1. Introduction
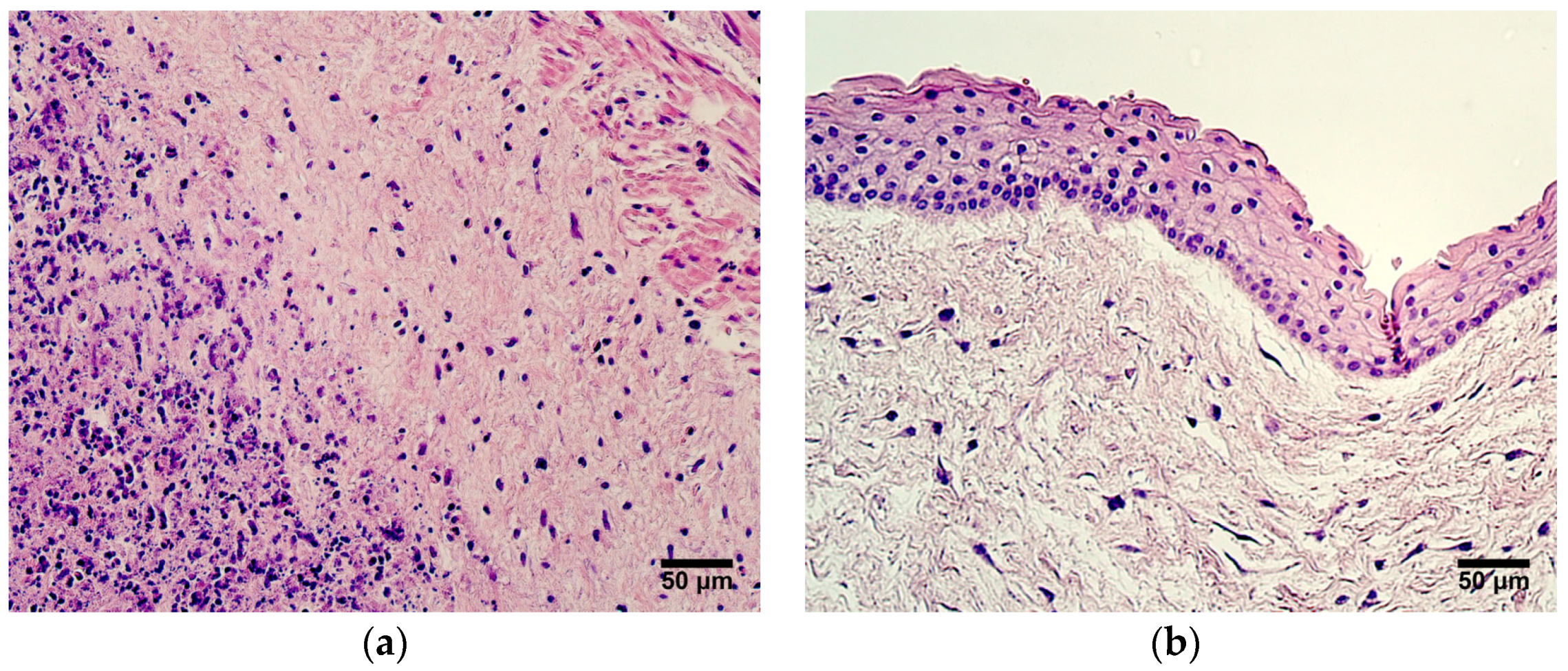
The banking of umbilical cord tissue and its use in clinical practice has gained popularity in recent years, although research has mainly focused on mesenchymal stem cells (MSCs) [
1]. Several factors cause umbilical cord tissue collection and preservation to be an attractive method for treating different diseases, i.e, the procedure is painless, noninvasive, and ethical, since the umbilical cord is considered to be a medical waste [
2]. Processes such as extracellular matrix remodeling, angiogenesis, progenitor activity, and immunomodulation are targets worth exploring when discussing potential modern therapeutic approaches to various pathologies. Accordingly, the exploration of the expression of markers representing these essential events in umbilical cord tissue can further characterize MSCs and their biological properties, as well as provide an additional understanding regarding whether other umbilical cord cells, aside from MSCs, could be suitable for use in clinical practice. Also, little is known about morphogenetic changes in the umbilical cord during the maturation process, and investigation of the representative markers can improve the understanding of physiological events occurring in the umbilical cord.
Matrix metalloproteinase-2 (MMP2), also known as gelatinase A or type IV collagenase, is a zinc-dependent endopeptidase that cleaves elements of the extracellular matrix (ECM) [
3]. The mRNA and the MMP-2 protein are found in various tissues, including the heart, smooth muscles, colon, kidney, urinary bladder, lungs, testis and prostate, uterine cervix, endometrium, and placenta. MMP-2 is made up of extra- and peri-cellular substrates, such as type I-VI collagen, elastin, fibronectin, proteoglycans, and cytokines, i.e., interleukin-1β [
4] and monocyte chemoattractant protein-3 [
5]. The intracellular activity of MMP2 has also been described [
6]. MMP2 contributes to physiological, as well as pathological, events. The functions of this protein include promoting angiogenesis, facilitating cell migration and cancer cell invasion and metastasis via degradation of the basement membrane [
7], and regulating endometrial remodeling during pregnancy [
3]. Overall matrix metalloproteinases (MMPs) can be induced by a large number of stimuli, some of which include inflammatory cytokines, growth factors, mechanical movement, and phagocytosis. Under physiological conditions, the basal expression of matrix metalloproteinases is low, and transient increases are seen during normal matrix remodeling, including wound healing, ovulation, and uterine resorption after pregnancy [
8].
In the extracellular compartment, the proteolytic functions of MMPs are affected by tissue inhibitors of metalloproteinases (TIMPs). TIMP2 is the most abundant member of the TIMP family; therefore, it plays a crucial role in maintaining the balance of the extracellular matrix remodeling [
9]. TIMP2 acts as an inhibitor of multiple MMPs, namely MMP-1, -2, -3, -7, -8, -9, -11, -13, -14, -16, and -24 [
10], while also taking part in the cell-surface activation of pro-MMP2 by interacting with membrane type 1-MMP. TIMP2 also inhibits angiogenesis independent of MMP inhibition [
11]. However, almost nothing is known regarding changes in TIMP2 expression in umbilical cords of different ages.
CD34 is a transmembrane glycoprotein typically associated with hematopoietic stem and progenitor cells. It is also expressed by mesenchymal stromal cells, muscle satellite cells, corneal keratocytes, interstitial cells, epithelial progenitors, and vascular endothelial progenitors [
12]. The possible functions of CD34 include enhancing proliferation and blocking differentiation, promoting lymphocyte adhesion, and enhancing trafficking and migration of hematopoietic cells [
13]. Most endothelial cells in larger veins and arteries are CD34-negative; CD34-positive endothelial cells are found within smaller blood vessels [
12]. It is also reported that CD34-positive human umbilical vein endothelial cells show an increased mRNA expression of all known tip cell markers; therefore, they might play an important role during sprouting angiogenesis [
14].
Vascular endothelial growth factor (VEGF, also known as VEGF-A) is a member of the VEGF family, along with VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) [
15]. VEGF plays a major role in regulating vasculogenesis and angiogenesis, since its actions include inducing endothelial cell migration and invasion into the basement membrane, promoting proliferation, the formation of fenestrations, and the survival of endothelial cells [
16]. The production of VEGF is stimulated by the presence of hypoxia or growth factors, i.e., transforming growth factor β (TGFβ), interleukins, or platelet-derived growth factors (PDGFs) [
17], and it is expressed in nearly all vascularized adult tissues, including select endothelium [
18]. Many findings broaden the possible therapeutic applications for VEGF, meaning that it could be used in pro-angiogenic therapy for various ischemic diseases, as well as for some degenerative diseases [
19].
Human β-defensin 2 (HBD2) is an antimicrobial peptide and an important component of innate immunity. HBD2 provides protection against bacterial, viral, fungal, and parasitic pathogens [
20]. Cationic β-defensins bind to negatively charged microbial membranes, additional pores are formed, and microbial death occurs due to the permeabilization of the membranes [
21]. This peptide not only exhibits direct bactericidal action but also participates in chemotaxis and Toll-like receptor activation, resulting in the modulation of immunocompetent cell responses [
22]. The expression of HBD2 is detected in several types of human epithelia such as epithelia from the skin, lungs, trachea, urogenital system [
23], and gastrointestinal tract [
21]. HBD2 is generally expressed at low levels in normal physiological states and is induced in response to microbial stimuli; the expression of HBD2 is stimulated by pro-inflammatory mediators or bacterial products [
24]. It is suggested that HBD2 could be applicable in various inflammatory diseases as a tool for modulating the response of the immune system [
20].
Consequently, the aim of the study was to determine the expression and distribution of markers MMP2, TIMP2, CD34, VEGF, and HBD2 in preterm and full-term human umbilical cord tissue.
4. Discussion
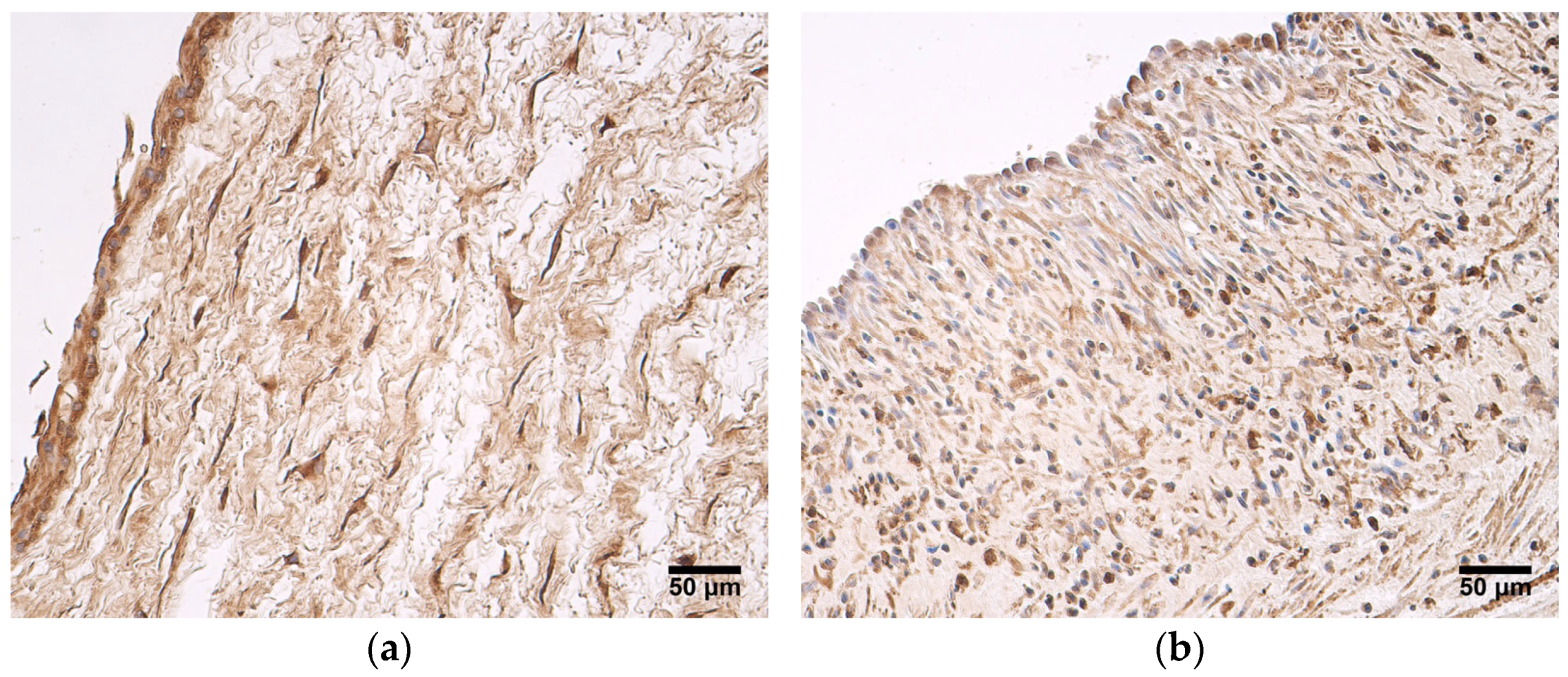
In this study, we found that the expression of MMP2 was more pronounced in very preterm and moderate preterm birth umbilical cords than in late preterm birth and full-term birth umbilical cords. MMP2 was mainly produced in the amniotic epithelium and the extraembryonic mesenchyme. Statistically significant differences in MMP2 expression between the two groups were seen in the amniotic epithelium. These findings suggest that the MMP2 activity decreases with an increase in the umbilical cord’s age. MMPs play a major role in the degradation of ECM, subsequently affecting processes such as cell migration, adhesion, and differentiation. ECM remodeling is prominent during organogenesis [
27]. MMP-2 also mediates ribosomal RNA transcription by cleaving nucleolar histones and it plays a role in cell proliferation [
28]. Research shows that the number of MMP2s found in umbilical cord vessels is several times higher than that of other MMPs, and pre-eclampsia causes a decrease in MMP2 levels [
29]. The reduction of MMP2 expression in late preterm birth and full-term birth umbilical cords could be explained with the proposition that ECM remodeling is not as essential at this stage as it is in earlier weeks of umbilical cord development. The findings of this study are in accordance withthose of other researchers. A different study also reported the expression of MMP2 in umbilical cord epithelial cells and in Wharton’s jelly fibroblasts [
30]. Interestingly, Mauro et al. [
30] revealed that MMP2 expression was found in cultured human umbilical vein endothelial cells, while it was not detected in the whole umbilical cord. The authors of said study suggest that blood vessel endothelium is a fragile structure, and that it could easily be damaged during the fixation and embedding procedures used when performing the immunohistochemistry assays. This possibility cannot be excluded in our study, since approximately half of the samples examined did not show the expression of MMP2 in the endothelium of the umbilical vein. MMP2 can take part in pathological processes as well. It can be considered a prognostic factor for several tumors—overexpression of MMP2 is associated with poor prognosis in oral cancers, retinoblastoma, bladder cancer, epithelial cancer, and breast cancer [
31]. It is also proposed that MMP2 may be involved in ECM degradation and placental barrier dysfunction, leading to pathogen transmission [
32]; therefore, while it is an important part of maintaining the physiological remodeling processes in the umbilical cord, its overexpression should be viewed with caution.
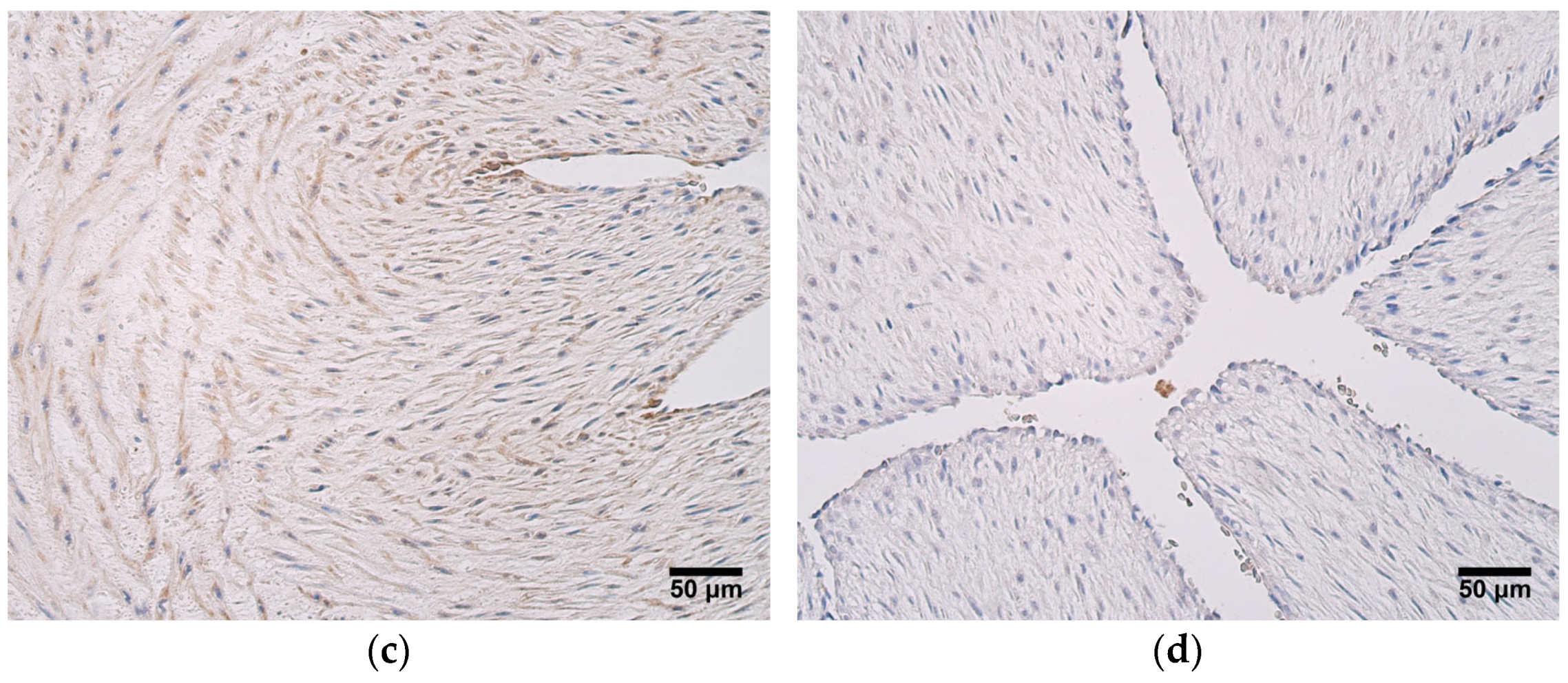
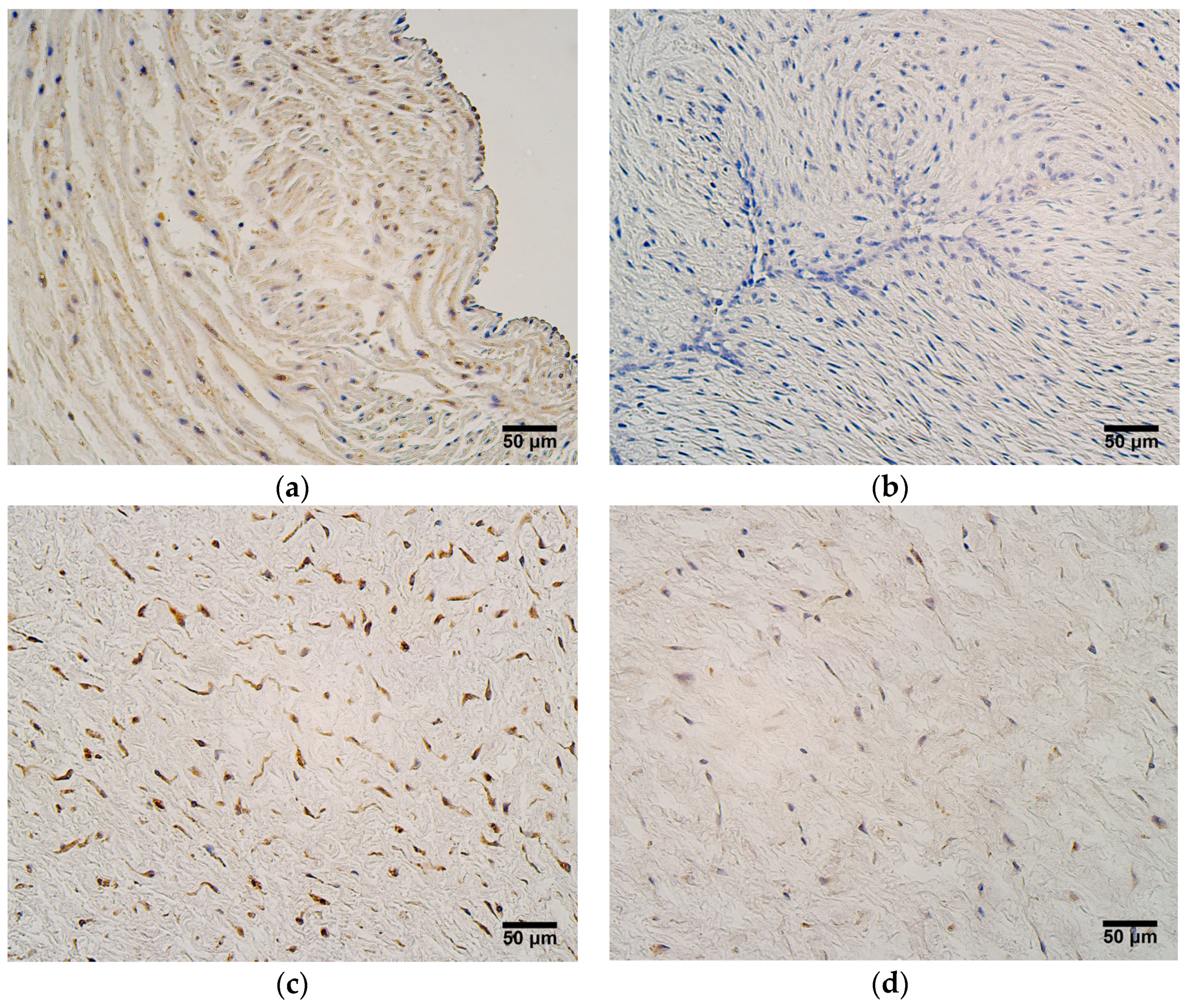
One factor that opposes the actions of MMP2 is TIMP2, and its presence was noteworthy in all umbilical cord tissue compartments in both patient groups. Statistically significant differences in TIMP2 expression between very preterm and moderate preterm birth umbilical cords and late preterm birth and full-term birth umbilical cords were seen in the blood vessel wall, the extraembryonic mesenchyme, and the amniotic epithelium; greater TIMP2 expression levels were observed in very preterm and moderate preterm birth umbilical cords. A very strong correlation was also found between TIMP2 and MMP2 expression in both patient groups, revealing that ECM remodeling in the umbilical cord is strongly regulated. When compared with the number of MMP2-positive cells, in the umbilical cord, TIMP2 shows overall greater expression. TIMP2 is known to form complexes with other MMPs aside from just MMP2 [
33]; at the same time, other MMPs, such as MMP9, can also be found in umbilical cord tissue [
30]. For that reason, it is possible that a considerable amount of TIMP2 is necessary to maintain tissue homeostasis in the umbilical cord. It is important to note that TIMP2 can also have stimulatory effects by interacting with pro-MMP2 and forming a trimolecular complex with MMP14, further participating in activation of MMP2. It is suggested that after this cascade, TIMP2 can be either degraded or recycled as an intact TIMP2 molecule [
9]. In quiescent adult tissues, sources of TIMP2 include stromal fibroblasts, perivascular smooth muscle, and endothelial cells. In such an environment, TIMP2 inhibits endothelial cell responses to minor fluctuations in angiogenic growth factors by binding to available α3β1-integrin receptors on the endothelial cells. In a situation where angiogenic factor concentrations rise due to hypoxia or other pathological stimuli, endothelial cells increase the production of MMPs and decrease the synthesis of TIMP2, while simultaneously, excess TIMP2 binds to proteases, leading to a reduction in local TIMP2 concentration [
11]. We speculate that this model of TIMP2 action could also be applicable to the human umbilical cord, where inhibitory activity dominates with regard to ECM turnover, including the appearance of angiogenesis.
In this study, the extraembryonic mesenchyme contained more VEGF-positive cells than did other tissue compartments in both the very preterm and moderate preterm birth umbilical cords and the late preterm birth and full-term birth umbilical cords. The number of VEGF-positive cells in the endothelium of the umbilical arteries and veins and the blood vessel wall varied, suggesting the presence of some individual factor affecting VEGF expression in these structures. A potent stimulus for VEGF expression is hypoxia; however, several other factors can participate in VEGF induction as well. Fibroblast growth factor, transforming growth factors (TGF-α and TGF-β), keratinocyte growth factor, insulin-like growth factor 1 (IGF-1), platelet-derived growth factor, as well as the inflammatory cytokines interleukin IL-1α and IL-6, can all up-regulate VEGF expression [
34]. No statistically significant differences in VEGF expression were detected between the two patient groups, suggesting that the VEGF activity in the umbilical cord is constant throughout the pregnancy. There have been reports that umbilical VEGF levels are higher in preterm births [
35]; however, the distinction of whether these differences account for individual factors or the age of the umbilical cord still needs to be confirmed. While most commonly known as an angiogenesis promoting factor, VEGF mediates other crucial functions as well, such as the reduction of apoptosis in endothelial cells [
36]. Having this essential purpose—the maintenance of endothelial cell survival—might explain why all samples examined in this study showed VEGF-positive cells in the extraembryonic mesenchyme, independent of the umbilical cord’s age. The expression pattern observed in this study is in agreement with those noted by previous researchers, where VEGF was also detected in the endothelial, stromal, and amniotic cells of the umbilical cord [
37]. In addition to its physiological properties, VEGF is thought to contribute to the pathogenesis of preeclampsia by disturbing the umbilical cord vascular endothelium, where its expression is significantly elevated [
38]. While VEGF is a common and essential component of umbilical cord tissue, its signalling must not be disregulated.
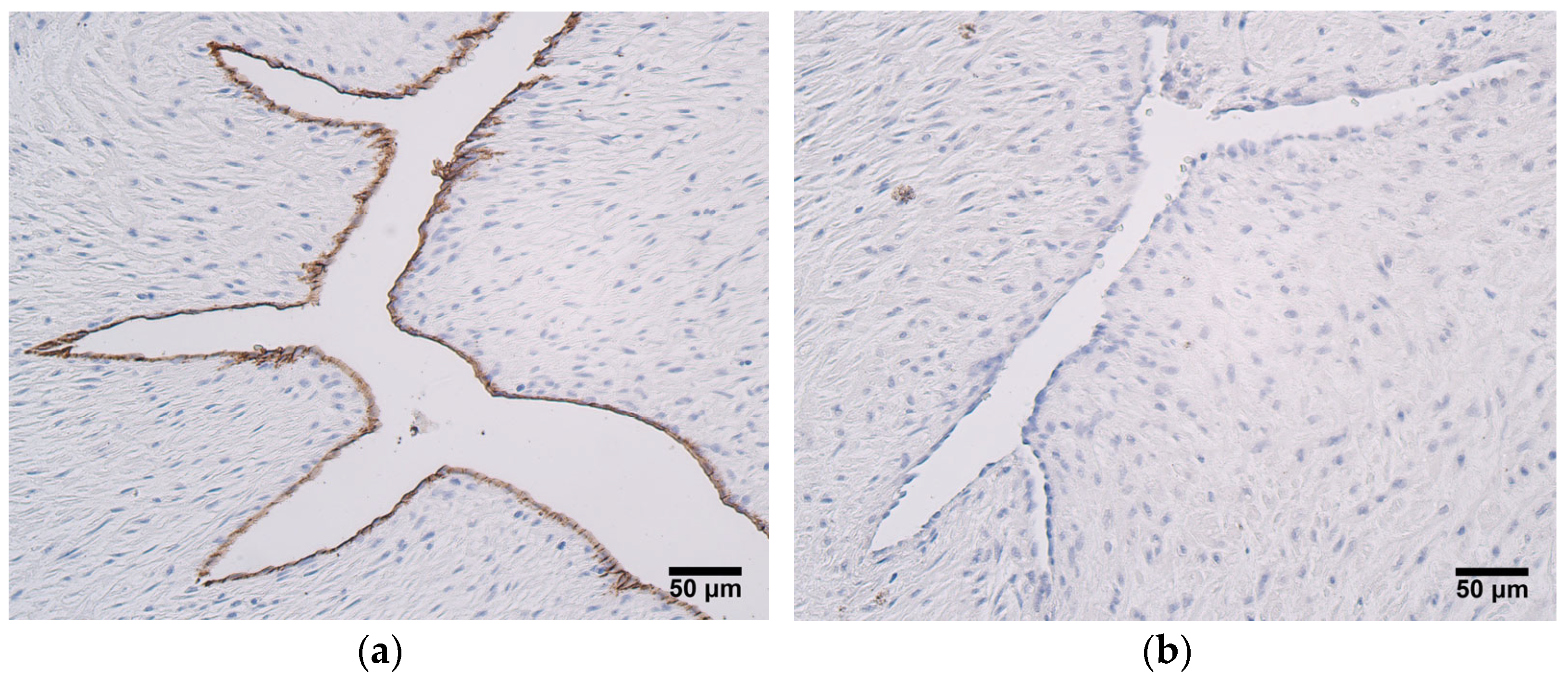
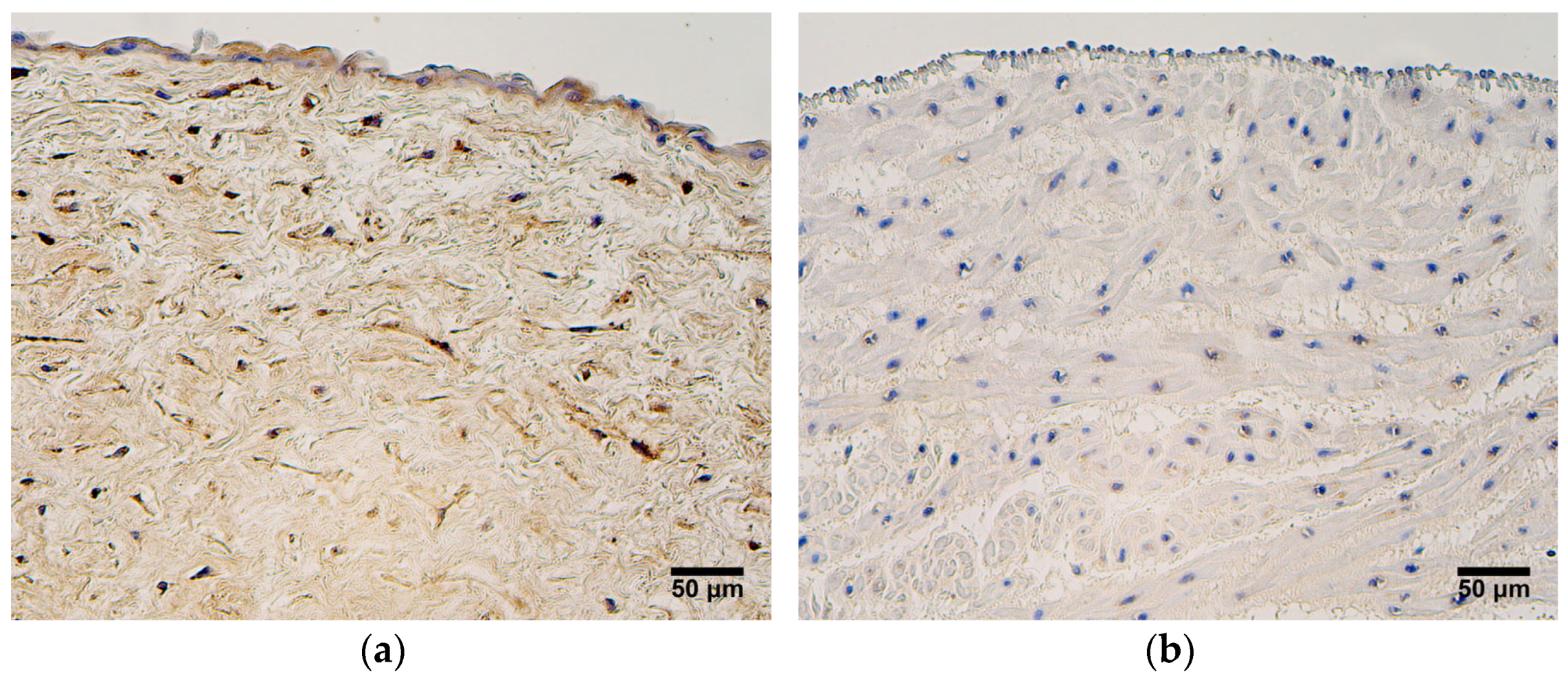
In this study, CD34-positivity was found only in the endothelium of the umbilical arteries and veins. The most prominent expression of CD34 was seen in the endothleium of the umbilical arteries in very preterm and moderate preterm birth umbilical cords, and statistically significant differences in CD34 expression between the two patient groups were also detected. It is proposed that CD34 can be considered a marker of subpopulations of progenitor cells from larger non-hematopoietic cell populations [
12], and it could be possible that this progenitor phenotype is lost with an increase in the umbilical cord’s age. Functional endothelial precursor cells (EPC) are characterized by the presence of the following markers—CD34, CD133, and vascular endothelial growth factor receptor-2 (VEGFR-2). More mature and differentiated EPCs are found in peripheral circulation, and they show a decreased expression of CD133. Finally, mature endothelial cells located in large vessels are CD34-negative [
39]. In only a few samples of this study did the umbilical cord vein endothelial cells stain positive for CD34. It has been reported that mature human umbilical vein endothelial cells are CD133-negative; thus, a loss of CD133 and subsequently, of CD34, can be observed during the transformation from an EPC to a mature endothelial cell [
39]. Previous research shows that CD34-positive human umbilical vein endothelial cells possess the properties of tip cells, which are essential components of sprouting angiogenesis. In addition, the number of CD34-positive cells increased upon stimulation with VEGF in vitro [
14]. We hypothesize that human umbilical artery endothelial cells could also carry the characteristics of tip cells, since in this research, CD34 expression was more noteable in the umbilical arteries.
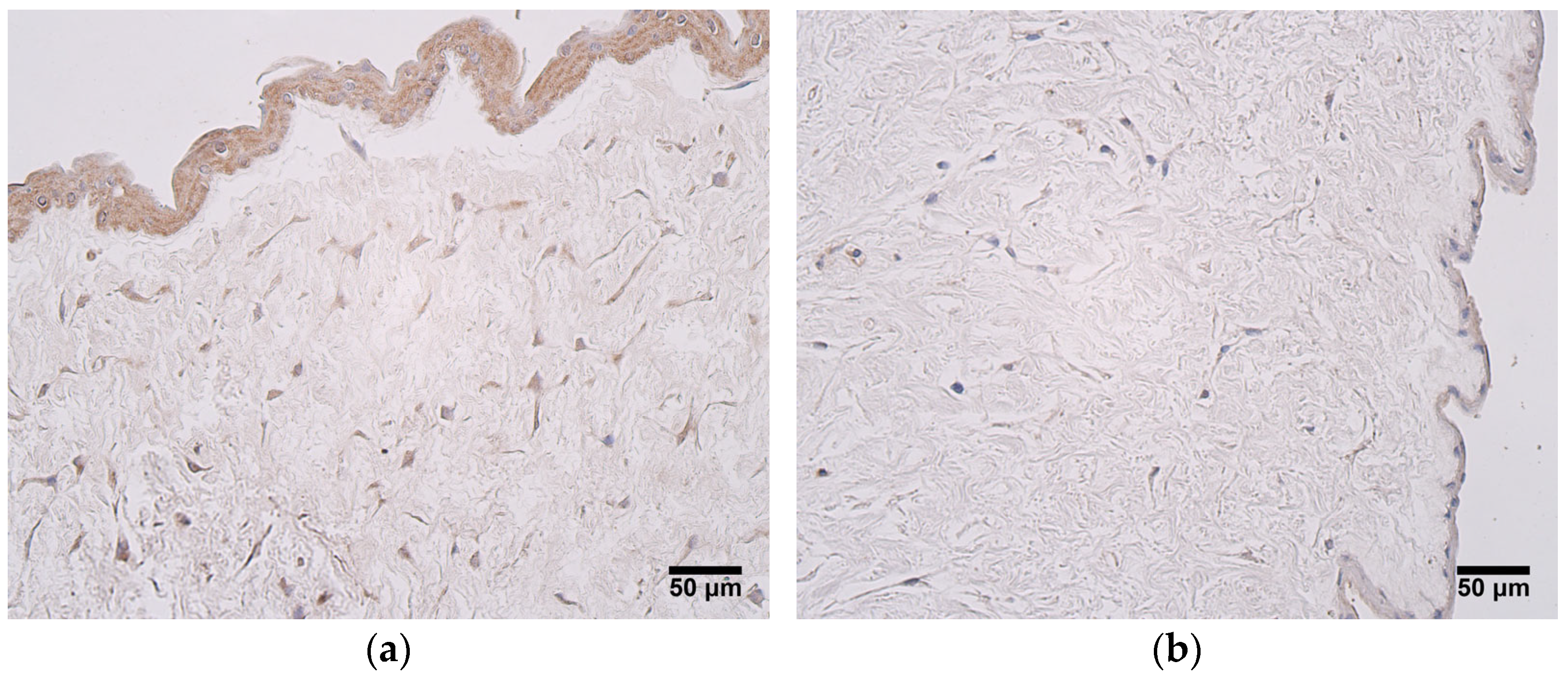
The expression of HBD2 was consistently seen in the extraembryonic mesenchyme and the amniotic epithelium of very preterm and moderate preterm birth umbilical cords, as well as in late preterm birth and full-term birth umbilical cords. No significant differences were found in HBD2 expression among the two patient groups. These findings suggest that the presence of antimicrobial molecules in the umbilical cord are consistent throughout pregnancy, and that these molecules are mainly produced outside of umbilical vessels. It is known that HBD2 plays a crucial role in mesenchymal stem cell-mediated microbicidal effects [
40]. In addition, HBD2 might play a role in protection against the uncontrolled activation of the complement system by inhibiting the classical pathway [
41]. In 2013, Olbrich et al. [
42] reported that HBD2 serum levels were lower in preterm neonates than in term infants, and that low HBD2 levels might be an independent risk factor for late-onset sepsis. It can be speculated that this HBD2 deficiency in preterm neonates arises from a region different than the umbilical cord, since the expression of HBD2 in umbilical cord tissues was similar in differently aged umbilical cords. Interestingly, a very strong correlation was found between the expression of CD34 in the endothelium of the umbilical vein and the expression of HBD2 in the amniotic epithelium. CD34-positive endothelial progenitor cells are thought to participate in NO-dependent vasodilatation and hyperpermeability [
43]. Research suggests that HBD2 opens Ca
2+-activated potassium channels and induces vasodilation in monkeys [
44]; therefore, the two markers—CD34 and HBD2—could be cooperating in modulating vascular function.
All things considered, in umbilical cords of different ages, VEGF and HBD2 were expressed in a constant manner, while MMP2, TIMP2, and CD34 were seen more frequently in very preterm and moderate preterm birth umbilical cords. These findings reveal that VEGF and HBD2 are stable markers when compared among very preterm and moderate preterm birth umbilical cords and late preterm birth and full-term birth umbilical cords. MMP2, TIMP2, and CD34, however, might play a role that is especially important during earlier periods of umbilical cord maturation.
This study exhibits a few limitations, such as relatively small number of patients and a lack of information about clinical outcomes. A larger number of study samples would contribute to more representative results, as well as allow for dividing the patients into smaller groups, perhaps observing a treshold of the umbilical cord’s age for change in certain marker expression levels. This study could be expanded by evaluating maternal factors that might affect specific marker expression, as well as by following the clinical outcomes with regard to specific marker levels in the umbilical cord. Tissue factor levels could also be further characterized using enzyme-linked immunosorbent assay (ELISA), leading to fully quantitative results. The evaluation of gene expression can provide further insight into the pysiological function of the umbilical cord; thus, mRNA levels could be assessed. Additional extension of this research could be achieved by exploring the possible association of tissue factor expression in the umbilical cord with the placental health status and the molecular and immunohistochemical qualities of placenta, as well as by detecting the expression different genes and gene proteins that might affect the development of the umbilical cord and placental vessels, i.e., placental growth factor and angiopoietins [
45].
5. Conclusions
Extracellular matrix remodeling in very preterm and moderate preterm birth umbilical cords and late preterm birth and full-term birth umbilical cords is strongly regulated, and tissue factors MMP2 and TIMP2 take part in this process by showing the decrease in MMP2 and the consistent increase in TIMP2 positive cells.
The expression of VEGF is not changed by the umbilical cord’s age; however, individual patient factors can affect the production of VEGF, suggesting the constant suppression of ischemia throughout the placenta development.
Numerous CD34-positive cells in the endothelium of the umbilical arteries suggest a significant role of progenitor cells in very preterm and moderate preterm birth umbilical cords.
Antimicrobial activity provided by HBD2 is essential and constant in very preterm and moderate preterm birth umbilical cords, as well as in late preterm birth and full-term birth umbilical cords.
The extraembryonic mesenchyme cells are the most active cell producers, expressing MMP2, TIMP2, VEGF, and HBD2 at notable levels in very preterm and moderate preterm birth umbilical cords, as well as in late preterm birth and full-term birth umbilical cords.