Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of SCNT and AI Fetuses
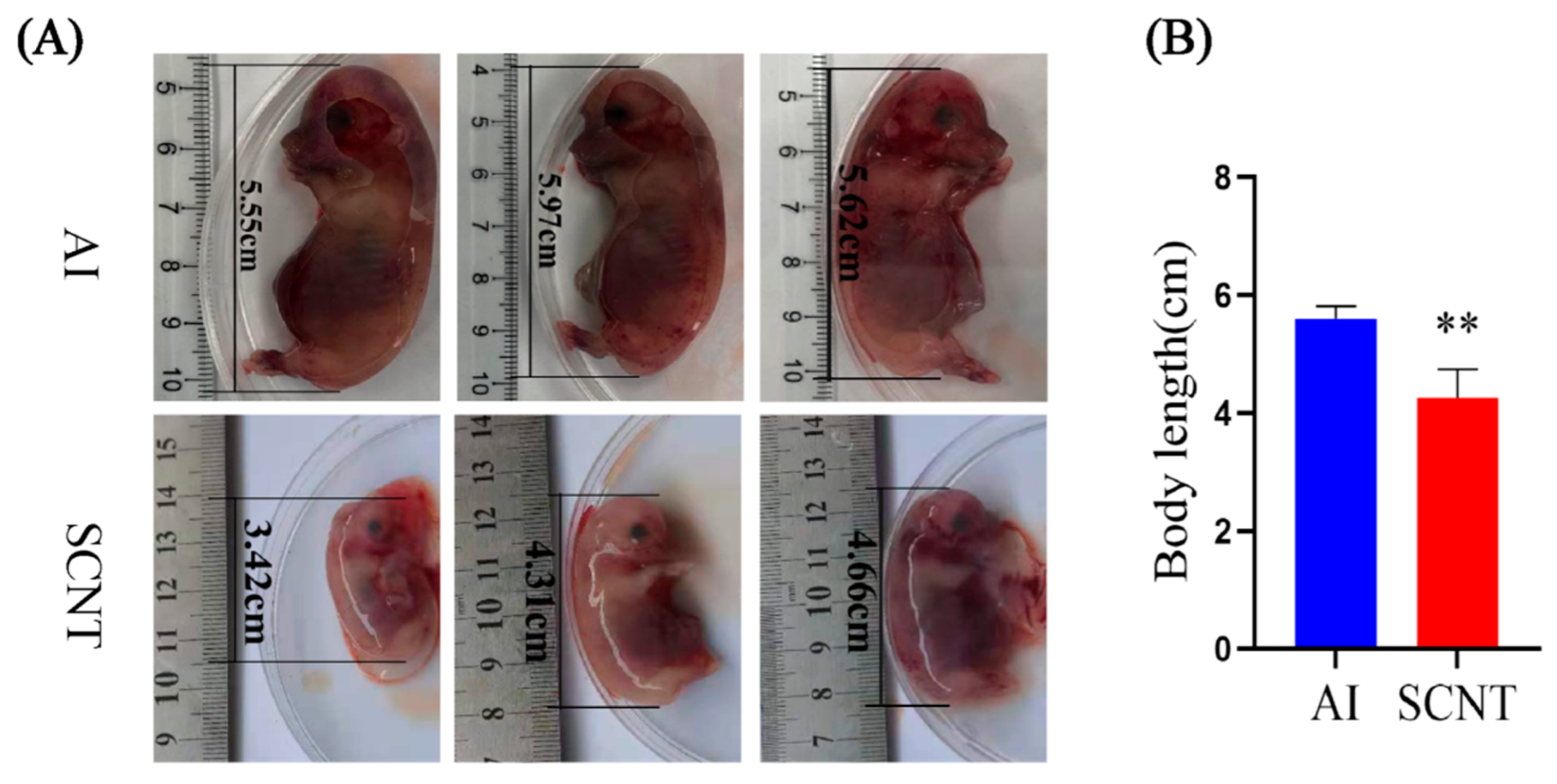
2.2. Placenta Sample Collection and Fetus Body Length Measurement
2.3. RNA Sequencing
2.4. Lipidomics Analysis
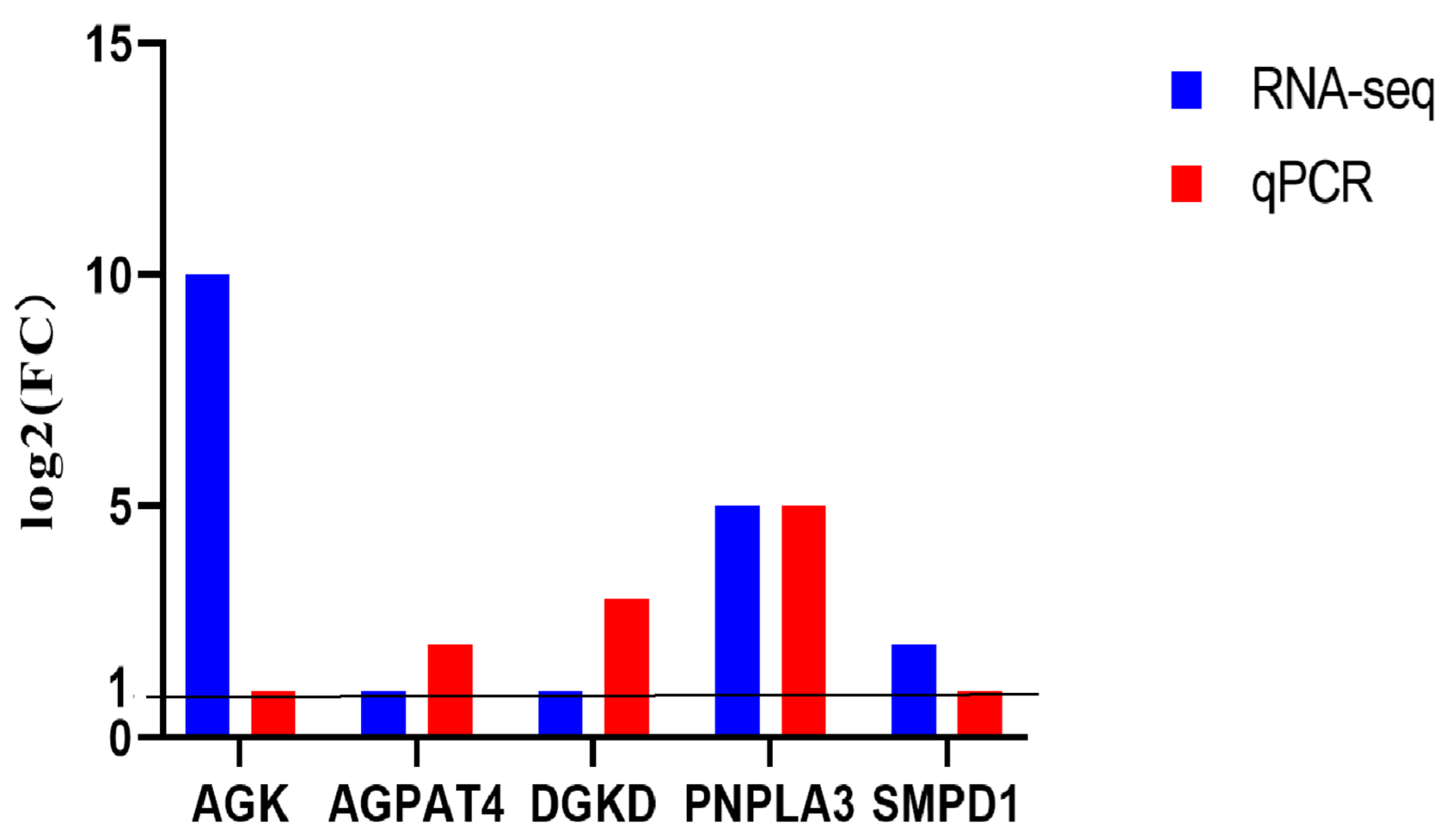
2.5. qPCR
2.6. Cell Culture
2.7. Cell Counting Kit-8 (CCK-8) Assay
2.8. EdU Assay
2.9. Cell Migration Assay
2.10. Statistical Analysis
3. Results
3.1. Comparison of Body Length Between AI and SCNT Fetuses
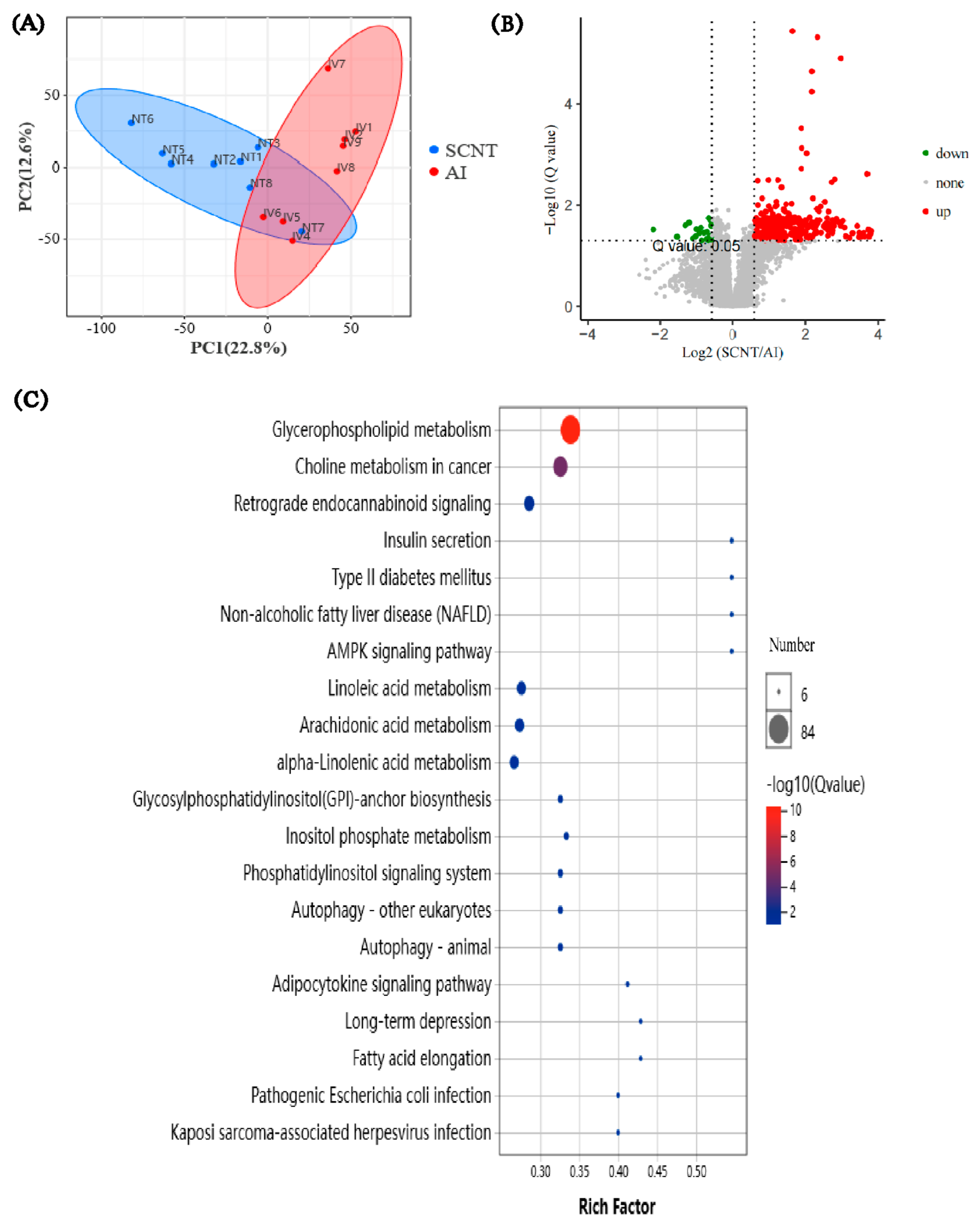
3.2. Transcriptome Analysis of AI and SCNT Fetal Placentas
3.3. Lipidomics Analysis of AI and SCNT Fetal Placentas
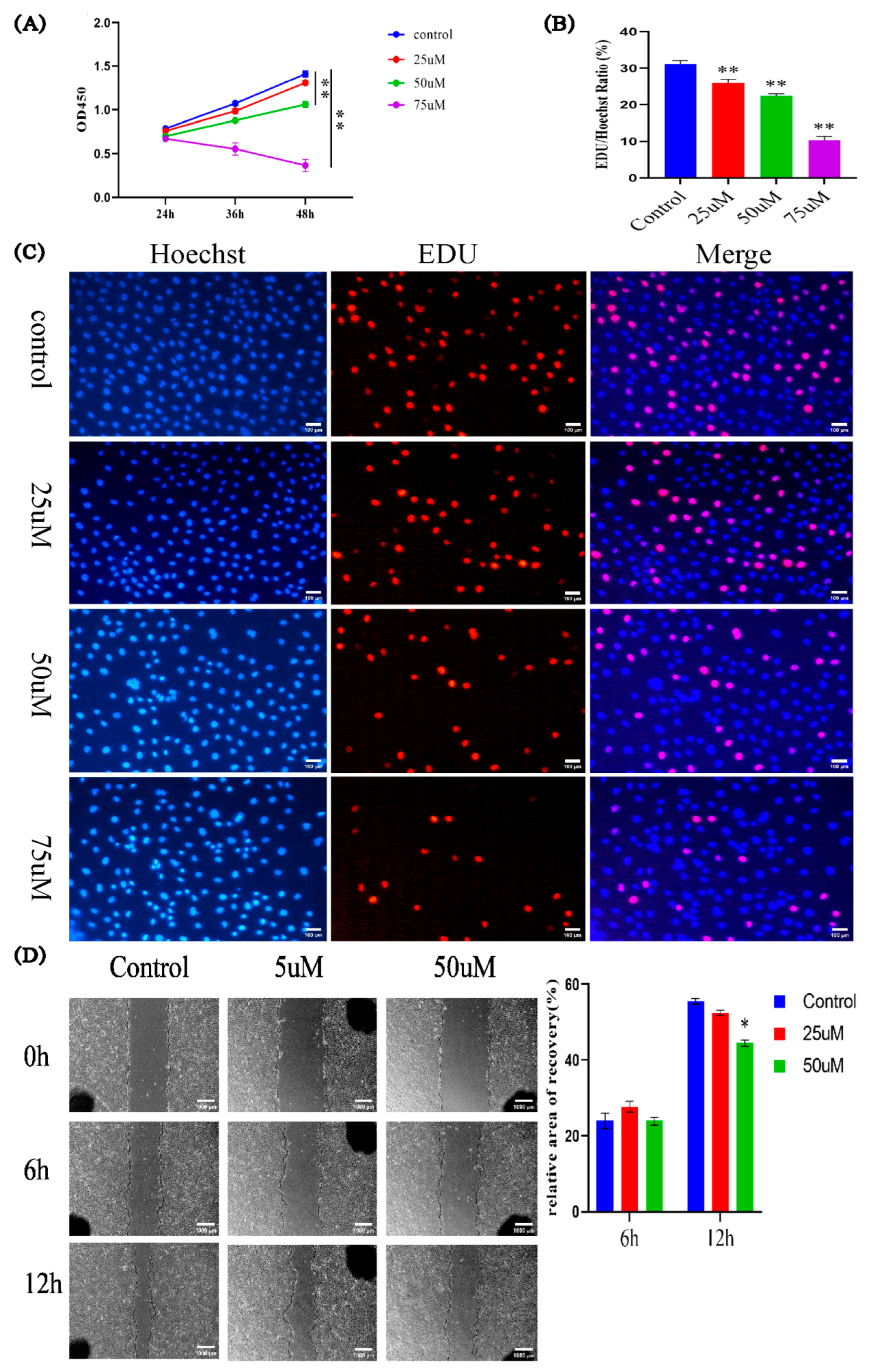
3.4. Effects of LPC on PTr2 Cell Proliferation and Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCNT | Somatic cell nuclear transfer |
| AI | Artificial insemination |
| LPC | Lysophosphatidylcholine |
| PZM-3 | Porcine fertilized egg medium 3 |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| QC | Quality control |
| TIC | Total ion chromatogram |
| OD | Optical density |
| PC | Phosphatidylcholine |
References
- Galli, C.; Lazzari, G. 25th anniversary of cloning by somatic-cell nuclear transfer: Current applications of SCNT in advanced breeding and genome editing in livestock. Reproduction 2021, 162, F23–F32. [Google Scholar] [PubMed]
- Gao, F.; Wang, Y.; Du, J.X.; Du, X.G.; Zhao, J.G.; Pan, D.K.; Wu, S.; Zhao, Y.F. Advances and applications of genetically modified pig models in biomedical and agricultural field. Yi Chuan 2023, 45, 6–28. [Google Scholar]
- Gomez, M.C.; Pope, C.E.; Ricks, D.M.; Lyons, J.; Dumas, C.; Dresser, B.L. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod. Fert. Dev. 2009, 21, 76–82. [Google Scholar] [CrossRef]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.S.; Sritanaudomchai, H.; et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef]
- Vajta, G.; Zhang, Y.; Machaty, Z. Somatic cell nuclear transfer in pigs: Recent achievements and future possibilities. Reprod. Fert. Dev. 2007, 19, 403–423. [Google Scholar] [CrossRef]
- Wang, X.; Qu, J.; Li, J.; He, H.; Liu, Z.; Huan, Y. Epigenetic Reprogramming During Somatic Cell Nuclear Transfer: Recent Progress and Future Directions. Front. Genet. 2020, 11, 205. [Google Scholar] [CrossRef]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Czernik, M.; Anzalone, D.A.; Palazzese, L.; Oikawa, M.; Loi, P. Somatic cell nuclear transfer: Failures, successes and the challenges ahead. Int. J. Dev. Biol. 2019, 63, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Wu, X.; Zhou, J.; Gu, T.; Wang, X.; Shi, J.; Zhao, C.; Cai, G.; Zheng, E.; Liu, D.; et al. Cloned pig fetuses exhibit fatty acid deficiency from impaired placental transport. Mol. Reprod. Dev. 2019, 86, 1569–1581. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Lovendahl, P.; Schmidt, M.; Larsen, K.; Callesen, H. In vitro manipulation techniques of porcine embryos: A meta-analysis related to transfers, pregnancies and piglets. Reprod. Fert. Dev. 2015, 27, 429–439. [Google Scholar] [CrossRef]
- Matoba, S.; Wang, H.; Jiang, L.; Lu, F.; Iwabuchi, K.A.; Wu, X.; Inoue, K.; Yang, L.; Press, W.; Lee, J.T.; et al. Loss of H3K27me3 Imprinting in Somatic Cell Nuclear Transfer Embryos Disrupts Post-Implantation Development. Cell Stem Cell 2018, 23, 343–354. [Google Scholar] [CrossRef]
- Almeida, F.; Dias, A. Pregnancy in pigs: The journey of an early life. Domest. Anim. Endocrin. 2022, 78, 106656. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R.; Gernand, A.D. Maternal-Fetal Inflammation in the Placenta and the Developmental Origins of Health and Disease. Front. Immunol. 2020, 11, 531543. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Friedman, A.M. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin. Perinatol. 2014, 38, 151–158. [Google Scholar] [CrossRef]
- Endler, M.; Saltvedt, S.; Cnattingius, S.; Stephansson, O.; Wikstrom, A.K. Retained placenta is associated with pre-eclampsia, stillbirth, giving birth to a small-for-gestational-age infant, and spontaneous preterm birth: A national register-based study. BJOG-Int. J. Obstet. Gynaecol. 2014, 121, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Vintzileos, A.M. Ischemic placental disease: Epidemiology and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 77–82. [Google Scholar] [CrossRef]
- Hashizume, K.; Ishiwata, H.; Kizaki, K.; Yamada, O.; Takahashi, T.; Imai, K.; Patel, O.V.; Akagi, S.; Shimizu, M.; Takahashi, S.; et al. Implantation and placental development in somatic cell clone recipient cows. Cloning Stem Cells 2002, 4, 197–209. [Google Scholar] [CrossRef]
- Hill, J.R.; Burghardt, R.C.; Jones, K.; Long, C.R.; Looney, C.R.; Shin, T.; Spencer, T.E.; Thompson, J.A.; Winger, Q.A.; Westhusin, M.E. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 2000, 63, 1787–1794. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.Y.; Choi, Y.J.; Cho, S.K.; Ahn, J.D.; Kwon, D.N.; Hwang, K.C.; Kang, S.J.; Paik, S.S.; Seo, H.G.; et al. Comparative proteomic analysis associated with term placental insufficiency in cloned pig. Proteomics 2007, 7, 1303–1315. [Google Scholar] [CrossRef]
- Pozor, M.A.; Sheppard, B.; Hinrichs, K.; Kelleman, A.A.; Macpherson, M.L.; Runcan, E.; Choi, Y.H.; Diaw, M.; Mathews, P.M. Placental abnormalities in equine pregnancies generated by SCNT from one donor horse. Theriogenology 2016, 86, 1573–1582. [Google Scholar] [CrossRef]
- Ao, Z.; Li, Z.; Wang, X.; Zhao, C.; Gan, Y.; Wu, X.; Zeng, F.; Shi, J.; Gu, T.; Hong, L.; et al. Identification of amniotic fluid metabolomic and placental transcriptomic changes associated with abnormal development of cloned pig fetuses. Mol. Reprod. Dev. 2019, 86, 278–291. [Google Scholar] [CrossRef]
- Ao, Z.; Liu, D.; Zhao, C.; Yue, Z.; Shi, J.; Zhou, R.; Cai, G.; Zheng, E.; Li, Z.; Wu, Z. Birth weight, umbilical and placental traits in relation to neonatal loss in cloned pigs. Placenta 2017, 57, 94–101. [Google Scholar] [CrossRef]
- Inoue, K.; Kohda, T.; Lee, J.; Ogonuki, N.; Mochida, K.; Noguchi, Y.; Tanemura, K.; Kaneko-Ishino, T.; Ishino, F.; Ogura, A. Faithful expression of imprinted genes in cloned mice. Science 2002, 295, 297. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, J.; Huan, Y.; Liu, Z.; Yang, C.; Zhang, X.; Mu, Y.; Xia, P.; Liu, Z. Aberrant expression and methylation status of putatively imprinted genes in placenta of cloned piglets. Cell Reprogram. 2010, 12, 213–222. [Google Scholar] [CrossRef]
- Guillomot, M.; Taghouti, G.; Constant, F.; Degrelle, S.; Hue, I.; Chavatte-Palmer, P.; Jammes, H. Abnormal expression of the imprinted gene Phlda2 in cloned bovine placenta. Placenta 2010, 31, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shi, L.; Zhang, M.; Yang, H.; Qin, Y.; Zhang, J.; Gong, D.; Zhang, X.; Li, D.; Li, J. Defects in Trophoblast Cell Lineage Account for the Impaired In Vivo Development of Cloned Embryos Generated by Somatic Nuclear Transfer. Cell Stem Cell 2011, 8, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zang, X.; Hu, Q.; He, Y.; Xu, Z.; Xie, Y.; Gu, T.; Yang, H.; Yang, J.; Shi, J.; et al. Uterine luminal-derived extracellular vesicles: Potential nanomaterials to improve embryo implantation. J. Nanobiotechnol. 2023, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Bellot, G.; Cartron, P.F.; Er, E.; Oliver, L.; Juin, P.; Armstrong, L.C.; Bornstein, P.; Mihara, K.; Manon, S.; Vallette, F.M. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2007, 14, 785–794. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Yang, C.; Li, C.; Xue, B.; Zhou, J. Clinical implication and potential function of ARHGEF6 in acute myeloid leukemia: An in vitro study. PLoS ONE 2023, 18, e283934. [Google Scholar] [CrossRef]
- Maiti, A.K. Gene network analysis of oxidative stress-mediated drug sensitivity in resistant ovarian carcinoma cells. Pharmacogenomics. J. 2010, 10, 94–104. [Google Scholar] [CrossRef][Green Version]
- Pan, Z.; Chen, C.; Long, H.; Lei, C.; Tang, G.; Li, L.; Feng, J.; Chen, F. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. Mol. Med. Rep. 2013, 7, 969–974. [Google Scholar] [CrossRef]
- Akutsu, N.; Yamamoto, H.; Sasaki, S.; Taniguchi, H.; Arimura, Y.; Imai, K.; Shinomura, Y. Association of glypican-3 expression with growth signaling molecules in hepatocellular carcinoma. World J. Gastroentero. 2010, 16, 3521–3528. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Y.; Song, Z.; Feng, S.; Tian, X.; Du, W.; Qiu, X.; Heese, K.; Wu, M. Livin promotes Smac/DIABLO degradation by ubiquitin-proteasome pathway. Cell Death Differ. 2006, 13, 2079–2088. [Google Scholar] [CrossRef]
- Vucic, D.; Stennicke, H.R.; Pisabarro, M.T.; Salvesen, G.S.; Dixit, V.M. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 2000, 10, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Ryborg, A.K.; Deleuran, B.; Sogaard, H.; Kragballe, K. Intracutaneous injection of lysophosphatidylcholine induces skin inflammation and accumulation of leukocytes. Acta Derm.-Venereol. 2000, 80, 242–246. [Google Scholar][Green Version]
- Ousman, S.S.; David, S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 2000, 30, 92–104. [Google Scholar] [CrossRef]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.J. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.A.; Spiegel, A.K.; Ing, N.H.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Functional effects of transforming growth factor beta on adhesive properties of porcine trophectoderm. Endocrinology 2005, 146, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.I.; Cho, S.K.; Seo, J.W.; Yoon, T.S.; Lee, K.S.; Kim, J.H.; Lee, K.K.; Han, Y.M.; Yu, K. Proteomic analysis of the extraembryonic tissue from cloned porcine embryos. Mol. Cell Proteom. 2006, 5, 1559–1566. [Google Scholar] [CrossRef][Green Version]
- Ka, H.; Seo, H.; Kim, M.; Moon, S.; Kim, H.; Lee, C.K. Gene expression profiling of the uterus with embryos cloned by somatic cell nuclear transfer on day 30 of pregnancy. Anim. Reprod. Sci. 2008, 108, 79–91. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, B.; Yang, S.; Zhang, M.; Wang, J.; Liu, M.; Chen, J. Role of autophagy in lysophosphatidylcholine-induced apoptosis in mouse Leydig cells. Env. Toxicol. 2022, 37, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, J.; Ma, B.; Wang, J.; Chen, J. Role of Autophagy in Lysophosphatidylcholine-Induced Apoptosis of Mouse Ovarian Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 1479. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Kang, Y.H.; Oh, W.J.; Roh, C.R.; Kim, D.K.; Kang, C.D. 17beta-Estradiol Inhibits Lysophosphatidylcholine-Induced Apoptosis in Cultured Vascular Smooth Muscle Cells. J. Menopausal. Med. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Leong, I.L.; Cheng, K.S.; Shiao, L.R.; Su, T.H.; Wong, K.L.; Chan, P.; Leung, Y.M. Lysophosphatidylcholine-induced cytotoxicity and protection by heparin in mouse brain bEND.3 endothelial cells. Fund. Clin. Pharmacol. 2019, 33, 52–62. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, G.R. TRPC1/TRPC3 channels mediate lysophosphatidylcholine-induced apoptosis in cultured human coronary artery smooth muscles cells. Oncotarget 2016, 7, 50937–50951. [Google Scholar] [CrossRef]
- Gauster, M.; Rechberger, G.; Sovic, A.; Horl, G.; Steyrer, E.; Sattler, W.; Frank, S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 2005, 46, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; Gansevoort, R.T.; Dikkeschei, B.D.; de Zeeuw, D.; de Jong, P.E.; Van Tol, A. Role of elevated lecithin: Cholesterol acyltransferase and cholesteryl ester transfer protein activities in abnormal lipoproteins from proteinuric patients. Kidney Int. 1993, 44, 91–97. [Google Scholar] [CrossRef]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef]
- Carneiro, A.B.; Iaciura, B.M.; Nohara, L.L.; Lopes, C.D.; Veas, E.M.; Mariano, V.S.; Bozza, P.T.; Lopes, U.G.; Atella, G.C.; Almeida, I.C.; et al. Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-kappaB translocation and MAPK/ERK phosphorylation. PLoS ONE 2013, 8, e76233. [Google Scholar] [CrossRef]
- Liang, G.H.; Park, S.; Kim, M.Y.; Kim, J.A.; Choi, S.; Suh, S.H. Modulation of nonselective cation current by oxidized LDL and lysophosphatidylcholine and its inhibitory contribution to endothelial damage. Life Sci. 2010, 86, 733–739. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, J.A.; Park, M.H.; Jung, S.C.; Suh, S.H.; Pang, M.G.; Kim, Y.J. Lysophosphatidylcholine induces endothelial cell injury by nitric oxide production through oxidative stress. J. Matern.-Fetal Neonatal Med. 2009, 22, 325–331. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Colles, S.M.; Damron, D.S.; Graham, L.M. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arter. Throm. Vas. 2003, 23, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Kawashima, S.; Yamashita, T.; Ueyama, T.; Ishido, S.; Hotta, H.; Hirata, K.; Yokoyama, M. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arter. Throm. Vas. 2000, 20, 1006–1012. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Guo, F.; Sun, X.; Yuan, K.; Wang, Q.; Lan, C. Lysophosphatidylcholine induces apoptosis and inflammatory damage in brain microvascular endothelial cells via GPR4-mediated NLRP3 inflammasome activation. Toxicol. Vitr. 2021, 77, 105227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, J.H.; Kim, N.H.; Lee, C.W.; Kim, M.J.; Kim, S.H.; Huh, S.O. Lysophosphatidylcholine-induced apoptosis in H19-7 hippocampal progenitor cells is enhanced by the upregulation of Fas Ligand. Biochim. Biophys. Acta 2009, 1791, 61–68. [Google Scholar] [CrossRef]
- Ojala, P.J.; Hirvonen, T.E.; Hermansson, M.; Somerharju, P.; Parkkinen, J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J. Leukoc. Biol. 2007, 82, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y. The Inhibitory Effect of Lysophosphatidylcholine on Proangiogenesis of Human CD34(+) Cells Derived Endothelial Progenitor Cells. Front. Mol. Biosci. 2021, 8, 682367. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liu, Y.; Yan, F.; Zeng, Y.; Song, Y.; Fang, H.; Song, D.; Wang, X. Lysophosphatidylcholine inhibits lung cancer cell proliferation by regulating fatty acid metabolism enzyme long-chain acyl-coenzyme A synthase 5. Clin. Transl. Med. 2023, 13, e1180. [Google Scholar] [CrossRef]
- Huang, F.; Subbaiah, P.V.; Holian, O.; Zhang, J.; Johnson, A.; Gertzberg, N.; Lum, H. Lysophosphatidylcholine increases endothelial permeability: Role of PKCalpha and RhoA cross talk. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L176–L185. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Song, F.; Xu, S.; Nian, L.; Zhou, X.; Wang, S. TSG attenuates LPC-induced endothelial cells inflammatory damage through notch signaling inhibition. Iubmb. Life 2016, 68, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.; Sandhya, R.M.; Gerber, C.E.; Mukhopadhyay, C.; Ransohoff, R.M.; Chisolm, G.M.; Kottke-Marchant, K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J. Mol. Cell. Cardiol. 2003, 35, 1375–1384. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.; Gao, X.; Zhang, G.; Wu, X.; Zhang, Y.; Wang, S.; Wu, Z.; Li, Z.; Xu, Z. Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity. J. Dev. Biol. 2025, 13, 41. https://doi.org/10.3390/jdb13040041
Lai J, Gao X, Zhang G, Wu X, Zhang Y, Wang S, Wu Z, Li Z, Xu Z. Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity. Journal of Developmental Biology. 2025; 13(4):41. https://doi.org/10.3390/jdb13040041
Chicago/Turabian StyleLai, Junkun, Xiaoyu Gao, Guke Zhang, Xiao Wu, Yiqian Zhang, Shunbo Wang, Zhenfang Wu, Zicong Li, and Zheng Xu. 2025. "Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity" Journal of Developmental Biology 13, no. 4: 41. https://doi.org/10.3390/jdb13040041
APA StyleLai, J., Gao, X., Zhang, G., Wu, X., Zhang, Y., Wang, S., Wu, Z., Li, Z., & Xu, Z. (2025). Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity. Journal of Developmental Biology, 13(4), 41. https://doi.org/10.3390/jdb13040041





