- Lou, J.; Tu, Y.; Li, S.; Manske, P.R. Involvement of ERK in BMP-2 Induced Osteoblastic Differentiation of Mesenchymal Progenitor Cell Line C3H10T1/2. Biochem. Biophys. Res. Commun. 2000, 268, 757–762. [Google Scholar] [CrossRef]
- Lysdahl, H.; Baatrup, A.; Foldager, C.B.; Bünger, C. Preconditioning Human Mesenchymal Stem Cells with a Low Concentration of BMP2 Stimulates Proliferation and Osteogenic Differentiation In Vitro. BioRes. Open Access 2014, 3, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Griffith, L.G.; Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 2010, 1, 32. [Google Scholar] [CrossRef]
- Chen, J.-R.; Plotkin, L.I.; Aguirre, J.I.; Han, L.; Jilka, R.L.; Kousteni, S.; Bellido, T.; Manolagas, S.C. Transient Versus Sustained Phosphorylation and Nuclear Accumulation of ERKs Underlie Anti-Versus Pro-apoptotic Effects of Estrogens. J. Biol. Chem. 2005, 280, 4632–4638. [Google Scholar] [CrossRef]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018, 19, 3004. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, D.; Wang, S.; Ding, J.; Zhao, L. Bone morphogenetic protein-9 promotes the differentiation of mouse spleen macrophages into osteoclasts via the ALK1 receptor and ERK 1/2 pathways in vitro. Mol. Med. Rep. 2016, 14, 4545–4550. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [PubMed]
- Baylink, D.J.; Finkelman, R.D.; Mohan, S. Growth factors to stimulate bone formation. J. Bone Miner. Res. 1993, 8, S565–S572. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Blair, H.C.; Teitelbaum, S.L.; Edwards, J.C. Characterization of the Osteoclast Ruffled Border Chloride Channel and Its Role in Bone Resorption *. J. Biol. Chem. 1997, 272, 18636–18643. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, M.; Takeuchi, Y.; Fukumoto, S.; Kato, S.; Ueno, N.; Miyazono, K.; Matsumoto, T.; Fujita, T. Extracellular Matrix-Associated Bone Morphogenetic Proteins Are Essential for Differentiation of Murine Osteoblastic Cells in Vitro. Endocrinology 1999, 140, 2125–2133. [Google Scholar] [CrossRef]
- Wang, C.-Y. Bone Morphogenic Proteins, Osteoblast Differentiation, and Cell Survival during Osteogenesis. In The Skeleton: Biochemical, Genetic, and Molecular Interactions in Development and Homeostasis; Massaro, E.J., Rogers, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 185–193. [Google Scholar]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mishina, Y.; Starbuck, M.W.; Gentile, M.A.; Fukuda, T.; Kasparcova, V.; Seedor, J.G.; Hanks, M.C.; Amling, M.; Pinero, G.J.; Harada, S.; et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J. Biol. Chem. 2004, 279, 27560–27566. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.; Wu, Z.Y.; Sankaran, J.S.; Sen, B.; Xie, Z.H.; Styner, M.; Zong, X.P.; Chen, W.Q.; Rubin, J.; Coleman, R.A. Exercise Effect on Marrow Adipose Tissue, Metabolic and Skeletal Health in Lipodystrophic SEIPIN Deficient Mice. J. Bone Miner. Res. 2017, 32, S204. [Google Scholar]
- Mosekilde, L.; Ebbesen, E.N.; Tornvig, L.; Thomsen, J.S. Trabecular bone structure and strength—Remodelling and repair. J. Musculoskelet. Neuronal Interact. 2000, 1, 25–30. [Google Scholar] [PubMed]
- McBride, S.H.; McKenzie, J.A.; Bedrick, B.S.; Kuhlmann, P.; Pasteris, J.D.; Rosen, V.; Silva, M.J. Long bone structure and strength depend on BMP2 from osteoblasts and osteocytes, but not vascular endothelial cells. PLoS ONE 2014, 9, e96862. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Hiepen, C.; Knaus, P.; ten Dijke, P. Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43–59. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Kahn, A.J.; Simmons, D.J. Investigation of cell lineage in bone using a chimaera of chick and quial embryonic tissue. Nature 1975, 258, 325–327. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Montanari, M.; Gemelli, C.; Tenedini, E.; Zanocco Marani, T.; Vignudelli, T.; Siena, M.; Zini, R.; Salati, S.; Chiossi, G.; Tagliafico, E.; et al. Correlation between differentiation plasticity and mRNA expression profiling of CD34+-derived CD14− and CD14+ human normal myeloid precursors. Cell Death Differ. 2005, 12, 1588–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Massey, H.M.; Flanagan, A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999, 106, 167–170. [Google Scholar] [CrossRef]
- Jin, X.; Kruth, H.S. Culture of Macrophage Colony-stimulating Factor Differentiated Human Monocyte-derived Macrophages. J. Vis. Exp. 2016, 112. [Google Scholar] [CrossRef]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sarrazin, S.; Kandalla, P.K.; Espinosa, L.; Stanley, E.R.; Nutt, S.L.; Moore, J.; Sieweke, M.H. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature 2013, 497, 239–243. [Google Scholar] [CrossRef]
- Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; Yasuda, H.; Yano, K.; Morinaga, T.; Higashio, K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 1998, 253, 395–400. [Google Scholar] [CrossRef]
- Quinn, J.M.; Elliott, J.; Gillespie, M.T.; Martin, T.J. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology 1998, 139, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hayashi, S.I.; Kunisada, T.; Ogawa, M.; Nishikawa, S.; Okamura, H.; Sudo, T.; Shultz, L.D.; Nishikawa, S.I. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Broege, A.; Pham, L.; Jensen, E.D.; Emery, A.; Huang, T.H.; Stemig, M.; Beppu, H.; Petryk, A.; O’Connor, M.; Mansky, K.; et al. Bone morphogenetic proteins signal via SMAD and mitogen-activated protein (MAP) kinase pathways at distinct times during osteoclastogenesis. J. Biol. Chem. 2013, 288, 37230–37240. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef]
- Kanatani, M.; Sugimoto, T.; Kaji, H.; Kobayashi, T.; Nishiyama, K.; Fukase, M.; Kumegawa, M.; Chihara, K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J. Bone Miner. Res. 1995, 10, 1681–1690. [Google Scholar] [CrossRef]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Li, A.; Cong, Q.; Xia, X.; Leong, W.F.; Yeh, J.; Miao, D.; Mishina, Y.; Liu, H.; Li, B. Pharmacologic Calcitriol Inhibits Osteoclast Lineage Commitment via the BMP-Smad1 and IκB-NF-κB Pathways. J. Bone Miner. Res. 2017, 32, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Murai, J.; Yoshikawa, H.; Tsumaki, N. Bone Morphogenetic Proteins in Bone Stimulate Osteoclasts and Osteoblasts During Bone Development. J. Bone Miner. Res. 2006, 21, 1022–1033. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, H.; Louie, K.A.; Mishina, Y.; Sun, H. BMP Signaling Mediated by BMPR1A in Osteoclasts Negatively Regulates Osteoblast Mineralization Through Suppression of Cx43. J. Cell. Biochem. 2017, 118, 605–614. [Google Scholar] [CrossRef]
- Sotillo Rodriguez, J.E.; Mansky, K.C.; Jensen, E.D.; Carlson, A.E.; Schwarz, T.; Pham, L.; MacKenzie, B.; Prasad, H.; Rohrer, M.D.; Petryk, A.; et al. Enhanced Osteoclastogenesis Causes Osteopenia in Twisted Gastrulation-Deficient Mice Through Increased BMP Signaling. J. Bone Miner. Res. 2009, 24, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.H.; Lee, C.K.; Lee, Y.I.; Paik, S.G.; Lee, H.J. The hematopoietic transcription factor PU.1 regulates RANK gene expression in myeloid progenitors. Biochem. Biophys. Res. Commun. 2005, 335, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tondravi, M.M.; McKercher, S.R.; Anderson, K.; Erdmann, J.M.; Quiroz, M.; Maki, R.; Teitelbaum, S.L. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 1997, 386, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Buckley, S.; Schouteden, S.; Ekker, S.; Petryk, A.; Delforge, M.; Zwijsen, A.; Verfaillie, C.M. A novel role of BMP4 in adult hematopoietic stem and progenitor cell homing via Smad independent regulation of integrin-α4 expression. Blood 2013, 121, 781–790. [Google Scholar] [CrossRef]
- Courtial, N.; Smink, J.J.; Kuvardina, O.N.; Leutz, A.; Göthert, J.R.; Lausen, J. Tal1 regulates osteoclast differentiation through suppression of the master regulator of cell fusion DC-STAMP. FASEB J. 2012, 26, 523–532. [Google Scholar] [CrossRef]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Mandal, C.C.; Das, F.; Ganapathy, S.; Harris, S.E.; Choudhury, G.G.; Ghosh-Choudhury, N. Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling. J. Biol. Chem. 2016, 291, 1148–1161. [Google Scholar] [CrossRef]
- Omi, M.; Kaartinen, V.; Mishina, Y. Activin A receptor type 1-mediated BMP signaling regulates RANKL-induced osteoclastogenesis via canonical SMAD-signaling pathway. J. Biol. Chem. 2019, 294, 17818–17836. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.; Yamamoto, M.; Taguchi, Y.; Lecka-Czernik, B.; O’Brien, C.A.; Economides, A.N.; Stahl, N.; Jilka, R.L.; Manolagas, S.C. Essential Requirement of BMPs-2/4 for Both Osteoblast and Osteoclast Formation in Murine Bone Marrow Cultures from Adult Mice: Antagonism by Noggin. J. Bone Miner. Res. 2000, 15, 663–673. [Google Scholar] [CrossRef]
- Huntley, R.; Davydova, J.; Petryk, A.; Billington, C.J., Jr.; Jensen, E.D.; Mansky, K.C.; Gopalakrishnan, R. The Function of Twisted Gastrulation in Regulating Osteoclast Differentiation is Dependent on BMP Binding. J. Cell. Biochem. 2015, 116, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Beyer, K.; Jensen, E.D.; Rodriguez, J.S.; Davydova, J.; Yamamoto, M.; Petryk, A.; Gopalakrishnan, R.; Mansky, K.C. Bone morphogenetic protein 2 signaling in osteoclasts is negatively regulated by the BMP antagonist, twisted gastrulation. J. Cell. Biochem. 2011, 112, 793–803. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Hollberg, K.; Nordahl, J.; Hultenby, K.; Mengarelli-Widholm, S.; Andersson, G.; Reinholt, F.P. Polarization and secretion of cathepsin K precede tartrate-resistant acid phosphatase secretion to the ruffled border area during the activation of matrix-resorbing clasts. J. Bone Min. Metab. 2005, 23, 441–449. [Google Scholar] [CrossRef]
- Jarrar, H.; Çetin Altındal, D.; Gümüşderelioğlu, M. Effect of melatonin/BMP-2 co-delivery scaffolds on the osteoclast activity. J. Mater. Sci. Mater. Med. 2021, 32, 32. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ghosh Choudhury, G.; Ghosh-Choudhury, N. Phosphatidylinositol 3 Kinase/Akt Signal Relay Cooperates with Smad in Bone Morphogenetic Protein-2-Induced Colony Stimulating Factor-1 (CSF-1) Expression and Osteoclast Differentiation. Endocrinology 2009, 150, 4989–4998. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Udagawa, N.; Kobayashi, Y.; Suda, T. Generation of Osteoclasts In Vitro, and Assay of Osteoclast Activity. In Arthritis Research: Methods and Protocols; Cope, A.P., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 1, pp. 285–301. [Google Scholar]
- Udagawa, N.; Takito, J.; Suda, T. [Mechanism of acid production and secretion by osteoclasts]. Nihon Rinsho Jpn. J. Clin. Med. 1992, 50, 2133–2138. [Google Scholar]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.L.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Goto, M.; Mochizuki, S.I.; Tsuda, E.; Morinaga, T.; Udagawa, N.; et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25, 109–113. [Google Scholar] [CrossRef]
- Bhargava, A.; Vagela, M.; Lennox, C.M. “Challenges in the management of fractures in osteopetrosis”! Review of literature and technical tips learned from long-term management of seven patients. Injury 2009, 40, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Graeff, C.; Marin, F.; Petto, H.; Kayser, O.; Reisinger, A.; Peña, J.; Zysset, P.; Glüer, C.-C. High resolution quantitative computed tomography-based assessment of trabecular microstructure and strength estimates by finite-element analysis of the spine, but not DXA, reflects vertebral fracture status in men with glucocorticoid-induced osteoporosis. Bone 2013, 52, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Granholm, S.; Henning, P.; Lindholm, C.; Lerner, U.H. Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone 2013, 52, 83–92. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 1998, 250, 776–781. [Google Scholar] [CrossRef]
- Krajewski, A.C.; Ghuman, M.; Reddi, D.; McKay, I.; Hughes, F.; Belibasakis, G. Influence of BMP-2 on RANKL/ OPG production in W-20-17 cells. In Proceedings of the IADR General Session, Barcelona, Spain, 14–17 July 2010. [Google Scholar]
- Tazoe, M.; Mogi, M.; Goto, S.; Togari, A. Involvement of p38MAP kinase in bone morphogenetic protein-4-induced osteoprotegerin in mouse bone-marrow-derived stromal cells. Arch. Oral Biol. 2003, 48, 615–619. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.-P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef]
- Valverde, P.; Kawai, T.; Taubman, M.A. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J. Bone Miner. Res. 2004, 19, 155–164. [Google Scholar] [CrossRef]
- Yun, T.J.; Chaudhary, P.M.; Shu, G.L.; Frazer, J.K.; Ewings, M.K.; Schwartz, S.M.; Pascual, V.; Hood, L.E.; Clark, E.A. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J. Immunol. 1998, 161, 6113–6121. [Google Scholar]
- Leibbrandt, A.; Penninger, J.M. Novel functions of RANK(L) signaling in the immune system. Adv. Exp. Med. Biol. 2010, 658, 77–94. [Google Scholar] [PubMed]
- Chen, L.; Wei, X.Q.; Evans, B.; Jiang, W.; Aeschlimann, D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur. J. Immunol. 2008, 38, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Hayakawa, N.; Mihara, M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-α and IL-17. Rheumatology 2008, 47, 1635–1640. [Google Scholar] [CrossRef]
- Komine, M.; Kukita, A.; Kukita, T.; Ogata, Y.; Hotokebuchi, T.; Kohashi, O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone 2001, 28, 474–483. [Google Scholar] [CrossRef]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, T.; Kyumoto-Nakamura, Y.; Kukita, A.; Uehara, N.; Zhang, J.; Koda, K.; Kamiya, M.; Badawy, T.; Tomoda, E.; Xu, X.; et al. IL-1β Induces Pathologically Activated Osteoclasts Bearing Extremely High Levels of Resorbing Activity: A Possible Pathological Subpopulation of Osteoclasts, Accompanied by Suppressed Expression of Kindlin-3 and Talin-1. J. Immunol. 2018, 200, 218. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The Mechanism of Osteoclast Differentiation Induced by IL-1. J. Immunol. 2009, 183, 1862. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Grassi, F.; Ryan, M.R.; Terauchi, M.; Page, K.; Yang, X.; Weitzmann, M.N.; Pacifici, R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007, 117, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Kohara, H.; Eguchi, T.; Yoshida, N. IL-18 inhibits TNF-alpha-induced osteoclastogenesis possibly via a T cell-independent mechanism in synergy with IL-12 in vivo. Calcif. Tissue Int. 2010, 86, 242–248. [Google Scholar] [CrossRef]
- Saleh, H.; Eeles, D.; Hodge, J.M.; Nicholson, G.C.; Gu, R.; Pompolo, S.; Gillespie, M.T.; Quinn, J.M.W. Interleukin-33, a Target of Parathyroid Hormone and Oncostatin M, Increases Osteoblastic Matrix Mineral Deposition and Inhibits Osteoclast Formation in Vitro. Endocrinology 2011, 152, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Saribal, D.; Hocaoglu-Emre, F.S.; Erdogan, S.; Bahtiyar, N.; Caglar Okur, S.; Mert, M. Inflammatory cytokines IL-6 and TNF-α in patients with hip fracture. Osteoporos. Int. 2019, 30, 1025–1031. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Hayashi, M.; Hayashi, N.; Aoki, S.; Suzuki, H. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J. Bone Miner. Res. 2013, 28, 1936–1949. [Google Scholar] [CrossRef]
- Gu, G.; Nars, M.; Hentunen, T.A.; Metsikkö, K.; Väänänen, H.K. Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res. 2006, 323, 263–271. [Google Scholar] [CrossRef]
- Fumoto, T.; Takeshita, S.; Ito, M.; Ikeda, K. Physiological functions of osteoblast lineage and T cell-derived RANKL in bone homeostasis. J. Bone Min. Res. 2014, 29, 830–842. [Google Scholar] [CrossRef]
- Wutzl, A.; Brozek, W.; Lernbass, I.; Rauner, M.; Hofbauer, G.; Schopper, C.; Watzinger, F.; Peterlik, M.; Pietschmann, P. Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J. Biomed. Mater. Res. A 2006, 77, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Teti, A. The tight relationship between osteoclasts and the immune system. Inflamm. Allergy Drug Targets 2012, 11, 181–187. [Google Scholar] [CrossRef]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef]
- Kamiya, N.; Ye, L.; Kobayashi, T.; Lucas, D.J.; Mochida, Y.; Yamauchi, M.; Kronenberg, H.M.; Feng, J.Q.; Mishina, Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J. Bone Min. Res. 2008, 23, 2007–2017. [Google Scholar] [CrossRef]
- Kusu, N.; Laurikkala, J.; Imanishi, M.; Usui, H.; Konishi, M.; Miyake, A.; Thesleff, I.; Itoh, N. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J. Biol. Chem. 2003, 278, 24113–24117. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Delgado-Calle, J.; Condon, K.W.; Maycas, M.; Zhang, H.; Carlesso, N.; Taketo, M.M.; Burr, D.B.; Plotkin, L.I.; Bellido, T. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 2015, 112, E478–E486. [Google Scholar] [CrossRef]
- Garimella, R.; Tague, S.E.; Zhang, J.; Belibi, F.; Nahar, N.; Sun, B.H.; Insogna, K.; Wang, J.; Anderson, H.C. Expression and synthesis of bone morphogenetic proteins by osteoclasts: A possible path to anabolic bone remodeling. J. Histochem. Cytochem. 2008, 56, 569–577. [Google Scholar] [CrossRef]
- Hayden, J.M.; Mohan, S.; Baylink, D.J. The insulin-like growth factor system and the coupling of formation to resorption. Bone 1995, 17 (Suppl. 2), 93s–98s. [Google Scholar] [CrossRef]
- Ota, K.; Quint, P.; Weivoda, M.M.; Ruan, M.; Pederson, L.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 2013, 57, 68–75. [Google Scholar] [CrossRef]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef]
- Aluganti Narasimhulu, C.; Singla, D.K. The Role of Bone Morphogenetic Protein 7 (BMP-7) in Inflammation in Heart Diseases. Cells 2020, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, E.; Guevara-Garcia, A.; Albiges-Rizo, C.; Picart, C. Learning from BMPs and their biophysical extracellular matrix microenvironment for biomaterial design. Bone 2020, 141, 115540. [Google Scholar] [CrossRef]
- Pang, D.-D.; Cai, L.; Zhang, J.-R.; Dai, S.-M. IL-23 induziert die Expression pro-osteogener Faktoren in Osteoklasten. Aktuelle Rheumatol. 2020, 45, 467–474. [Google Scholar] [CrossRef]
- Ghosh-Choudhury, N.; Singha, P.K.; Woodruff, K.; Clair, P.S.; Bsoul, S.; Werner, S.L.; Choudhury, G.G. Concerted Action of Smad and CREB-binding Protein Regulates Bone Morphogenetic Protein-2-stimulated Osteoblastic Colony-stimulating Factor-1 Expression. J. Biol. Chem. 2006, 281, 20160–20170. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Xiao, Y.; Luo, G.; Li, G.; Li, Z. Friend or Foe? Essential Roles of Osteoclast in Maintaining Skeletal Health. BioMed Res. Int. 2020, 2020, 4791786. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Okamoto, M.; Murai, J.; Imai, Y.; Ikegami, D.; Kamiya, N.; Kato, S.; Mishina, Y.; Yoshikawa, H.; Tsumaki, N. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice. J. Bone Miner. Res. 2011, 26, 2511–2522. [Google Scholar] [CrossRef]
- Shi, C.; Iura, A.; Terajima, M.; Liu, F.; Lyons, K.; Pan, H.; Zhang, H.; Yamauchi, M.; Mishina, Y.; Sun, H. Deletion of BMP receptor type IB decreased bone mass in association with compromised osteoblastic differentiation of bone marrow mesenchymal progenitors. Sci. Rep. 2016, 6, 24256. [Google Scholar] [CrossRef] [PubMed]
- Attisano, L.; Lee-Hoeflich, S.T. The Smads. Genome Biol. 2001, 2. [Google Scholar] [CrossRef]
- Fong, D.; Bisson, M.; Laberge, G.; McManus, S.; Grenier, G.; Faucheux, N.; Roux, S. Bone morphogenetic protein-9 activates Smad and ERK pathways and supports human osteoclast function and survival in vitro. Cell. Signal. 2013, 25, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.; Astleford, K.; Blixt, N.C.; Jensen, E.D.; Gopalakrishnan, R.; Mansky, K.C. SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors. PLoS ONE 2018, 13, e0203404. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yuan, J.; Wu, J.; Zheng, J.; Zheng, W.; Wang, F.; Wang, C.; Li, X.; Liu, S.; Shi, Z.; et al. Bone Morphogenetic Protein-2 Promotes Osteoclasts-mediated Osteolysis via Smad1 and p65 Signaling Pathways. Spine 2021, 46. [Google Scholar] [CrossRef]
- Qi, B.; Cong, Q.; Li, P.; Ma, G.; Guo, X.; Yeh, J.; Xie, M.; Schneider, M.D.; Liu, H.; Li, B. Ablation of Tak1 in osteoclast progenitor leads to defects in skeletal growth and bone remodeling in mice. Sci. Rep. 2014, 4, 7158. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Nagai, S.-I.; Ninomiya-Tsuji, J.; Nishita, M.; Tamai, K.; Irie, K.; Ueno, N.; Nishida, E.; Shibuya, H.; Matsumoto, K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1–TAK1 in the BMP signaling pathway. EMBO J. 1999, 18, 179–187. [Google Scholar] [CrossRef]
- Feng, X. RANKing Intracellular Signaling in Osteoclasts. IUBMB Life 2005, 57, 389–395. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, Z.; Wu, B.; Chen, F.; Tickner, J.; Wang, Z.; Qiu, H.; Wang, C.; Chen, K.; Tan, R.; et al. Modulating calcium-mediated NFATc1 and mitogen-activated protein kinase deactivation underlies the inhibitory effects of kavain on osteoclastogenesis and bone resorption. J. Cell. Physiol. 2019, 234, 789–801. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 Induces Osteoclast Fusion Via Up-Regulation of Atp6v0d2 and the Dendritic Cell-Specific Transmembrane Protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, B.; Yang, X.; Guo, H.; Zhang, K.; Zhu, Z.; Liu, J.; Hao, D. Picrasidine I from Picrasma Quassioides Suppresses Osteoclastogenesis via Inhibition of RANKL Induced Signaling Pathways and Attenuation of ROS Production. Cell. Physiol. Biochem. 2017, 43, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Kelly, S.; Wood, R.; Heubel, B.; Nohe, A. A Synthetic Peptide, CK2.3, Inhibits RANKL-Induced Osteoclastogenesis through BMPRIa and ERK Signaling Pathway. J. Dev. Biol. 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Andersen, P.E. Fracture patterns in two types of autosomal-dominant osteopetrosis. Acta Orthop. Scand. 1989, 60, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Sreehari, S.; Naik, D.R.; Eapen, M. Osteopetrosis: A rare cause of anemia. Hematol. Rep. 2011, 3, e1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell. Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef]
- Lane, N.E.; Thompson, J.M.; Haupt, D.; Kimmel, D.B.; Modin, G.; Kinney, J.H. Acute Changes in Trabecular Bone Connectivity and Osteoclast Activity in the Ovariectomized Rat In Vivo. J. Bone Miner. Res. 1998, 13, 229–236. [Google Scholar] [CrossRef]
- Legrand, E.; Chappard, D.; Pascaretti, C.; Duquenne, M.; Krebs, S.; Rohmer, V.; Basle, M.-F.; Audran, M. Trabecular Bone Microarchitecture, Bone Mineral Density, and Vertebral Fractures in Male Osteoporosis. J. Bone Miner. Res. 2000, 15, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Looker, A.C.; Tosteson, A.N.; Johansson, H.; Kanis, J.A.; Melton, L.J., 3rd. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos. Int. 2010, 21, 41–52. [Google Scholar] [CrossRef]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.S.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 24. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Nicks, K.M.; Fowler, T.W.; Akel, N.S.; Perrien, D.S.; Suva, L.J.; Gaddy, D. Bone turnover across the menopause transition: The role of gonadal inhibins. Ann. N. Y. Acad. Sci. 2010, 1192, 153–160. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38 (Suppl. 1), 4–9. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A.; Odén, A.; Sernbo, I.; Redlund-Johnell, I.; Petterson, C.; De Laet, C.; Jönsson, B. Mortality after osteoporotic fractures. Osteoporos. Int. 2004, 15, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Neuburger, J.; Currie, C.; Wakeman, R.; Tsang, C.; Plant, F.; De Stavola, B.; Cromwell, D.A.; van der Meulen, J. The impact of a national clinician-led audit initiative on care and mortality after hip fracture in England: An external evaluation using time trends in non-audit data. Med. Care 2015, 53, 686–691. [Google Scholar] [CrossRef]
- Blume, S.W.; Curtis, J.R. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos. Int. 2011, 22, 1835–1844. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Draper, J.; Roland, M. Perimenopausal women’s views on taking hormone replacement therapy to prevent osteoporosis. Br. Med. J. 1990, 300, 786. [Google Scholar] [CrossRef]
- Grady, D.; Rubin, S.M.; Petitti, D.B.; Fox, C.S.; Black, D.; Ettinger, B.; Ernster, V.L.; Cummings, S.R. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann. Intern. Med. 1992, 117, 1016–1037. [Google Scholar] [CrossRef]
- Colditz, G.A. Relationship Between Estrogen Levels, Use of Hormone Replacement Therapy, and Breast Cancer. JNCI J. Natl. Cancer Inst. 1998, 90, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Manson, J.E.; Joffe, M.; Rosner, B.; Fuchs, C.; Hankinson, S.E.; Hunter, D.J.; et al. Postmenopausal hormone therapy and mortality. N. Engl. J. Med. 1997, 336, 1769–1775. [Google Scholar] [CrossRef]
- Bodalia, P.N.; Balaji, V.; Kaila, R.; Wilson, L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery. Bone Jt. Res. 2016, 5, 145–152. [Google Scholar] [CrossRef]
- Deyo, R.A.; Ching, A.; Matsen, L.; Martin, B.I.; Kreuter, W.; Jarvik, J.G.; Angier, H.; Mirza, S.K. Use of bone morphogenetic proteins in spinal fusion surgery for older adults with lumbar stenosis: Trends, complications, repeat surgery, and charges. Spine 2012, 37, 222–230. [Google Scholar] [CrossRef]
- Huang, K.; Wu, G.; Zou, J.; Peng, S. Combination therapy with BMP-2 and psoralen enhances fracture healing in ovariectomized mice. Exp. Ther. Med. 2018, 16, 1655–1662. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.M.; Sasso, R.C. Resorptive response of rhBMP2 simulating infection in an anterior lumbar interbody fusion with a femoral ring. Clin. Spine Surg. 2006, 19, 130–134. [Google Scholar] [CrossRef]
- Vaidya, R.; Weir, R.; Sethi, A.; Meisterling, S.; Hakeos, W.; Wybo, C.D. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J. Bone Jt. Surg. Br. Vol. 2007, 89, 342–345. [Google Scholar] [CrossRef]
- Durbano, H.W.; Halloran, D.; Nguyen, J.; Stone, V.; McTague, S.; Eskander, M.; Nohe, A. Aberrant BMP2 Signaling in Patients Diagnosed with Osteoporosis. Int. J. Mol. Sci. 2020, 21, 6909. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.M.; Chambers, T.J. Inhibition of bone resorption by bisphosphonates: Interactions between bisphosphonates, osteoclasts, and bone. Calcif. Tissue Int. 1991, 49, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Coleman, R.; Gnant, M.; Green, J. The impact of menopause on bone, zoledronic acid, and implications for breast cancer growth and metastasis. Ann. Oncol. 2012, 23, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, D.G.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef]
- Woo, S.-B.; Hellstein, J.W.; Kalmar, J.R. Systematic Review: Bisphosphonates and Osteonecrosis of the Jaws. Ann. Intern. Med. 2006, 144, 753–761. [Google Scholar] [CrossRef]
- Hamdy, N.A. Denosumab: RANKL inhibition in the management of bone loss. Drugs Today 2008, 44, 7–21. [Google Scholar] [CrossRef]
- Törring, O. Effects of denosumab on bone density, mass and strength in women with postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 2015, 7, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Augustine, M.; Horwitz, M.J. Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr. Osteoporos. Rep. 2013, 11, 400–406. [Google Scholar] [CrossRef]
- Cho, M.; Han, S.; Kim, H.; Kim, K.S.; Hahn, S.K. Hyaluronate—Parathyroid hormone peptide conjugate for transdermal treatment of osteoporosis. J. Biomater. Sci. Polym. Ed. 2018, 29, 793–804. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Johnson, J.; Le Henaff, C.; Bitel, C.L.; Tamasi, J.A.; Partridge, N.C. Catabolic Effects of Human PTH (1–34) on Bone: Requirement of Monocyte Chemoattractant Protein-1 in Murine Model of Hyperparathyroidism. Sci. Rep. 2017, 7, 15300. [Google Scholar] [CrossRef]
- Camirand, A.; Goltzman, D.; Gupta, A.; Kaouass, M.; Panda, D.; Karaplis, A. The Role of Parathyroid Hormone-Related Protein (PTHrP) in Osteoblast Response to Microgravity: Mechanistic Implications for Osteoporosis Development. PLoS ONE 2016, 11, e0160034. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J. Clin. Investig. 2005, 115, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.J.; Tedesco, M.B.; Garcia-Ocaña, A.; Sereika, S.M.; Prebehala, L.; Bisello, A.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F. Parathyroid Hormone-Related Protein for the Treatment of Postmenopausal Osteoporosis: Defining the Maximal Tolerable Dose. J. Clin. Endocrinol. Metab. 2010, 95, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Shoback, D.; Lewiecki, E.M. Sclerostin inhibition: A novel therapeutic approach in the treatment of osteoporosis. Int. J. Womens Health 2015, 7, 565–580. [Google Scholar]
- Suen, P.K.; Qin, L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: A general review. J. Orthop. Transl. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Stolina, M.; Dwyer, D.; Niu, Q.T.; Villasenor, K.S.; Kurimoto, P.; Grisanti, M.; Han, C.Y.; Liu, M.; Li, X.; Ominsky, M.S.; et al. Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone 2014, 67, 305–313. [Google Scholar] [CrossRef]
- Boyce, R.W.; Brown, D.; Felx, M.; Mellal, N.; Locher, K.; Pyrah, I.; Ominsky, M.S.; Taylor, S. Decreased osteoprogenitor proliferation precedes attenuation of cancellous bone formation in ovariectomized rats treated with sclerostin antibody. Bone Rep. 2018, 8, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic Injection of CK2.3, a Novel Peptide Acting Downstream of Bone Morphogenetic Protein Receptor BMPRIa, Leads to Increased Trabecular Bone Mass. J. Orthop. Res. 2015, 33, 208–215. [Google Scholar] [CrossRef]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein Kinase 2 β-Subunit Is a Regulator of Bone Morphogenetic Protein 2 Signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef]
- Vrathasha, V.; Weidner, H.; Nohe, A. Mechanism of CK2.3, a Novel Mimetic Peptide of Bone Morphogenetic Protein Receptor Type IA, Mediated Osteogenesis. Int. J. Mol. Sci. 2019, 20, 2500. [Google Scholar] [CrossRef] [PubMed]
- Weidner, H.; Yuan Gao, V.; Dibert, D.; McTague, S.; Eskander, M.; Duncan, R.; Wang, L.; Nohe, A. CK2.3, a Mimetic Peptide of the BMP Type I Receptor, Increases Activity in Osteoblasts over BMP2. Int. J. Mol. Sci. 2019, 20, 5877. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. https://doi.org/10.3390/jdb9030024
Heubel B, Nohe A. The Role of BMP Signaling in Osteoclast Regulation. Journal of Developmental Biology. 2021; 9(3):24. https://doi.org/10.3390/jdb9030024
Chicago/Turabian StyleHeubel, Brian, and Anja Nohe. 2021. "The Role of BMP Signaling in Osteoclast Regulation" Journal of Developmental Biology 9, no. 3: 24. https://doi.org/10.3390/jdb9030024
APA StyleHeubel, B., & Nohe, A. (2021). The Role of BMP Signaling in Osteoclast Regulation. Journal of Developmental Biology, 9(3), 24. https://doi.org/10.3390/jdb9030024




