Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Pharmacological Exposures
2.3. Geometric Morphometric Analysis
2.4. Whole-Mount Immunohistochemistry
2.5. Alcian Blue Staining
2.6. Image Adjustments
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Statistical Analysis
2.9. Behavioral Scoring
3. Results
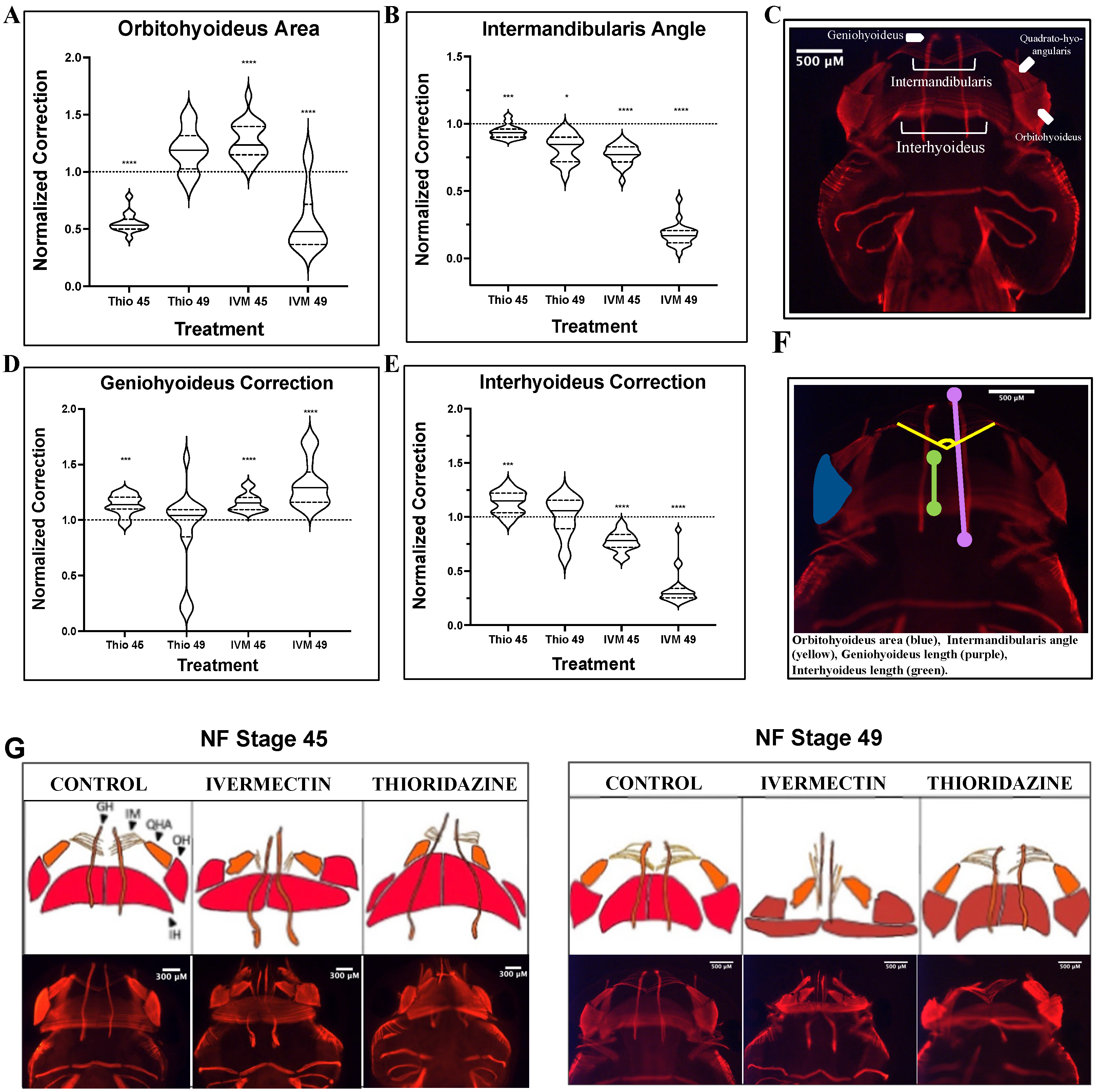
3.1. Ivermectin-Induced Craniofacial Defects Show Limited Remodeling Capabilities of Major Landmark Structures Through Pre-Metamorphic Stages
3.2. Major Musculature Defects in Ivermectin-Treatment Groups Fail to Show Evidence of Remodeling Through Pre-Metamorphic Periods
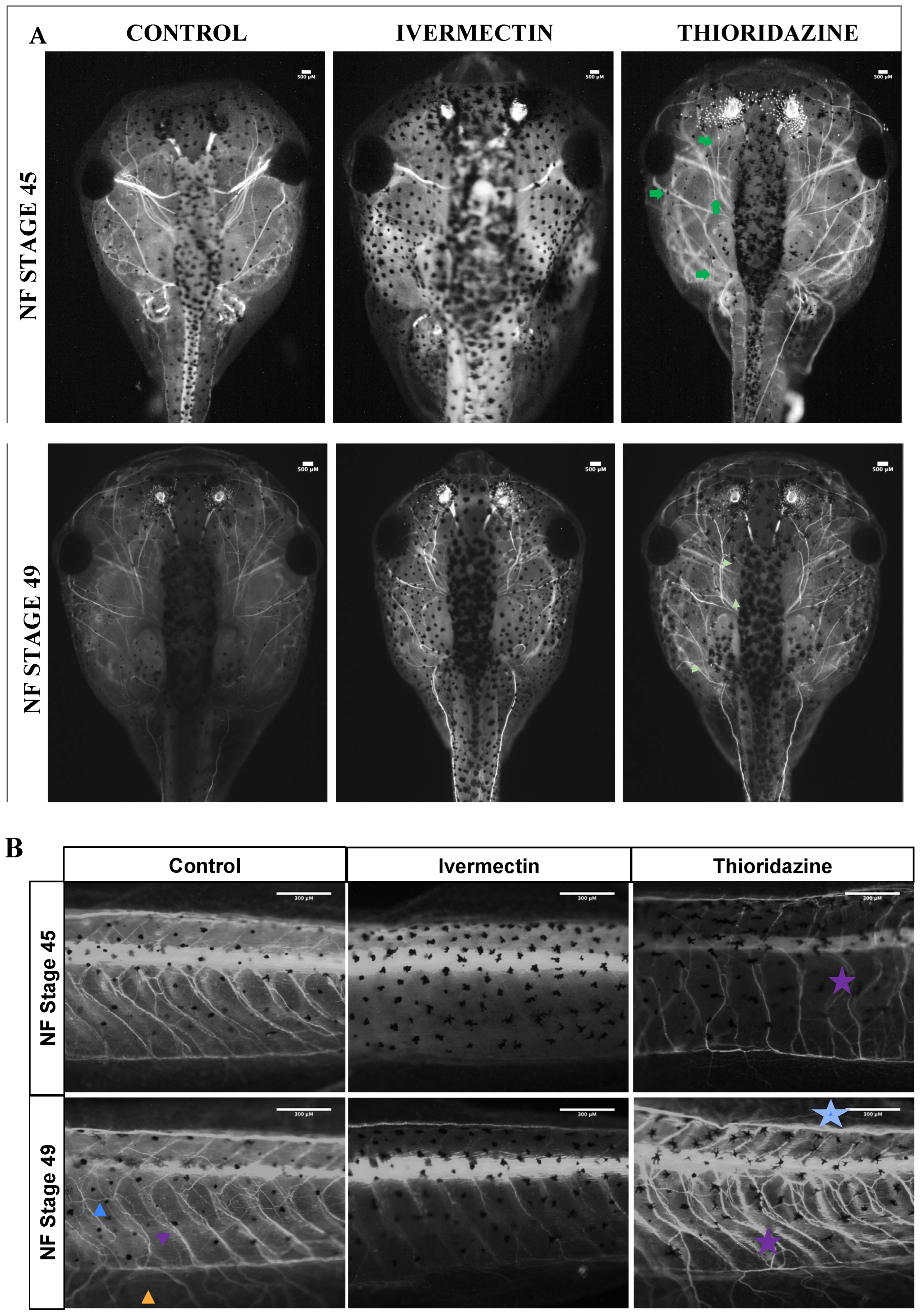
3.3. Remodeling Treatment Groups Display Distinct Peripheral Nerve Phenotypes Compared to Control Groups
3.4. Remodeling and Non-Remodeling Treatment Groups Display Distinct Expression of Remodeling-Associated Genes During Pre-Metamorphic Stages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Thio | Thioridazine |
| IVM | Ivermectin |
| Prl.2 | Prolactin 2 |
| MMP1 | Matrix Metalloproteinases 1 |
| MMP13 | Matrix Metalloproteinases 1 |
| Thra | Thyroid Hormone Receptor Alpha |
| Thrb | Thyroid Hormone Receptor Beta |
| Eef1a | Eukaryotic Elongation factor 1 |
| CNS | Central Nervous System |
| PNS | Peripheral Nervous System |
References
- CDC Data and Statistics on Birth Defects. Available online: https://www.cdc.gov/birth-defects/data-research/facts-stats/index.html (accessed on 11 October 2024).
- CDC Cleft Lip/Cleft Palate. Available online: https://www.cdc.gov/birth-defects/about/cleft-lip-cleft-palate.html (accessed on 11 October 2024).
- Goodwin, A.F.; Kim, R.; Bush, J.O.; Klein, O.D. From Bench to Bedside and Back: Improving Diagnosis and Treatment of Craniofacial Malformations Utilizing Animal Models. Curr. Top. Dev. Biol. 2015, 115, 459–492. [Google Scholar] [CrossRef] [PubMed]
- Benard, E.L.; Küçükaylak, I.; Hatzold, J.; Berendes, K.U.W.; Carney, T.J.; Beleggia, F.; Hammerschmidt, M. Wnt10a Is Required for Zebrafish Median Fin Fold Maintenance and Adult Unpaired Fin Metamorphosis. Dev. Dyn. 2024, 253, 566–592. [Google Scholar] [CrossRef] [PubMed]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal Table of Postembryonic Zebrafish Development: Staging by Externally Visible Anatomy of the Living Fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, S.; Ji, W.; Li, X.; Shi, Z.; Hou, J.; Li, W.; Fu, Y. Thyroid Hormone Signaling Is Required for Dynamic Variation in Opsins in the Retina during Metamorphosis of the Japanese Flounder (Paralichthys Olivaceus). Biology 2023, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Vagner, M.; de Montgolfier, B.; Sévigny, J.-M.; Tremblay, R.; Audet, C. Expression of Genes Involved in Key Metabolic Processes during Winter Flounder (Pseudopleuronectes Americanus) Metamorphosis. Can. J. Zool. 2013, 91, 156–163. [Google Scholar] [CrossRef]
- Zhou, H.; Shu, R.; Zhang, C.; Xiao, Y.; Jing, D.; Tang, J.; Cao, Z.; Chen, X.; Mei, Y.; Li, F. Developmental Correspondence of Juvenile Stages across the Locust, Harlequin Ladybird, and Diamondback Moth. iScience 2024, 27, 110898. [Google Scholar] [CrossRef] [PubMed]
- Pinet, K.; Deolankar, M.; Leung, B.; McLaughlin, K.A. Adaptive Correction of Craniofacial Defects in Pre-Metamorphic Xenopus Laevis Tadpoles Involves Thyroid Hormone-Independent Tissue Remodeling. Development 2019, 146, dev175893. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkoop, P.D.; Faber, E.J. (Eds.) Normal Table of Xenopus Laevis (DAUDIN): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; Garland Science: New York, NY, USA, 1967. [Google Scholar]
- Vandenberg, L.N.; Adams, D.S.; Levin, M. Normalized Shape and Location of Perturbed Craniofacial Structures in the Xenopus Tadpole Reveal an Innate Ability to Achieve Correct Morphology. Dev. Dyn. 2012, 241, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Ataliotis, P.; Ivins, S.; Mohun, T.J.; Scambler, P.J. XTbx1 Is a Transcriptional Activator Involved in Head and Pharyngeal Arch Development in Xenopus Laevis. Dev. Dyn. 2005, 232, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Devotta, A.; Juraver-Geslin, H.; Gonzalez, J.A.; Hong, C.-S.; Saint-Jeannet, J.-P. Sf3b4-Depleted Xenopus Embryos: A Model to Study the Pathogenesis of Craniofacial Defects in Nager Syndrome. Dev. Biol. 2016, 415, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.J.G. Using Frogs Faces to Dissect the Mechanisms Underlying Human Orofacial Defects. Semin. Cell Dev. Biol. 2016, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.E.; Dickinson, A.J.G. Median Facial Clefts in Xenopus Laevis: Roles of Retinoic Acid Signaling and Homeobox Genes. Dev. Biol. 2012, 365, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Liu, K.J.; Kwan, M.D.; Quarto, N.; Longaker, M.T. Cranial Osteogenesis and Suture Morphology in Xenopus Laevis: A Unique Model System for Studying Craniofacial Development. PLoS ONE 2009, 4, e3914. [Google Scholar] [CrossRef] [PubMed]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, N.; Krol, K.M.; Macdonald, D.R.; Kawaja, M.D. Comparison of Target Innervation by Sympathetic Axons in Adult Wild Type and Heterozygous Mice for Nerve Growth Factor or Its Receptor trkA. J. Pineal Res. 2004, 37, 230–240. [Google Scholar] [CrossRef] [PubMed]
- McBratney-Owen, B.; Iseki, S.; Bamforth, S.D.; Olsen, B.R.; Morriss-Kay, G.M. Development and Tissue Origins of the Mammalian Cranial Base. Dev. Biol. 2008, 322, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Rajderkar, S.S.; Paraiso, K.; Amaral, M.L.; Kosicki, M.; Cook, L.E.; Darbellay, F.; Spurrell, C.H.; Osterwalder, M.; Zhu, Y.; Wu, H.; et al. Dynamic Enhancer Landscapes in Human Craniofacial Development. Nat. Commun. 2024, 15, 2030. [Google Scholar] [CrossRef] [PubMed]
- Thirumangalathu, S.; Harlow, D.E.; Driskell, A.L.; Krimm, R.F.; Barlow, L.A. Fate Mapping of Mammalian Embryonic Taste Bud Progenitors. Development 2009, 136, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Lee, P.G.; Uchimura, T.; Seufert, C.R.; Kwon, H.; Zeng, L. The Role of Muscle Cells in Regulating Cartilage Matrix Production. J. Orthop. Res. 2010, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.J.; Keshishian, H. Motoneurons Regulate Myoblast Proliferation and Patterning in Drosophila. Dev. Biol. 2005, 277, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Roddy, K.A.; Prendergast, P.J.; Murphy, P. Mechanical Influences on Morphogenesis of the Knee Joint Revealed through Morphological, Molecular and Computational Analysis of Immobilised Embryos. PLoS ONE 2011, 6, e17526. [Google Scholar] [CrossRef] [PubMed]
- Tokita, M.; Nakayama, T. Development of the Trigeminal Motor Neurons in Parrots: Implications for the Role of Nervous Tissue in the Evolution of Jaw Muscle Morphology. J. Morphol. 2014, 275, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Fini, J.B.; Le Mével, S.; Palmier, K.; Darras, V.M.; Punzon, I.; Richardson, S.J.; Clerget-Froidevaux, M.S.; Demeneix, B.A. Thyroid Hormone Signaling in the Xenopus Laevis Embryo Is Functional and Susceptible to Endocrine Disruption. Endocrinology 2012, 153, 5068–5081. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Sterner, Z.R.; Buchholz, D.R.; Shi, Y.-B.; Sachs, L.M. Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells 2022, 11, 1595. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B.; Sachs, L.M.; Jones, P.; Li, Q.; Ishizuya-Oka, A. Thyroid Hormone Regulation of Xenopus Laevis Metamorphosis: Functions of Thyroid Hormone Receptors and Roles of Extracellular Matrix Remodeling. Wound Repair Regen. 1998, 6, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Matsuda, H.; Shi, Y.-B. Developmental Regulation and Function of Thyroid Hormone Receptors and 9-Cis Retinoic Acid Receptors during Xenopus Tropicalis Metamorphosis. Endocrinology 2008, 149, 5610–5618. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; He, C.; Sifuentes, C.J.; Denver, R.J. Thyroid Hormone Receptor Alpha Is Required for Thyroid Hormone-Dependent Neural Cell Proliferation During Tadpole Metamorphosis. Front. Endocrinol. 2019, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Flores-Espinosa, P.; Olmos-Ortíz, A.; Granados-Cepeda, M.; Quesada-Reyna, B.; Vega-Sánchez, R.; Velázquez, P.; Zaga-Clavellina, V. Prolactin Protects the Structural Integrity of Human Fetal Membranes by Downregulating Inflammation-Induced Secretion of Matrix Metalloproteinases. Immunol. Investig. 2022, 51, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Hrabia, A.; Wolak, D.; Sechman, A. Response of the Matrix Metalloproteinase System of the Chicken Ovary to Prolactin Treatment. Theriogenology 2021, 169, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Brown, D.D. Prolactin Is Not a Juvenile Hormone in Xenopus Laevis Metamorphosis. Proc. Natl. Acad. Sci. USA 2000, 97, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-C.; West-Mays, J.A.; Stramer, B.M.; Byrne, M.H.; Scott, S.; Mody, M.K.; Sadow, P.M.; Krane, S.M.; Fini, M.E. Activity and Expression of Xenopus Laevis Matrix Metalloproteinases: Identification of a Novel Role for the Hormone Prolactin in Regulating Collagenolysis in Both Amphibians and Mammals. J. Cell. Physiol. 2004, 201, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Stellwag, E.J.; Zhu, Y. Prolactin-Dependent Modulation of Organogenesis in the Vertebrate: Recent Discoveries in Zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Plumb, D.A.; Tsuchimochi, K.; Dragomir, C.L.; Hashimoto, K.; Peng, H.; Olivotto, E.; Bevilacqua, M.; Tan, L.; Yang, Z.; et al. E74-like Factor 3 (ELF3) Impacts on Matrix Metalloproteinase 13 (MMP13) Transcriptional Control in Articular Chondrocytes under Proinflammatory Stress. J. Biol. Chem. 2012, 287, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix Remodeling by MMPs during Wound Repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sive, H.; Grainger, R.; Harland, R. Early Development of Xe-Nopus Laevis: A Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Olsen, A.M.; Westneat, M.W. StereoMorph: An R Package for the Collection of 3D Landmarks and Curves Using a Stereo Camera Set-Up. Methods Ecol. Evol. 2015, 6, 351–356. [Google Scholar] [CrossRef]
- Lukas, P.; Schmidt, J.; Olsson, L. Knockdown of Zax in Xenopus Laevis Leads to Craniofacial Malformations and the Absence of the Intramandibular Joint. Vertebr. Zool. 2019, 70, 9–22. [Google Scholar] [CrossRef]
- Deniz, E.; Jonas, S.; Hooper, M.; N.Griffin, J.; Choma, M.A.; Khokha, M.K. Analysis of Craniocardiac Malformations in Xenopus Using Optical Coherence Tomography. Sci. Rep. 2017, 7, 42506. [Google Scholar] [CrossRef] [PubMed]
- Lukas, P.; Ziermann, J.M. Sequence of Chondrocranial Development in Basal Anurans—Let’s Make a Cranium. Front. Zool. 2022, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, E.M.; Atkins, J.B.; Korneisel, D.E.; Cantelon, A.S.; McKinnell, I.W.; Maddin, H.C. Normal Development in Xenopus Laevis: A Complementary Staging Table for the Skull Based on Cartilage and Bone. Dev. Dyn. 2022, 251, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.S. The Cellular Basis of Cartilage Growth and Shape Change in Larval and Metamorphosing Xenopus Frogs. PLoS ONE 2023, 18, e0277110. [Google Scholar] [CrossRef] [PubMed]
- Bassel-Duby, R.; Olson, E.N. Signaling Pathways in Skeletal Muscle Remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-Induced Skeletal Muscle Remodeling and Metabolic Adaptation: Redox Signaling and Role of Autophagy. Antioxid. Redox Signal 2014, 21, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Flann, K.L.; LaStayo, P.C.; McClain, D.A.; Hazel, M.; Lindstedt, S.L. Muscle Damage and Muscle Remodeling: No Pain, No Gain? J. Exp. Biol. 2011, 214, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Shingo, T.; Gregg, C.; Enwere, E.; Fujikawa, H.; Hassam, R.; Geary, C.; Cross, J.C.; Weiss, S. Pregnancy-Stimulated Neurogenesis in the Adult Female Forebrain Mediated by Prolactin. Science 2003, 299, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Torner, L.; Karg, S.; Blume, A.; Kandasamy, M.; Kuhn, H.-G.; Winkler, J.; Aigner, L.; Neumann, I.D. Prolactin Prevents Chronic Stress-Induced Decrease of Adult Hippocampal Neurogenesis and Promotes Neuronal Fate. J. Neurosci. 2009, 29, 1826. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.L.; Vukovic, J.; Koudijs, M.M.; Blackmore, D.G.; Mackay, E.W.; Sykes, A.M.; Overall, R.W.; Hamlin, A.S.; Bartlett, P.F. Prolactin Stimulates Precursor Cells in the Adult Mouse Hippocampus. PLoS ONE 2012, 7, e44371. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Yung, P.S.-H.; Lee, Y.-W.; Fu, S.-C.; Chen, C.-H.; Rolf, C.G.; Chan, K.-M. Differential MMP 1 and MMP 13 Expression in Proliferation and Ligamentization Phases of Graft Remodeling in Anterior Cruciate Ligament Reconstruction. Connect. Tissue Res. 2021, 62, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Vaalamo, M.; Mattila, L.; Johansson, N.; Kariniemi, A.-L.; Karjalainen-Lindsberg, M.-L.; Kähäri, V.-M.; Saarialho-Kere, U. Distinct Populations of Stromal Cells Express Collagenase-3 (MMP-13) and Collagenase-1 (MMP-1) in Chronic Ulcers but Not in Normally Healing Wounds. J. Investig. Dermatol. 1997, 109, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional Regulation of Collagenase (MMP-1, MMP-13) Genes in Arthritis: Integration of Complex Signaling Pathways for the Recruitment of Gene-Specific Transcription Factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Anderson, G.M.; Rahman, N.; Bieck, C.; Levin, M. A Novel Method for Inducing Nerve Growth via Modulation of Host Resting Potential: Gap Junction-Mediated and Serotonergic Signaling Mechanisms. Neurotherapeutics 2015, 12, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Giordano, J.E.; Conte, S.; Levin, M.; Kaplan, D.L. Ivermectin Promotes Peripheral Nerve Regeneration during Wound Healing. ACS Omega 2018, 3, 12392–12402. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; DeMatteo, C.; Gjertsen, D.; Packham, T.; Galea, V.; Harper, J.A. Limb Length Differences after Obstetrical Brachial Plexus Injury: A Growing Concern. Plast. Reconstr. Surg. 2012, 130, 558e–571e. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Bubenik, A.B. Horns, Pronghorns, and Antlers: Evolution, Morphology, Physiology, and Social Significance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4613-8966-8. [Google Scholar]
- Fairfax, S.T.; Padilla, J.; Vianna, L.C.; Davis, M.J.; Fadel, P.J. Spontaneous Bursts of Muscle Sympathetic Nerve Activity Decrease Leg Vascular Conductance in Resting Humans. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H759–H766. [Google Scholar] [CrossRef] [PubMed]
- Popiela, H. In Vivo Limb Tissue Development in the Absence of Nerves: A Quantitative Study. Exp. Neurol. 1976, 53, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Waugh, M. The Primary Development of the Skeleton in Nerveless and Poorly Innervated Limb Transplants of Chick Embryos. Physiol. Zool. 1940, 13, 367–382. [Google Scholar] [CrossRef]
- Long, H.; Ahmed, M.; Ackermann, P.; Stark, A.; Li, J. Neuropeptide Y Innervation during Fracture Healing and Remodeling. Acta Orthop. 2010, 81, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Alfandari, D.; Cousin, H.; Marsden, M. Mechanism of Xenopus Cranial Neural Crest Cell Migration. Cell Adhes. Migr. 2010, 4, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Duband, J.-L. Neural Crest Delamination and Migration: Integrating Regulations of Cell Interactions, Locomotion, Survival and Fate. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Sadaghiani, B.; Thiébaud, C.H. Neural Crest Development in the Xenopus Laevis Embryo, Studied by Interspecific Transplantation and Scanning Electron Microscopy. Dev. Biol. 1987, 124, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Zahn, N.; James-Zorn, C.; Ponferrada, V.G.; Adams, D.S.; Grzymkowski, J.; Buchholz, D.R.; Nascone-Yoder, N.M.; Horb, M.; Moody, S.A.; Vize, P.D.; et al. Normal Table of Xenopus Development: A New Graphical Resource. Development 2022, 149, dev200356. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, Y.; Dubuc, A.M.; Hashizume, R.; Zhang, W.; Reimand, J.; Yang, H.; Wang, T.A.; Stehbens, S.J.; Younger, S.; et al. EAG2 Potassium Channel with Evolutionarily Conserved Function as a Brain Tumor Target. Nat. Neurosci. 2015, 18, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, J.; Cai, B.; Bai, X.; Zhu, Y. Ivermectin Accelerates Autophagic Death of Glioma Cells by Inhibiting Glycolysis through Blocking GLUT4 Mediated JAK/STAT Signaling Pathway Activation. Environ. Toxicol. 2022, 37, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhang, P.; Zhang, C.; Wang, W.; Wu, M.; Xu, W.; Tao, L.; Li, Z.; Zhang, Y. Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells. Molecules 2023, 28, 2201. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Sharma, A.; Gupta, S.; Mishra, A.; Singh, A. Antitumor Potential of Ivermectin against T-Cell Lymphoma-Bearing Hosts. Med. Oncol. 2025, 42, 169. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-W.; Ko, H.-J.; Chou, C.-H.; Cheng, T.-S.; Cheng, H.-W.; Liang, Y.-H.; Lai, Y.-L.; Lin, C.-Y.; Wang, C.; Loh, J.-K.; et al. Thioridazine Enhances P62-Mediated Autophagy and Apoptosis Through Wnt/β-Catenin Signaling Pathway in Glioma Cells. Int. J. Mol. Sci. 2019, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Kilts, C.D.; Knight, D.L.; Mailman, R.B.; Widerlöv, E.; Breese, G.R. Effects of Thioridazine and Its Metabolites on Dopaminergic Function: Drug Metabolism as a Determinant of the Antidopaminergic Actions of Thioridazine. J. Pharmacol. Exp. Ther. 1984, 231, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, T.I.; Yablonsky-Alter, E.; Zuck, L.G.; Banerjee, S.P. Antipsychotic Drug Effects on Glutamatergic Activity. Brain Res. 1997, 764, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Picton, L.D.; Sillar, K.T. Mechanisms Underlying the Endogenous Dopaminergic Inhibition of Spinal Locomotor Circuit Function in Xenopus Tadpoles. Sci. Rep. 2016, 6, 35749. [Google Scholar] [CrossRef] [PubMed]
- Pio-Lopez, L.; Kuchling, F.; Tung, A.; Pezzulo, G.; Levin, M. Active Inference, Morphogenesis, and Computational Psychiatry. Front. Comput. Neurosci. 2022, 16, 988977. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, D.A.; Jackvony, T.N.; Bookland, M.J.; Tang-Schomer, M.D. Targeting Ion Channels: Blockers Suppress Calcium Signals and Induce Cytotoxicity Across Medulloblastoma Cell Models. Bioengineering 2025, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Mondragon, A.; Lynch, J.W. Functional Characterization of Ivermectin Binding Sites in α1β2γ2L GABA(A) Receptors. Front. Mol. Neurosci. 2015, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Fuse, T.; Kita, T.; Nakata, Y.; Ozoe, F.; Ozoe, Y. Electrophysiological Characterization of Ivermectin Triple Actions on Musca Chloride Channels Gated by l-Glutamic Acid and γ-Aminobutyric Acid. Insect Biochem. Mol. Biol. 2016, 77, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Krůsek, J.; Zemková, H. Effect of Ivermectin on Gamma-Aminobutyric Acid-Induced Chloride Currents in Mouse Hippocampal Embryonic Neurones. Eur. J. Pharmacol. 1994, 259, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Trailovic, S.M.; Nedeljkovic, J.T. Central and Peripheral Neurotoxic Effects of Ivermectin in Rats. J. Vet. Med. Sci. 2011, 73, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; McLaughlin, K.A. A Novel Perspective on Neuronal Control of Anatomical Patterning, Remodeling, and Maintenance. Int. J. Mol. Sci. 2023, 24, 13358. [Google Scholar] [CrossRef] [PubMed]
- Pagella, P.; Jiménez-Rojo, L.; Mitsiadis, T.A. Roles of Innervation in Developing and Regenerating Orofacial Tissues. Cell Mol. Life Sci. 2014, 71, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Sudiwala, S.; Knox, S.M. The Emerging Role of Cranial Nerves in Shaping Craniofacial Development. Genesis 2019, 57, e23282. [Google Scholar] [CrossRef] [PubMed]
- Blanchi, D.; Camino, E.; Guardabassi, A. Chemoreceptors of the Lateral-Line Organs in Intact, Hypophysectomized, and Prolactin-Treated Hypophysectomized Xenopus Laevis Specimens. Comp. Biochem. Physiol. Part A Physiol. 1976, 55, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Porreca, F.; Navratilova, E. Prolactin and Pain of Endometriosis. Pharmacol. Ther. 2023, 247, 108435. [Google Scholar] [CrossRef] [PubMed]
- Scotland, P.E.; Patil, M.; Belugin, S.; Henry, M.A.; Goffin, V.; Hargreaves, K.M.; Akopian, A.N. Endogenous Prolactin Generated during Peripheral Inflammation Contributes to Thermal Hyperalgesia. Eur. J. Neurosci. 2011, 34, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Bowers, M.B., Jr. Thioridazine: Central Dopamine Turnover and Clinical Effects of Antipsychotic Drugs. Clin. Pharmacol. Ther. 1975, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ichikawa, J.; Meltzer, H.Y. Effect of Loxapine on Dopamine and Acetylcholine Release. Psychopharmacology 2003, 167, 315. [Google Scholar] [CrossRef] [PubMed]
- Parisi, D.P.; Santos, S.A.R.; Cabral, D.; Queiroz-Hazarbassanov, N.; Flório, J.C.; Bernardi, M.M.; Kirsten, T.B. Therapeutical Doses of Ivermectin and Its Association with Stress Disrupt Motor and Social Behaviors of Juvenile Rats and Serotonergic and Dopaminergic Systems. Res. Vet. Sci. 2019, 124, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, H.A.; Warnecke, A.M.P.; Barlow, J.C.; Robinson, J.K.; Steimle, E.; Ronström, J.W.; Williams, P.E.; Galbraith, C.J.; Baldridge, J.; Jakowec, M.W.; et al. Ivermectin Increases Striatal Cholinergic Activity to Facilitate Dopamine Terminal Function. Cell Biosci. 2024, 14, 50. [Google Scholar] [CrossRef] [PubMed]


| Landmark | Orientation | Morphology Assessed |
|---|---|---|
| Eye Ratios | Dorsal | Shape of the eye |
| Mouth Angle | Dorsal | Overall width of the most anterior jaw |
| Meckel’s Spread | Ventral | Specific width of the anterior jaw structures |
| Quadrate Spread | Ventral | Specific width of the posterior jaw structures |
| Ceratohyal Angle | Ventral | The curvature and lengths of the ceratohyal cartilages |
| Landmark | Corresponding Cartilage | Morphology Assessed |
|---|---|---|
| Orbitohyoideus Area | Quadrate | Growth and expansion of this muscle |
| Intermandibularis Angle | Meckel’s | Widening or condensing of the anterior jaw |
| Geniohyoideus Correction | N/A | Lengthening of the overall jaw |
| Interhyoideus Correction | Ceratohyal | Anterior-posterior expansion of this muscle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, E.; Fonticella, J.M.; McLaughlin, K.A. Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles. J. Dev. Biol. 2025, 13, 26. https://doi.org/10.3390/jdb13030026
Jones E, Fonticella JM, McLaughlin KA. Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles. Journal of Developmental Biology. 2025; 13(3):26. https://doi.org/10.3390/jdb13030026
Chicago/Turabian StyleJones, Emilie, Jay Miguel Fonticella, and Kelly A. McLaughlin. 2025. "Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles" Journal of Developmental Biology 13, no. 3: 26. https://doi.org/10.3390/jdb13030026
APA StyleJones, E., Fonticella, J. M., & McLaughlin, K. A. (2025). Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles. Journal of Developmental Biology, 13(3), 26. https://doi.org/10.3390/jdb13030026







