In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals and Experimental Design
2.3. Post-Mortem and Tissue Collection
2.4. Quantification of Cardiac mRNA Expression
2.5. Quantification of Cardiac Protein Expression
2.6. Quantification of Cardiac Concentration of Glucocorticoid and Thyroid Hormones
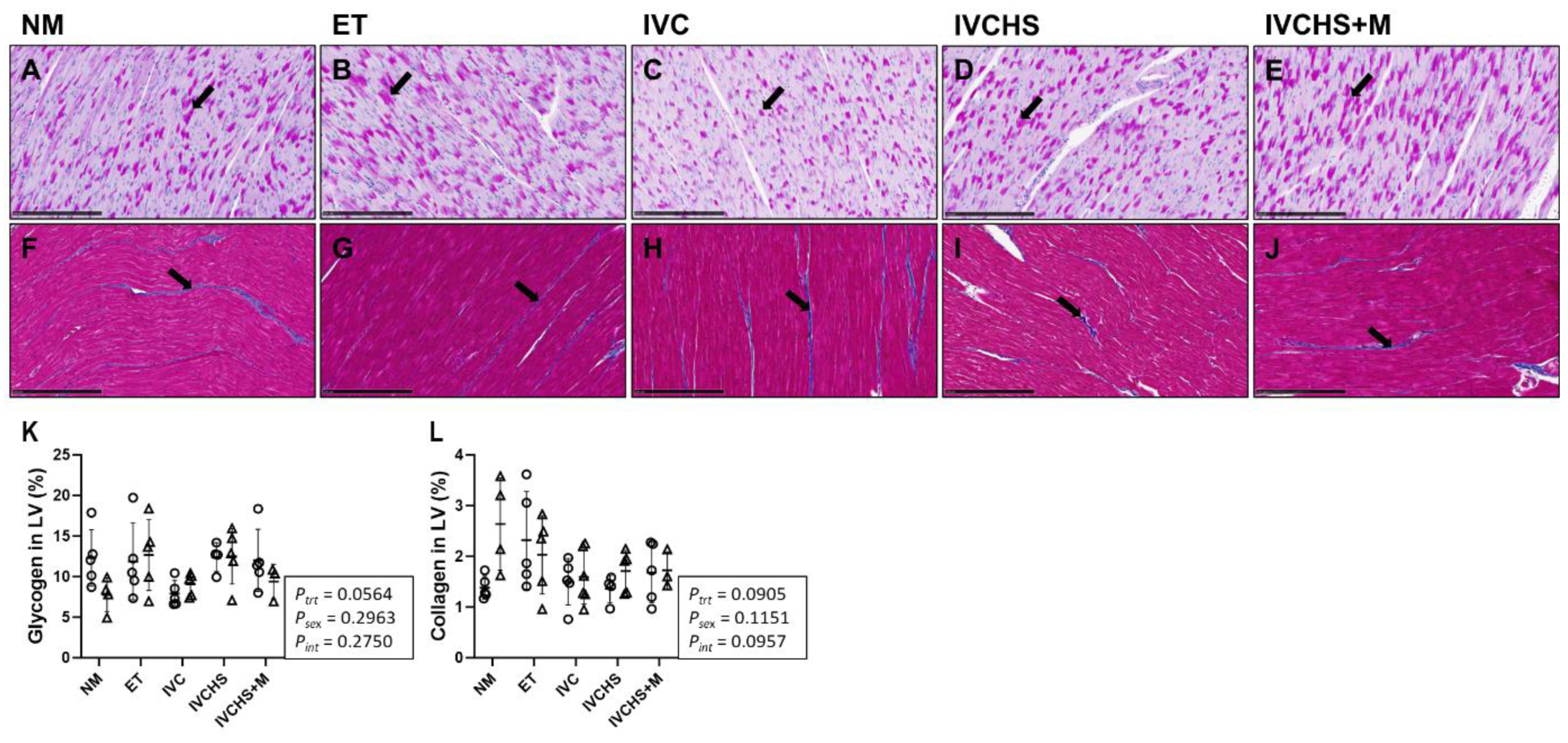
2.7. Quantification of Cardiac Glycogen and Collagen Staining
2.8. Statistical Analyses
3. Results
3.1. IVC Did Not Impact Cardiac Hormones and Glucocorticoid Receptors
3.2. IVC Reduced Mitochondrial Abundance and OXPHOS, Particularly in Males
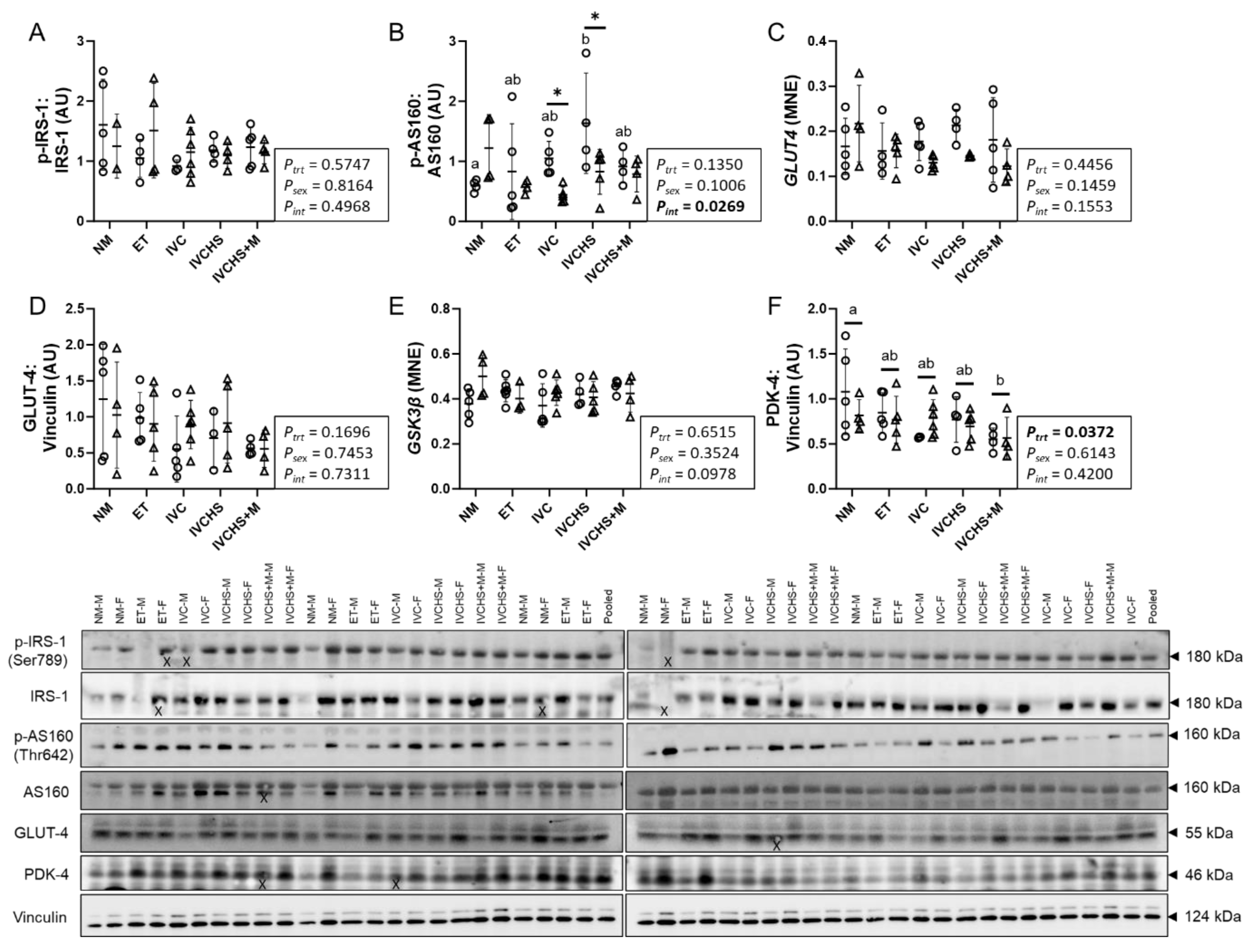
3.3. IVC Altered a Marker of Cardiac Metabolic Switch
3.4. IVC Did Not Alter Markers of Cardiac Fatty Acid Metabolism
3.5. IVC Reduced a Marker of Cardiac Antioxidant Defense, with No Effect on Markers of Contractility
3.6. IVC Did Not Alter Cardiac Glycogen and Collagen Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I. ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar]
- de Castro, R.C.F.; Buranello, T.W.; Recchia, K.; de Souza, A.F.; Pieri, N.C.G.; Bressan, F.F. Emerging Contributions of Pluripotent Stem Cells to Reproductive Technologies in Veterinary Medicine. J. Dev. Biol. 2024, 12, 14. [Google Scholar] [CrossRef]
- Celermajer, D.S. Manipulating nature: Might there be a cardiovascular price to pay for the miracle of assisted conception? Circulation 2012, 125, 1832–1834. [Google Scholar] [CrossRef]
- Padhee, M.; Lock, M.C.; McMillen, I.C.; Zhang, S.; Botting, K.J.; Nyengaard, J.R.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K.; Kelly, J.M.; et al. Sex-specific effects of in vitro culture and embryo transfer on cardiac growth in sheep offspring. J. Mol. Cell. Cardiol. Plus 2023, 5, 100039. [Google Scholar] [CrossRef]
- Fernández-Gonzalez, R.; Moreira, P.; Bilbao, A.; Jiménez, A.; Pérez-Crespo, M.; Ramírez, M.A.; De Fonseca, F.R.; Pintado, B.; Gutiérrez-Adán, A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 5880–5885. [Google Scholar] [CrossRef]
- Padhee, M.; Zhang, S.; Lie, S.; Wang, K.C.; Botting, K.J.; McMillen, I.C.; MacLaughlin, S.M.; Morrison, J.L. The Periconceptional Environment and Cardiovascular Disease: Does In Vitro Embryo Culture and Transfer Influence Cardiovascular Development and Health? Nutrients 2015, 7, 1378–1425. [Google Scholar] [CrossRef]
- Padhee, M.; McMillen, I.C.; Zhang, S.; MacLaughlin, S.M.; Armitage, J.A.; Head, G.A.; Darby, J.R.; Kelly, J.M.; Rudiger, S.R.; Kleemann, D.O. Impact of in vitro embryo culture and transfer on blood pressure regulation in the adolescent lamb. J. Dev. Orig. Health Dis. 2021, 12, 731–737. [Google Scholar] [CrossRef]
- Talwar, P. Manual of Assisted Reproductive Technologies and Clinical Embryology; JP Medical Ltd.: Delhi, India, 2012. [Google Scholar]
- Young, L.E.; Fernandes, K.; McEvoy, T.G.; Butterwith, S.C.; Gutierrez, C.G.; Carolan, C.; Broadbent, P.J.; Robinson, J.J.; Wilmut, I.; Sinclair, K.D. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 2001, 27, 153–154. [Google Scholar] [CrossRef]
- Sinclair, K.D.; McEvoy, T.; Maxfield, E.; Maltin, C.; Young, L.; Wilmut, I.; Broadbent, P.; Robinson, J. Aberrant fetal growth and development after in vitro culture of sheep zygotes. Reproduction 1999, 116, 177–186. [Google Scholar] [CrossRef]
- Farin, P.W.; Farin, C.E. Transfer of bovine embryos produced in vivo or in vitro: Survival and fetal development. Biol. Reprod. 1995, 52, 676–682. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Harper, J.C. IVF culture media: Past, present and future. Hum. Reprod. Update 2015, 21, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E. Culture media in IVF: Decisions for the laboratory. In In Vitro Fertilization: A Textbook of Current and Emerging Methods and Devices; Springer: Berlin/Heidelberg, Germany, 2019; pp. 105–119. [Google Scholar]
- Feuer, S.; Rinaudo, P. From embryos to adults: A DOHaD perspective on in vitro fertilization and other assisted reproductive technologies. Healthcare 2016, 4, 51. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Aljahdali, A.; Airina, R.R.I.; Velazquez, M.A.; Sheth, B.; Wallen, K.; Osmond, C.; Watkins, A.J.; Eckert, J.J.; Smyth, N.R.; Fleming, T.P. The duration of embryo culture after mouse IVF differentially affects cardiovascular and metabolic health in male offspring. Hum. Reprod. 2020, 35, 2497–2514. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Elhakeem, A.; Taylor, A.E.; Inskip, H.M.; Huang, J.Y.; Mansell, T.; Rodrigues, C.; Asta, F.; Blaauwendraad, S.M.; Håberg, S.E.; Halliday, J. Long-term cardiometabolic health in people born after assisted reproductive technology: A multi-cohort analysis. Eur. Heart J. 2023, 44, 1464–1473. [Google Scholar] [CrossRef]
- Wijs, L.; Doherty, D.; Keelan, J.; Burton, P.; Yovich, J.; Beilin, L.; Mori, T.; Huang, R.; Adams, L.; Olynyk, J.K. Comparison of the cardiometabolic profiles of adolescents conceived through ART with those of a non-ART cohort. Hum. Reprod. 2022, 37, 1880–1895. [Google Scholar] [CrossRef]
- Morrison, J.L.; Berry, M.J.; Botting, K.J.; Darby, J.R.; Frasch, M.G.; Gatford, K.L.; Giussani, D.A.; Gray, C.L.; Harding, R.; Herrera, E.A. Improving pregnancy outcomes in humans through studies in sheep. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R1123–R1153. [Google Scholar] [CrossRef]
- Dimasi, C.G.; Darby, J.R.T.; Morrison, J.L. A change of heart: Understanding the mechanisms regulating cardiac proliferation and metabolism before and after birth. J. Physiol. 2023, 601, 1319–1341. [Google Scholar] [CrossRef]
- Chattergoon, N.N.; Giraud, G.D.; Louey, S.; Stork, P.; Fowden, A.L.; Thornburg, K.L. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012, 26, 397. [Google Scholar] [CrossRef]
- Thornburg, K.; Jonker, S.; O’Tierney, P.; Chattergoon, N.; Louey, S.; Faber, J.; Giraud, G. Regulation of the cardiomyocyte population in the developing heart. Prog. Biophys. Mol. Biol. 2011, 106, 289–299. [Google Scholar] [CrossRef]
- Giraud, G.D.; Louey, S.; Jonker, S.; Schultz, J.; Thornburg, K.L. Cortisol Stimulates Cell Cycle Activity in the Cardiomyocyte of the Sheep Fetus. Endocrinology 2006, 147, 3643–3649. [Google Scholar] [CrossRef]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.-M.; Weigel, N.L. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef]
- Fowden, A.L.; Giussani, D.A.; Forhead, A.J. Intrauterine programming of physiological systems: Causes and consequences. Physiology 2006, 21, 29–37. [Google Scholar] [CrossRef]
- Saif, Z.; Hodyl, N.A.; Hobbs, E.; Tuck, A.R.; Butler, M.S.; Osei-Kumah, A.; Clifton, V.L. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta 2014, 35, 260–268. [Google Scholar] [CrossRef]
- Clifton, V.L.; McDonald, M.; Morrison, J.L.; Holman, S.L.; Lock, M.C.; Saif, Z.; Meakin, A.; Wooldridge, A.L.; Gatford, K.L.; Wallace, M.J.; et al. Placental glucocorticoid receptor isoforms in a sheep model of maternal allergic asthma. Placenta 2019, 83, 33–36. [Google Scholar] [CrossRef]
- Orgeig, S.; McGillick, E.V.; Botting, K.J.; Zhang, S.; McMillen, I.C.; Morrison, J.L. Increased lung prolyl hydroxylase and decreased glucocorticoid receptor are related to decreased surfactant protein in the growth-restricted sheep fetus. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L84–L97. [Google Scholar] [CrossRef]
- Amanollahi, R.; Holman, S.L.; Bertossa, M.R.; Meakin, A.S.; Thornburg, K.L.; McMillen, I.C.; Wiese, M.D.; Lock, M.C.; Morrison, J.L. Ontogeny of Fetal Cardiometabolic Pathways: The Potential Role of Cortisol and Thyroid Hormones in Driving the Transition from Preterm to Near-term Heart Development in Sheep. J. Cardiovasc. Dev. Dis. 2025, 12, 36. [Google Scholar] [CrossRef]
- Davies, K.L.; Camm, E.J.; Smith, D.J.; Miles, J.; Forhead, A.J.; Murray, A.J.; Fowden, A.L. Developmental programming of mitochondrial substrate metabolism in skeletal muscle of adult sheep by cortisol exposure before birth. J. Dev. Orig. Health Dis. 2023, 14, 77–87. [Google Scholar] [CrossRef]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N. Mitochondrial function, biology, and role in disease: A scientific statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Bhullar, S.K.; Dhalla, N.S. Status of Mitochondrial Oxidative Phosphorylation during the Development of Heart Failure. Antioxidants 2023, 12, 1941. [Google Scholar] [CrossRef]
- Shao, D.; Tian, R. Glucose transporters in cardiac metabolism and hypertrophy. Compr. Physiol. 2015, 6, 331. [Google Scholar] [CrossRef]
- Dimasi, C.G.; Darby, J.R.T.; Cho, S.K.S.; Saini, B.S.; Holman, S.L.; Meakin, A.S.; Wiese, M.D.; Macgowan, C.K.; Seed, M.; Morrison, J.L. Reduced in utero substrate supply decreases mitochondrial abundance and alters the expression of metabolic signalling molecules in the fetal sheep heart. J. Physiol. 2024, 602, 5901–5922. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef]
- Seckl, J.R.; Holmes, M.C. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 479–488. [Google Scholar] [CrossRef]
- Dancause, K.N.; Veru, F.; Andersen, R.E.; Laplante, D.P.; King, S. Prenatal stress due to a natural disaster predicts insulin secretion in adolescence. Early Hum. Dev. 2013, 89, 773–776. [Google Scholar] [CrossRef]
- Barnes, J.; Sutcliffe, A.; Kristoffersen, I.; Loft, A.; Wennerholm, U.; Tarlatzis, B.; Kantaris, X.; Nekkebroeck, J.; Hagberg, B.; Madsen, S. The influence of assisted reproduction on family functioning and children’s socio-emotional development: Results from a European study. Hum. Reprod. 2004, 19, 1480–1487. [Google Scholar] [CrossRef]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 2015, 593, 2547–2549. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation of Animal Welfare: Wheathampstead, UK, 1959. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- AFRC. Energy and protein requirements of ruminants. In An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Walker, S.; Smith, D.; Seamark, R. Timing of multiple ovulations in the ewe after treatment with FSH or PMSG with and without GnRH. Reproduction 1986, 77, 135–142. [Google Scholar] [CrossRef]
- Holm, P.; Walker, S.; Seamark, R. Embryo viability, duration of gestation and birth weight in sheep after transfer of in vitro matured and in vitro fertilized zygotes cultured in vitro or in vivo. Reproduction 1996, 107, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kakar, M.; Maddocks, S.; Lorimer, M.; Kleemann, D.; Rudiger, S.; Hartwich, K.; Walker, S. The effect of peri-conception nutrition on embryo quality in the superovulated ewe. Theriogenology 2005, 64, 1090–1103. [Google Scholar] [CrossRef]
- Kleemann, D.; Walker, S.; Seamark, R. Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy. Reproduction 1994, 102, 411–417. [Google Scholar] [CrossRef]
- Walker, S.; Hill, J.; Kleemann, D.; Nancarrow, C. Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biol. Reprod. 1996, 55, 703–708. [Google Scholar] [CrossRef]
- Kleemann, D.; Walker, S.; Hartwich, K.; Fong, L.; Seamark, R.; Robinson, J.; Owens, J. Fetoplacental growth in sheep administered progesterone during the first three days of pregnancy. Placenta 2001, 22, 14–23. [Google Scholar] [CrossRef]
- Walker, S.; Heard, T.; Seamark, R. In vitro culture of sheep embryos without co-culture: Successes and perspectives. Theriogenology 1992, 37, 111–126. [Google Scholar] [CrossRef]
- Wang, K.C.; Zhang, L.; McMillen, I.C.; Botting, K.J.; Duffield, J.A.; Zhang, S.; Suter, C.M.; Brooks, D.A.; Morrison, J.L. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J. Physiol. 2011, 589, 4709–4722. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE Guidelines: M inimum I nformation for Publication of Q uantitative Real-Time PCR E xperiments. Clin Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Wang, K.C.; Lim, C.H.; McMillen, I.C.; Duffield, J.A.; Brooks, D.A.; Morrison, J.L. Alteration of cardiac glucose metabolism in association to low birth weight: Experimental evidence in lambs with left ventricular hypertrophy. Metabolism 2013, 62, 1662–1672. [Google Scholar] [CrossRef]
- Botting, K.J.; McMillen, I.C.; Forbes, H.; Nyengaard, J.R.; Morrison, J.L. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J. Am. Heart Assoc. 2014, 3, e000531. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McGillick, E.V.; Morrison, J.L.; McMillen, I.C.; Orgeig, S. Intrafetal glucose infusion alters glucocorticoid signaling and reduces surfactant protein mRNA expression in the lung of the late-gestation sheep fetus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R538–R545. [Google Scholar] [CrossRef] [PubMed]
- Lie, S.; Morrison, J.L.; Williams-Wyss, O.; Ozanne, S.E.; Zhang, S.; Walker, S.K.; Kleemann, D.O.; MacLaughlin, S.M.; Roberts, C.T.; McMillen, I.C. Impact of embryo number and periconceptional undernutrition on factors regulating adipogenesis, lipogenesis, and metabolism in adipose tissuein the sheep fetus. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E931–E941. [Google Scholar] [CrossRef]
- Lie, S.; Sim, S.; McMillen, I.C.; Williams-Wyss, O.; MacLaughlin, S.; Kleemann, D.; Walker, S.; Roberts, C.T.; Morrison, J. Maternal undernutrition around the time of conception and embryo number each impact on the abundance of key regulators of cardiac growth and metabolism in the fetal sheep heart. J. Dev. Orig. Health Dis. 2013, 4, 377–390. [Google Scholar] [CrossRef]
- Soo, P.S.; Hiscock, J.; Botting, K.J.; Roberts, C.T.; Davey, A.K.; Morrison, J.L. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod. Toxicol. 2012, 33, 374–381. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, 1–14. [Google Scholar] [CrossRef]
- Nicholas, L.M.; Rattanatray, L.; MacLaughlin, S.M.; Ozanne, S.E.; Kleemann, D.O.; Walker, S.K.; Morrison, J.L.; Zhang, S.; Muhlhäusler, B.S.; Martin-Gronert, M.S. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013, 27, 3786–3796. [Google Scholar] [CrossRef]
- Darby, J.R.; Sorvina, A.; Bader, C.A.; Lock, M.C.; Soo, J.Y.; Holman, S.L.; Seed, M.; Kuchel, T.; Brooks, D.A.; Plush, S.E. Detecting metabolic differences in fetal and adult sheep adipose and skeletal muscle tissues. J. Biophotonics 2020, 13, e201960085. [Google Scholar] [CrossRef]
- Darby, J.R.; Chiu, J.; Regnault, T.R.; Morrison, J.L. Placental insufficiency induces a sexually dimorphic response in the expression of cardiac growth and metabolic signalling molecules upon exposure to a postnatal western diet in guinea pigs. J. Dev. Orig. Health Dis. 2022, 13, 345–357. [Google Scholar] [CrossRef]
- Botting, K.J.; Loke, X.Y.; Zhang, S.; Andersen, J.B.; Nyengaard, J.R.; Morrison, J.L. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex-and cause-of-IUGR-specific manner. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R48–R67. [Google Scholar] [CrossRef]
- Lie, S.; Hui, M.; McMillen, I.C.; Muhlhausler, B.S.; Posterino, G.S.; Dunn, S.L.; Wang, K.C.; Botting, K.J.; Morrison, J.L. Exposure to rosiglitazone, a PPAR-γ agonist, in late gestation reduces the abundance of factors regulating cardiac metabolism and cardiomyocyte size in the sheep fetus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 306, R429–R437. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Tosh, D.N.; Zhang, S.; McMillen, I.C.; Duffield, J.A.; Brooks, D.A.; Morrison, J.L. IGF-2R-Gαq signaling and cardiac hypertrophy in the low-birth-weight lamb. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R627–R635. [Google Scholar] [CrossRef] [PubMed]
- Darby, J.R.T.; Saini, B.S.; Soo, J.Y.; Lock, M.C.; Holman, S.L.; Bradshaw, E.L.; McInnes, S.J.P.; Voelcker, N.H.; Macgowan, C.K.; Seed, M.; et al. Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant ewe and increases fetal but not cardiac growth. J. Physiol. 2019, 597, 5063–5077. [Google Scholar] [CrossRef]
- McBride, G.M.; Meakin, A.S.; Soo, J.Y.; Darby, J.R.; Varcoe, T.J.; Bradshaw, E.L.; Lock, M.C.; Holman, S.L.; Saini, B.S.; Macgowan, C.K. Intrauterine growth restriction alters the activity of drug metabolising enzymes in the maternal-placental-fetal unit. Life Sci. 2021, 285, 120016. [Google Scholar] [CrossRef]
- Meakin, A.S.; Nathanielsz, P.W.; Li, C.; Huber, H.F.; Clifton, V.L.; Wiese, M.D.; Morrison, J.L. Maternal obesogenic diet during pregnancy and its impact on fetal hepatic function in baboons. Obesity 2024, 32, 1910–1922. [Google Scholar] [CrossRef]
- Lock, M.C.; Darby, J.R.T.; Soo, J.Y.; Brooks, D.A.; Perumal, S.R.; Selvanayagam, J.B.; Seed, M.; Macgowan, C.K.; Porrello, E.R.; Tellam, R.L.; et al. Differential Response to Injury in Fetal and Adolescent Sheep Hearts in the Immediate Post-myocardial Infarction Period. Front. Physiol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Roy, B.N.; Reid, R.L.; Van Vugt, D.A. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology 1999, 140, 2191–2198. [Google Scholar] [CrossRef]
- Qureshi, A.C.; Bahri, A.; Breen, L.A.; Barnes, S.C.; Powrie, J.K.; Thomas, S.M.; Carroll, P.V. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin. Endocrinol. 2007, 66, 632–635. [Google Scholar] [CrossRef]
- Bohler Jr, H.C.; Zoeller, R.T.; King, J.C.; Rubin, B.S.; Weber, R.; Merriam, G.R. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Mol. Brain Res. 1990, 8, 259–262. [Google Scholar] [CrossRef]
- Burgess, L.H.; Handa, R.J. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 1992, 131, 1261–1269. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Torpy, D.J.; Gold, P.W. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann. Intern. Med. 1998, 129, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mbiydzenyuy, N.E.; Qulu, L.-A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 2024, 39, 1613–1636. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, D.R.; Roca, C.A.; Schmidt, P.J.; Danaceau, M.A.; Putnam, K.; Cizza, G.; Chrousos, G.; Nieman, L. Testosterone Suppression of CRH-Stimulated Cortisol in Men. Neuropsychopharmacology 2005, 30, 1906–1912. [Google Scholar] [CrossRef]
- Lu, N.Z.; Cidlowski, J.A. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006, 16, 301–307. [Google Scholar] [CrossRef]
- Caramori, G.; Mumby, S.; Girbino, G.; Chung, K.F.; Adcock, I.M. Corticosteroids. In Nijkamp and Parnham’s Principles of Immunopharmacology; Springer: Cham, Switzerland, 2019; pp. 661–688. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Lu, N.Z.; Collins, J.B.; Grissom, S.F.; Cidlowski, J.A. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol. Cell. Biol. 2007, 27, 7143–7160. [Google Scholar] [CrossRef]
- Lu, N.Z.; Cidlowski, J.A. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 2005, 18, 331–342. [Google Scholar] [CrossRef]
- Nehmé, A.; Lobenhofer, E.K.; Stamer, W.D.; Edelman, J.L. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med. Genom. 2009, 2, 58. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Lopez-Crisosto, C.; Pennanen, C.; Vasquez-Trincado, C.; Morales, P.E.; Bravo-Sagua, R.; Quest, A.F.; Chiong, M.; Lavandero, S. Sarcoplasmic reticulum–mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 2017, 14, 342–360. [Google Scholar] [CrossRef]
- Delbridge, L.M.; Mellor, K.M.; Taylor, D.J.; Gottlieb, R.A. Myocardial stress and autophagy: Mechanisms and potential therapies. Nat. Rev. Cardiol. 2017, 14, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vizarra, E.; Enríquez, J.A.; Pérez-Martos, A.; Montoya, J.; Fernández-Silva, P. Tissue-specific differences in mitochondrial activity and biogenesis. Mitochondrion 2011, 11, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Borghetti, G.; Von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Chang, E.I.; Stremming, J.; Knaub, L.A.; Wesolowski, S.R.; Rozance, P.J.; Sucharov, C.C.; Reusch, J.E.B.; Brown, L.D. Mitochondrial respiration is lower in the intrauterine growth-restricted fetal sheep heart. J. Physiol. 2024, 602, 2697–2715. [Google Scholar] [CrossRef]
- Fassone, E.; Rahman, S. Complex I deficiency: Clinical features, biochemistry and molecular genetics. J. Med. Genet. 2012, 49, 578–590. [Google Scholar] [CrossRef]
- Rowles, J.; Scherer, S.W.; Xi, T.; Majer, M.; Nickle, D.C.; Rommens, J.M.; Popov, K.M.; Harris, R.A.; Riebow, N.L.; Xia, J. Cloning and characterization of PDK4 on 7q21. 3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J. Biol. Chem. 1996, 271, 22376–22382. [Google Scholar] [CrossRef]
- Sugden, M.C.; Holness, M.J. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol.-Endocrinol. Metab. 2003, 284, E855–E862. [Google Scholar] [CrossRef]
- Bowker-Kinley, M.M.; Davis, I.W.; Wu, P.; Harris, A.R.; Popov, M.K. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem. J. 1998, 329, 191–196. [Google Scholar] [CrossRef]
- Kim, D.H.; Chauhan, S. The role of dichloroacetate in improving acute hypoxic tolerance and cardiac function: Translation to failing hearts? J. Physiol. 2018, 596, 2967. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Bowker-Kinley, M.M.; Huang, B.; Wu, P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv. Enzym. Regul. 2002, 42, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, F.L.; Angerosa, J.; Liaw, N.Y.; Cheung, M.M.; Pepe, S. Adaptations in Protein Expression and Regulated Activity of Pyruvate Dehydrogenase Multienzyme Complex in Human Systolic Heart Failure. Oxidative Med. Cell. Longev. 2019, 2019, 4532592. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Zhabyeyev, P.; Gandhi, M.; Mori, J.; Basu, R.; Kassiri, Z.; Clanachan, A.; Lopaschuk, G.D.; Oudit, G.Y. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc. Res. 2013, 97, 676–685. [Google Scholar] [CrossRef]
- Zhang, L.; Jaswal, J.S.; Ussher, J.R.; Sankaralingam, S.; Wagg, C.; Zaugg, M.; Lopaschuk, G.D. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ. Heart Fail. 2013, 6, 1039–1048. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Lijnen, P.J.; van Pelt, J.F.; Fagard, R.H. Downregulation of Manganese Superoxide Dismutase by Angiotensin II in Cardiac Fibroblasts of Rats: Association With Oxidative Stress in Myocardium. Am. J. Hypertens. 2010, 23, 1128–1135. [Google Scholar] [CrossRef]
- Suzuki, K.; Murtuza, B.; Sammut, I.A.; Latif, N.; Jayakumar, J.; Smolenski, R.T.; Kaneda, Y.; Sawa, Y.; Matsuda, H.; Yacoub, M.H. Heat Shock Protein 72 Enhances Manganese Superoxide Dismutase Activity During Myocardial Ischemia-Reperfusion Injury, Associated With Mitochondrial Protection and Apoptosis Reduction. Circulation 2002, 106, I-270–I-276. [Google Scholar] [CrossRef]
- Nojiri, H.; Shimizu, T.; Funakoshi, M.; Yamaguchi, O.; Zhou, H.; Kawakami, S.; Ohta, Y.; Sami, M.; Tachibana, T.; Ishikawa, H. Oxidative stress causes heart failure with impaired mitochondrial respiration. J. Biol. Chem. 2006, 281, 33789–33801. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Ide, T.; Yamato, M.; Matsusaka, H.; Hattori, F.; Ikeuchi, M.; Kubota, T.; Sunagawa, K.; Hasegawa, Y.; Kurihara, T. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 2006, 113, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.; Kerstetter, D.L.; Pimental, D.R.; Mulukutla, S.; Tabaee, A.; Bristow, M.R.; Colucci, W.S.; Sawyer, D.B. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J. Card. Fail. 2005, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Turton, M.; Luderer, U. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 2006, 147, 1224–1236. [Google Scholar] [CrossRef]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sato, E.F.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Inoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanollahi, R.; Holman, S.L.; Meakin, A.S.; Padhee, M.; Botting-Lawford, K.J.; Zhang, S.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K.; Kelly, J.M.; et al. In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep. J. Dev. Biol. 2025, 13, 17. https://doi.org/10.3390/jdb13020017
Amanollahi R, Holman SL, Meakin AS, Padhee M, Botting-Lawford KJ, Zhang S, MacLaughlin SM, Kleemann DO, Walker SK, Kelly JM, et al. In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep. Journal of Developmental Biology. 2025; 13(2):17. https://doi.org/10.3390/jdb13020017
Chicago/Turabian StyleAmanollahi, Reza, Stacey L. Holman, Ashley S. Meakin, Monalisa Padhee, Kimberley J. Botting-Lawford, Song Zhang, Severence M. MacLaughlin, David O. Kleemann, Simon K. Walker, Jennifer M. Kelly, and et al. 2025. "In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep" Journal of Developmental Biology 13, no. 2: 17. https://doi.org/10.3390/jdb13020017
APA StyleAmanollahi, R., Holman, S. L., Meakin, A. S., Padhee, M., Botting-Lawford, K. J., Zhang, S., MacLaughlin, S. M., Kleemann, D. O., Walker, S. K., Kelly, J. M., Rudiger, S. R., McMillen, I. C., Wiese, M. D., Lock, M. C., & Morrison, J. L. (2025). In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep. Journal of Developmental Biology, 13(2), 17. https://doi.org/10.3390/jdb13020017






