How Weird is The Worm? Evolution of the Developmental Gene Toolkit in Caenorhabditis elegans
Abstract
1. Introduction
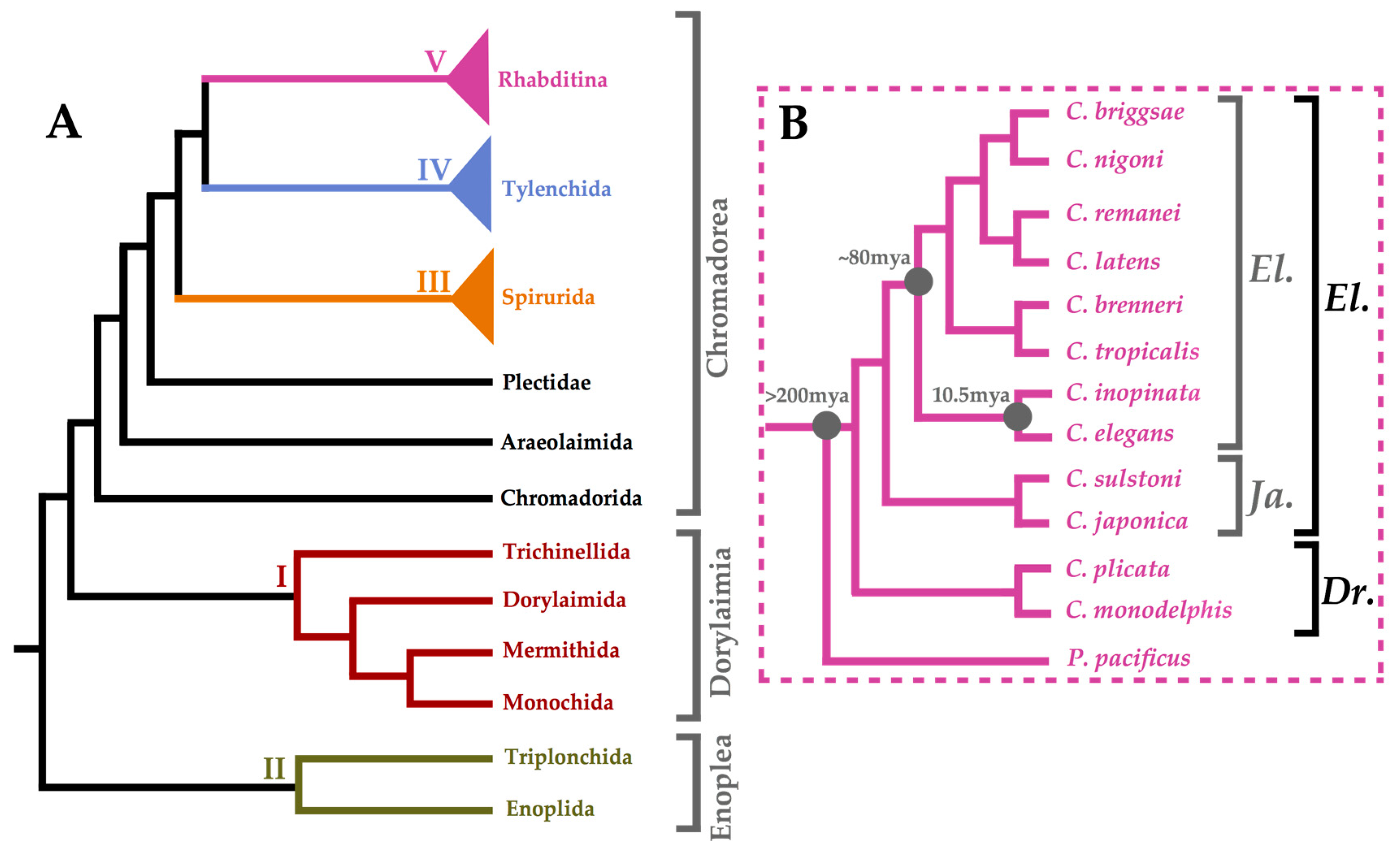
2. The Nematode Phylum: Beyond ‘The Worm’
3. Phyletic Body Plans and the Phylotypic Stage
4. Tools to Make a Worm
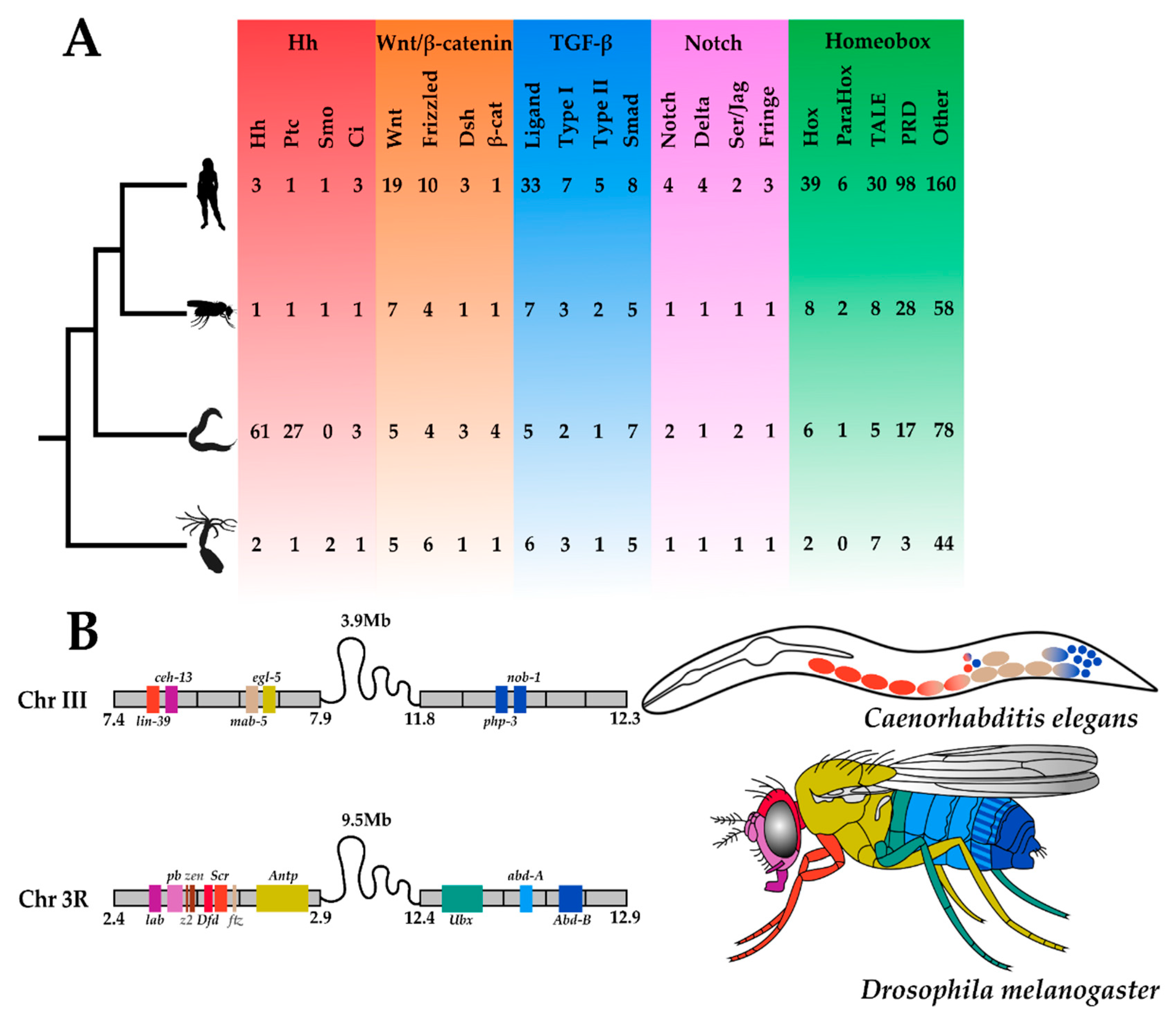
4.1. Gaining Tools: The Hedgehog Pathway
4.2. Losing Tools: The Hox Complement
4.3. Changing Tools: Wnt and Notch Signalling
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Mb | MegaBase |
| mab-5 | MAle aBnormal 5 |
| Ceh (gene class) | C. elegans Homeobox |
| tbx-43 | T-BoX 43 |
| RNAi | RNA Interference |
| PAR proteins | PARtitioning proteins |
| TGF-β | Transforming Growth Factor β |
| ORFs | Open Reading Frames |
| Gli | GLIoma-associated oncogene homologue |
| Ci | Cubitus Interruptus |
| tra-1 | TRAnsformer XX animals transformed into males |
| Lin (gene class) | LINeage defective |
| egl-5 | Egg Laying Defective 5 |
| php-3 | Posterior Hox gene Paralogue 3 |
| nob-1 | kNOB-like posterior (NO Backside) 1 |
| Cdx | CauDal Type HomeoboX |
| pal-1 | Posterior ALae in Males 1 |
| sop-2 | Suppressor of Pal-1 |
| SYS-1 | SYmmetrical Sister cell hermaphrodite gonad defect |
| WRM-1 | Worm aRMadillo |
| BAR-1 | Beta-catenin/Armadillo Related |
| HMP-2 | HuMPback (dorsal lumps) |
| TCF | T-Cell Factor |
| POP-1 | POsterior Pharynx defect |
| glp-1 | abnormal Germ Line Proliferation 1 |
| BLAST | Basic Local Alignment Search Tool |
References
- Cook, D.E.; Zdraljevic, S.; Roberts, J.P.; Andersen, E.C. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 2016, 45, D650–D657. [Google Scholar] [CrossRef] [PubMed]
- Haag, E.S. The evolution of nematode sex determination: C. elegans as a reference point for comparative biology. WormBook Online C. elegans Biol. 2005, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Samuel, A.C. C. elegans: A model system for systems neuroscience. Curr. Opin. Neurobiol. 2009, 19, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, P.W. Vulval development. WormBook Online C. elegans Biol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.J. Evolution of development in nematodes related to C. elegans. WormBook Online C. elegans Biol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Evolution at Two Levels: On Genes and Form. PLoS Biol. 2005, 3, e245. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.F.; Baxevanis, A.D. Hox, Wnt, and the evolution of the primary body axis: Insights from the early-divergent phyla. Biol. Direct. 2007, 2, 1–37. [Google Scholar] [CrossRef]
- Abzhanov, A.; Protas, M.; Grant, B.R.; Grant, P.R.; Tabin, C.J. Bmp4 and morphological variation of beaks in Darwin's finches. Science 2004, 305, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. How great wings can look alike. Science 2011, 333, 1100–1101. [Google Scholar] [CrossRef]
- Gillis, J.A.; Hall, B.K. A shared role for sonic hedgehog signalling in patterning chondrichthyan gill arch appendages and tetrapod limbs. Development 2016, 143, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M. Gene duplication and evolution. Science 2002, 297, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Woollard, A. Gene duplications and genetic redundancy in C. elegans. WormBook Online C. elegans Biol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Sulston, J.E.; Horvitz, H.R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.D.Z.; Bao, D.; Blasiar, T.; Blumenthalm, M.R.; Brent, N. The genome sequence of Caenorhabditis briggsae: A platform for comparative genomics. PLoS Biol. 2003, 1, e45. [Google Scholar] [CrossRef] [PubMed]
- Fierst, J.L.; Willis, J.H.; Thomas, C.G.; Wang, W.; Reynolds, R.M. Reproductive mode and the evolution of genome size and structure in Caenorhabditis nematodes. PLoS Genet. 2015, 11, e1005323. [Google Scholar]
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Smythe, A.B.; Holovachov, O.; Kocot, K.M. Improved phylogenomic sampling of free-living nematodes enhances resolution of higher-level nematode phylogeny. BMC Evol. Biol. 2019, 19, 121. [Google Scholar] [CrossRef]
- Osche, G. Systematik und Phylogenie der Gattung Rhabditis (Nematoda). Zool. Jahrb. 1952, 81, 90–280. [Google Scholar]
- Kanzaki, N.; Tsai, I.J.; Tanaka, R.; Hunt, V.L.; Tsuyama, K.; Liu, D.; Maeda, Y.; Namai, S.; Kumagai, R.; Tracey, A.; et al. Biology and genome of a newly discovered sibling species of Caenorhabditis elegans. Nat. Commun. 2018, 9, 3216. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Félix, M.-A.; Beltran, T.; Braendle, C.; Caurcel, C.; Fausett, S. Comparative genomics of ten new Caenorhabditis species. Evol. Lett. 2019, 3, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Haag, E.S.; Fitch, D.H.A.; Delattre, M. From “the worm” to “the worms” and back again: The evolutionary developmental biology of nematodes. Genetics 2018, 210, 397–433. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Schierenberg, E. Evolution of embryonic development in nematodes. EvoDevo 2011, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Haag, E.S.; Doty, A.V. Sex determination across evolution: Connecting the dots. PLoS Biol. 2005, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, A.; Cumbo, P.; Bojanala, N.; Gupta, B.P. Conserved mechanism of Wnt signalling function in the specification of vulval precursor fates in C. elegans and C. briggsae. Dev. Biol. 2010, 346, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sharanya, D.; Thillainathan, B.; Marri, S.; Bojanala, N.; Taylor, J. Genetic control of vulval development in Caenorhabditis briggsae. G3 Genes Genomes Genet. 2012, 2, 1625–1641. [Google Scholar]
- Jarrell, T.A.; Wang, Y.; Bloniarz, A.E.; Brittin, C.A.; Xu, M. The connectome of a decision-making neural network. Science 2012, 337, 437–444. [Google Scholar] [CrossRef]
- Schafer, W. Nematode nervous systems. Curr. Biol. 2016, 26, R955–R959. [Google Scholar] [CrossRef]
- Memar, N.; Schiemann, S.; Hennig, C.; Findeis, D.; Condradt, B.; Schnabel, R. Twenty million years of evolution: The embryogenesis of four Caenorhabditis species are indistinguishable despite extensive genome divergence. Dev. Biol. 2019, 447, 182–199. [Google Scholar] [CrossRef]
- Cutter, A.D. Divergence times in Caenorhabditis and Drosophila inferred from direct estimates of the neutral mutation rate. Mol. Biol. Evol. 2008, 25, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Hashimshony, T.; Wagner, F.; Yanai, I. Developmental Milestones Punctuate Gene Expression in the Caenorhabditis Embryo. Dev. Cell 2012, 22, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Fraser, A.G.; Dong, Y.; Poulin, G.; Durbin, R.; Gotta, M.; Kanapin, A.; Le Bot, N.; Moreno, S.; Sohrmann, M.; et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 2003, 421, 231–237. [Google Scholar] [CrossRef]
- Graveley, B.R.; Brooks, A.N.; Carlson, J.W.; Duff, M.O.; Landolin, J.M.; Yang, L.; Artieri, C.G.; van Baren, M.J.; Boley, N.; Booth, B.W.; et al. The developmental transcriptome of Drosophila melanogaster. Nature 2011, 471, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yanai, I.; Peshkin, L.; Jorgensen, P.; Kirschner, M.W. Mapping gene expression in two Xenopus species: Evolutionary constraints and developmental flexibility. Dev. Cell 2011, 20, 483–496. [Google Scholar] [CrossRef]
- Kalinka, A.T.; Varga, K.M.; Gerrard, D.T.; Preibisch, S.; Corcoran, D.L.; Jarrells, J.; Ohler, U.; Bergman, C.M.; Tomancak, P. Gene expression divergence recapitulates the developmental hourglass model. Nature 2010, 468, 811–814. [Google Scholar] [CrossRef]
- Slack, J.M.; Holland, P.W.; Graham, C.F. The zootype and the phylotypic stage. Nature 1993, 361, 490–492. [Google Scholar] [CrossRef]
- Duboule, D. Temporal colinearity and the phylotypic progression: A basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development 1994, 1994, 135–142. [Google Scholar]
- Vangestel, S.; Houthoofd, W.; Bert, W.; Borgonie, G. The early embryonic development of the satellite organism Pristionchus pacificus: Differences and similarities with Caenorhabditis elegans. Nematology 2008, 10, 301–312. [Google Scholar]
- Goldstein, B.; Macara, I.G. The PAR proteins: Fundamental players in animal cell polarization. Dev. Cell 2007, 5, 609–622. [Google Scholar] [CrossRef]
- De Robertis, E.M. Evo-Devo: Variations on ancestral themes. Cell 2008, 132, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.W.H.; Marlétaz, F.; Maeso, I.; Dunwell, T.L.; Paps, J. New genes from old: Asymmetric divergence of gene duplicates and the evolution of development. Philos. Trans. R Soc. B 2017, 372, 20150480. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev. 2008, 18, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Zhao, Z.; Ingham, P.W. Hedgehog signalling. Development 2016, 143, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Dray, N.; Tessmar-Raible, K.; Le Gouar, M.; Vibert, L.; Christodoulou, F.; Schipany, K.; Guillou, A.; Zantke, J.; Snyman, H.; Béhague, J.; et al. Hedgehog signaling regulates segment formation in the annelid Platynereis. Science 2010, 329, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Matus, D.Q.; Magie, C.R.; Pang, K.; Martindale, M.Q.; Thomsen, G.H. The Hedgehog gene family of the cnidarian, Nematostella vectensis, and implications for understanding metazoan Hedgehog pathway evolution. Dev. Biol. 2008, 313, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; (Lee Kong Chian School of Medicine Nanyang Technological University Experimental Medicine, Building 59 Nanyang Drive, Singapore). Personal Communications, 2018.
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011, 12, 393–406. [Google Scholar] [CrossRef]
- Hall, T.M.; Porter, J.A.; Beachy, P.A.; Leahy, D.J. A potential catalytic site revealed by the 1.7-Å crystal structure of the amino-terminal signalling domain of Sonic hedgehog. Nature 1995, 378, 212–216. [Google Scholar] [CrossRef]
- Bürglin, T.R. Warthog and Groundhog, novel families related to Hedgehog. Curr. Biol. 1996, 6, 1047–1050. [Google Scholar] [CrossRef][Green Version]
- Aspöck, G.; Kagoshima, H.; Niklaus, G.; Bürglin, T.R. Caenorhabditis elegans has scores of hedgehog-related genes: Sequence and expression analysis. Genome Res. 1999, 9, 909–923. [Google Scholar] [CrossRef]
- Bürglin, T.R.; Kuwabara, P.E. Homologs of the Hh signalling network in C. elegans. WormBook Online C. elegans Biol. 2006, 1–14. [Google Scholar]
- Zugasti, O.; Rajan, J.; Kuwabara, P.E. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res. 2005, 15, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Bürglin, T.R. The Hedgehog Protein Family. Genome Biol. 2008, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R. Taxonomically Restricted Genes Are Fundamental to Biology and Evolution. Front. Genet. 2018, 9, 407–412. [Google Scholar] [CrossRef]
- Johnson, B.R.; Tsutsui, N.D. Taxonomically restricted genes are associated with the evolution of sociality in the honey bee. BMC Genom. 2011, 12, 164. [Google Scholar] [CrossRef]
- Santos, M.E.; Le Bouquin, A.; Crumiere, A.J.J.; Khila, A. Taxon-restricted genes at the origin of a novel trait allowing access to a new environment. Science 2017, 358, 386–390. [Google Scholar] [CrossRef]
- Werner, M.S.; Sieriebriennikov, B.; Prabh, N.; Loschko, T.; Lanz, C.; Sommer, R.J. Young genes have distinct gene structure, epigenetic profiles, and transcriptional regulation. Genome Res. 2018, 28, 1675–1687. [Google Scholar] [CrossRef]
- Prabh, N.; Roeseler, W.; Witte, H.; Eberhardt, G.; Sommer, R.J.; Rödelsperger, C. Deep taxon sampling reveals the evolutionary dynamics of novel gene families in Pristionchus nematodes. Genome Res. 2018, 28, 1664–1674. [Google Scholar] [CrossRef]
- Lightfoot, J.W.; Wilecki, M.; Rödelsperger, C.; Moreno, E.; Susoy, V.; Witte, H.; Sommer, R.J. Small peptide–mediated self-recognition prevents cannibalism in predatory nematodes. Science 2019, 364, 86–89. [Google Scholar] [CrossRef]
- Baker, E.A.; Gilbert, S.P.R.; Shimeld, S.M.; Woollard, A. Extensive Non-Redundancy in a Recently Duplicated Developmental Gene Family. 2019; unpublished work. [Google Scholar]
- Hao, L.; Mukherjee, K.; Liegeois, S.; Baillie, D.; Labouesse, M.; Burglin, T.R. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans. Dev. Dyn. 2006, 235, 1469–1481. [Google Scholar] [CrossRef]
- Hao, L.; Aspock, G.; Burglin, T.R. The hedgehog-related gene wrt-5 is essential for hypodermal development in Caenorhabditis elegans. Dev. Biol. 2006, 290, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–2254. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, P.E.; Lee, M.H.; Schedl, T.; Jefferis, G.S. A C. elegans patched gene, ptc-1, functions in germ-line cytokinesis. Genes Dev. 2000, 14, 1933–1944. [Google Scholar] [PubMed]
- Perens, E.A.; Shaham, S. C. elegans daf-6 encodes a Patched-related protein required for lumen formation. Dev. Cell 2005, 8, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, J.A. Molecular cloning and duplication of the nematode sex-determining gene tra-1. Genetics 1993, 133, 543–560. [Google Scholar] [PubMed]
- Aboobaker, A.; Blaxter, M. Hox gene evolution in nematodes: Novelty conserved. Curr. Opin. Genet. Dev. 2003, 13, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Aboobaker, A.; Blaxter, M. Hox Gene Loss during Dynamic Evolution of the Nematode Cluster. Curr. Biol. 2003, 13, 37–40. [Google Scholar] [CrossRef]
- Duboule, D. The rise and fall of Hox gene clusters. Development 2007, 134, 2549–2560. [Google Scholar] [CrossRef]
- Aboobaker, A.; Blaxter, M. The nematode story: Hox gene loss and rapid evolution. Adv. Exp. Med. Biol. 2010, 689, 101–110. [Google Scholar]
- Gauchat, D.; Mazet, F.; Berney, C.; Schummer, M.; Kreger, S.; Pawlowski, J.; Galliot, B. Evolution of Antp-class genes and differential expression of Hydra Hox/paraHox genes in anterior patterning. Proc. Natl. Acad. Sci. USA 2000, 97, 4493–4498. [Google Scholar] [CrossRef]
- Sekigami, Y.; Kobayashi, T.; Omi, A.; Nishitsuji, K.; Ikuta, T.; Fujiyama, A.; Satoh, N.; Saiga, H. Hox gene cluster of the ascidian, Halocynthia roretzi, reveals multiple ancient steps of cluster disintegration during ascidian evolution. Zool. Lett. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.J.; Wu, W.; Hirai, H.; Ivens, A.; Murphy, L.D.; Noel, C.; Johnston, D.A.; Artiguenave, F.; Adams, M.; Cornette, J. Evidence for a dispersed Hox gene cluster in the platyhelminth parasite Schistosoma mansoni. Mol. Biol. Evol. 2005, 12, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Streit, A.; Kohler, R.; Marty, T.; Belfiore, M.; Takacs-Vellai, K.; Vigano, M.A.; Schnabel, R.; Affolter, M.; Muller, F. Conserved regulation of the Caenorhabditis elegans labial/Hox1 gene ceh-13. Dev. Biol. 2002, 242, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Van Auken, K.; Weaver, D.C.; Edgar, L.G.; Wood, W.B. Caenorhabditis elegans embryonic axial patterning requires two recently discovered posterior-group Hox genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4499–4503. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J.; Austin, J.; Costa, M.; Cowing, D.W.; Harris, J.M.; Honigberg, L.; Hunter, C.P.; Maloof, J.N.; Muller-Immergluck, M.M.; Salser, S.J. The dance of the Hox genes: Patterning the anteroposterior body axis of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 1997, 62, 293–305. [Google Scholar]
- Brooke, N.M.; Garcia-Fernàndez, J.; Holland, P.W.H. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature 1998, 392, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Lohnes, D. The Cdx1 homeodomain protein: An integrator of posterior signaling in the mouse. BioEssays 2003, 25, 971–980. [Google Scholar] [CrossRef]
- Copf, T.; Schroder, R.; Averof, M. Ancestral role of caudal genes in axis elongation and segmentation. Proc. Natl. Acad. Sci. USA 2004, 101, 17711–17715. [Google Scholar] [CrossRef]
- Zhang, H.; Azevedo, R.B.; Lints, R.; Doyle, C.; Teng, Y.; Haber, D.; Emmons, S.W. Global regulation of Hox gene expression in C. elegans by a SAM domain protein. Dev. Cell 2003, 4, 903–915. [Google Scholar] [CrossRef]
- Ross, J.M.; Zarkower, D. Polycomb group regulation of Hox gene expression in C. elegans. Dev. Cell 2003, 4, 891–901. [Google Scholar] [CrossRef]
- Hench, J.; Henriksson, J.; Akram, M.; Lüppert, M.; Dethlefsen, J.; Mukherjee, K.; Tong, G.; Tang, L.; Gangishetti, U.; Baillie, D.; et al. The Homeobox Genes of Caenorhabditis elegans and Insights into Their Spatio-Temporal Expression Dynamics during Embryogenesis. PLoS ONE 2015, 10, e0126947. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.W.H.; (Department of Zoology, University of Oxford, Oxford, UK). Personal Communications, 2019.
- Bürglin, T.; Affolter, M. Homeodomain proteins: An update. Chromosoma 2016, 125, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, T.L.; Holland, P.W.H. Diversity of human and mouse homeobox gene expression in development and adult tissues. BMC Dev. Biol. 2016, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Royall, A.H.; Maeso, I.; Dunwell, T.L.; Holland, P.W.H. Mouse Obox and Crxos modulate preimplantation transcriptional profiles revealing similarity between paralogous mouse and human homeobox genes. EvoDevo 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.R. Nusse Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Sawa, H.; Korswagen, H.C. Wnt signaling in C. elegans. WormBook Online C. elegans Biol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Phillips, B.T.; Amaya, M.F.; Kimble, J.; Xu, W. The C. elegans SYS-1 protein is a bona fide β-catenin. Dev. Cell 2008, 14, 751–761. [Google Scholar] [CrossRef]
- Phillips, B.T.; Kimble, J. A New Look at TCF and β-Catenin through the Lens of a Divergent C. elegans Wnt Pathway. Dev. Cell 2009, 17, 27–34. [Google Scholar] [CrossRef]
- Costa, M.; Raich, W.; Agbunag, C.; Leung, B.; Hardin, J.; Priess, J.R. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 1998, 141, 297–308. [Google Scholar] [CrossRef]
- Korswagen, H.C.; Herman, M.A.; Clevers, H.C. Distinct β-catenins mediate adhesion and signalling functions in C. elegans. Nature 2000, 406, 527–532. [Google Scholar] [CrossRef]
- Jackson, B.M.; Eisenmann, D.M. β-catenin-dependent Wnt signaling in C. elegans: Teaching an old Dog a new trick. Cold Spring Harb. Perspect. Biol. 2012, 4, a007948. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, I.; Kovall, R. Notch signaling: Genetics and structure. WormBook Online C. elegans Biol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Gazave, E.; Lapébie, P.; Richards, G.S.; Brunet, F.; Ereskovsky, A.V.; Degnan, B.M.; Borchiellini, C.; Vervoort, M.; Renard, E. Origin and evolution of the notch signalling pathway: An overview from eukaryotic genomes. BMC Evol. Biol. 2009, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Lambie, E.J.; Kimble, J. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development 1991, 112, 231–240. [Google Scholar] [PubMed]
- Austin, J.; Kimble, J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 1987, 51, 589–599. [Google Scholar] [CrossRef]
- Greenwald, I.S.; Sternberg, P.W.; Horvitz, H.R. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell 1983, 34, 435–444. [Google Scholar] [CrossRef]
- Fitzgerald, K. Wilkinson, H.A. and Greenwald, I. glp-1 can substitute for lin-12 in specifying cell fate decisions in Caenorhabditis elegans. Development 1993, 119, 1019–1022. [Google Scholar] [PubMed]
- Auderset, F.; Schuster, S.; Coutaz, M.; Koch, U.; Desgranges, F.; Merck, E.; Macdonald, H.R.; Radtke, F.; Tacchini-Cottier, F. Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with Leishmania major. PLoS Pathog. 2012, 8, e1002560. [Google Scholar] [CrossRef]
- Kitamoto, T.; Takahashi, K.; Takimoto, H.; Tomizuka, K.; Hayasaka, M.; Tabira, T.; Hanaoka, K. Functional redundancy of the Notch gene family during mouse embryogenesis: Analysis of Notch gene expression in Notch3-deficient mice. Biochem. Biophys. Res. Commun. 2005, 331, 1154–1162. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, E.A.; Woollard, A. How Weird is The Worm? Evolution of the Developmental Gene Toolkit in Caenorhabditis elegans. J. Dev. Biol. 2019, 7, 19. https://doi.org/10.3390/jdb7040019
Baker EA, Woollard A. How Weird is The Worm? Evolution of the Developmental Gene Toolkit in Caenorhabditis elegans. Journal of Developmental Biology. 2019; 7(4):19. https://doi.org/10.3390/jdb7040019
Chicago/Turabian StyleBaker, Emily A., and Alison Woollard. 2019. "How Weird is The Worm? Evolution of the Developmental Gene Toolkit in Caenorhabditis elegans" Journal of Developmental Biology 7, no. 4: 19. https://doi.org/10.3390/jdb7040019
APA StyleBaker, E. A., & Woollard, A. (2019). How Weird is The Worm? Evolution of the Developmental Gene Toolkit in Caenorhabditis elegans. Journal of Developmental Biology, 7(4), 19. https://doi.org/10.3390/jdb7040019




