Effect of Cyclic Adenosine Monophosphate on Connexin 37 Expression in Sheep Cumulus-Oocyte Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sheep Cumulus-Oocyte Complex (COC) Collection
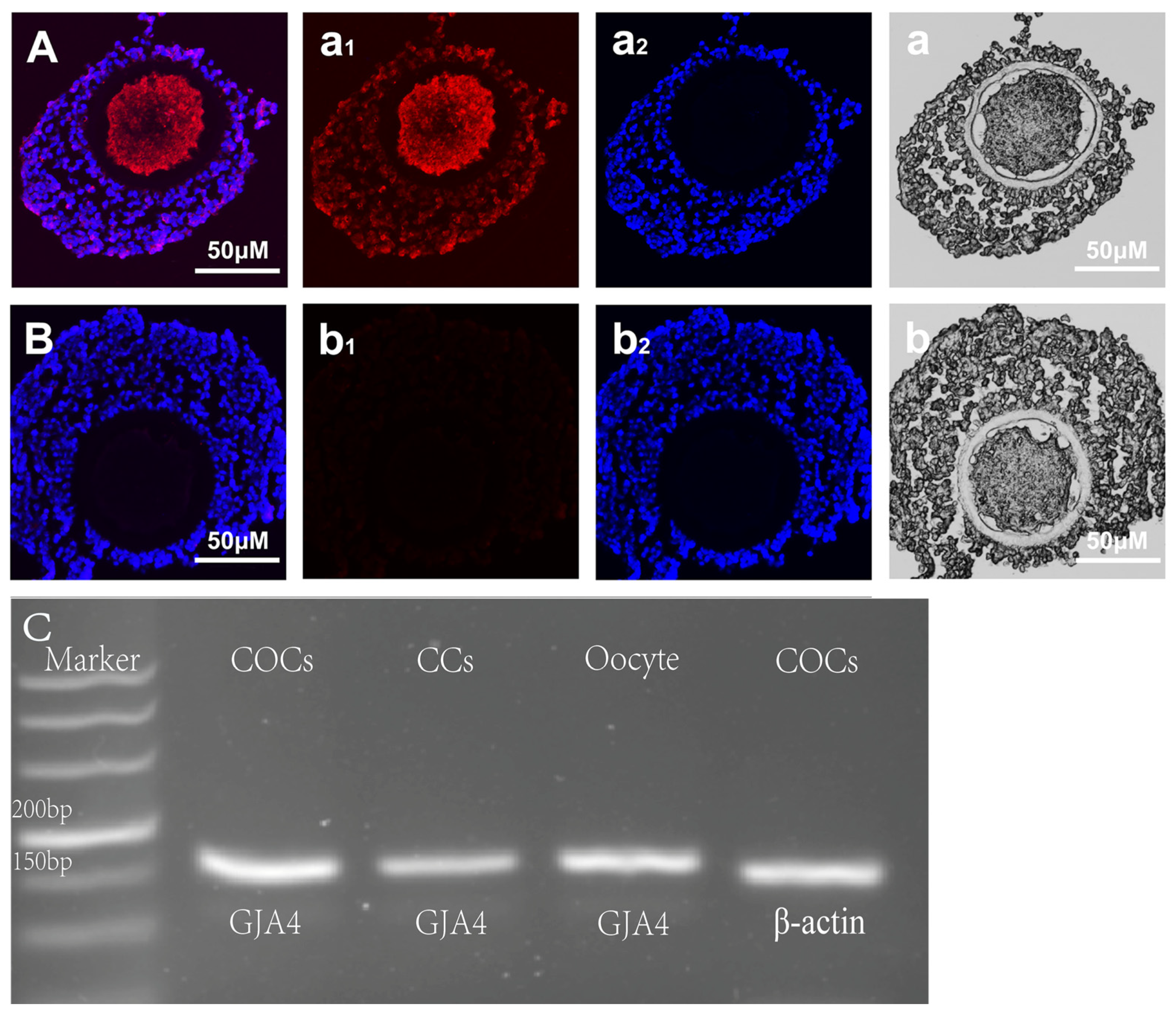
2.2. Indirect Immunofluorescence Staining
2.3. PCR (Polymerase Chain Reaction)
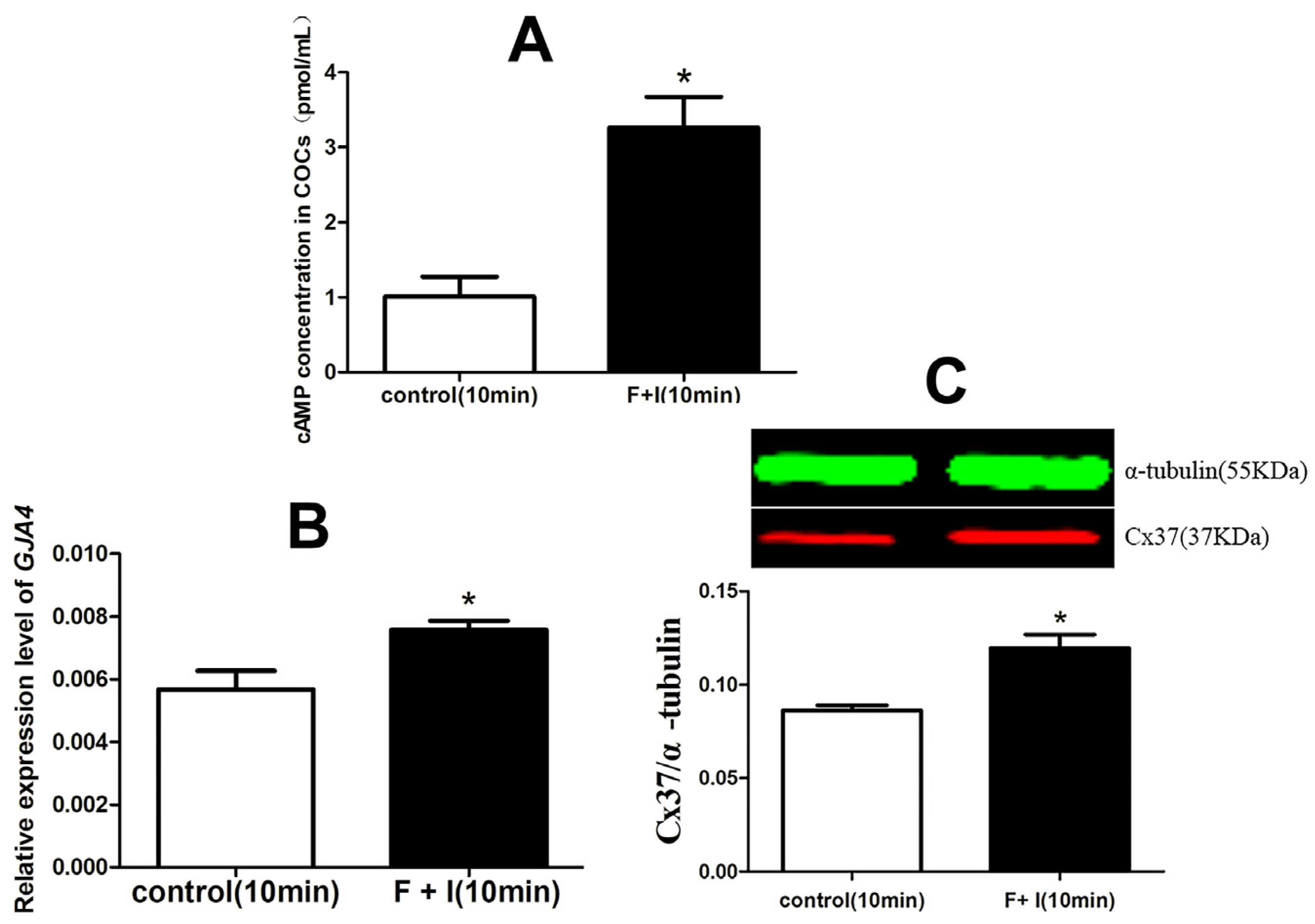
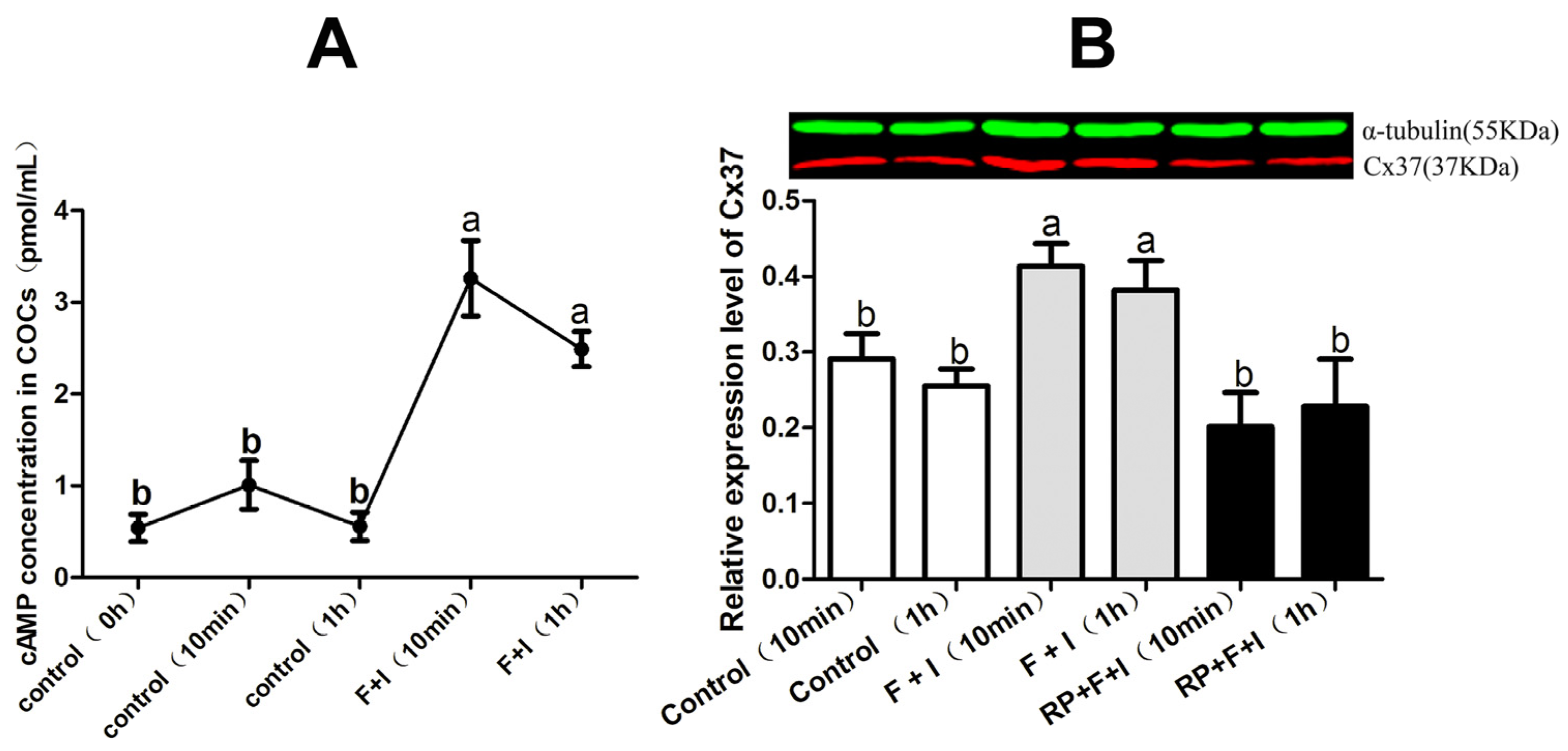
2.4. ELISA
2.5. Quantitative Real-Time PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Cx37 Expression in Sheep COC
3.2. The Effect of cAMP on the Expression of Cx37 in Sheep COC
3.3. Effect of cAMP-PKA on Cx37 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.J.T. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Ritter, L.J.; Armstrong, D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82–83, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.R.; Monteiro, C.A.S.; Souza-Fabjan, J.M.G.; Vasconcelos, C.O.d.P.; Nogueira, L.A.G.; Ferreira, A.M.R.; Serapião, R.V. Role of cAMP modulator supplementations during oocyte in vitro maturation in domestic animals. Anim. Reprod. Sci. 2018, 199, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.D.; Ji, Z.Y.; Tsalkova, T.; Mei, F. Epac and PKA: A tale of two intracellular cAMP receptors. Acta Biochim. Biophys. Sin. 2008, 40, 651–662. [Google Scholar] [CrossRef]
- Conti, M.; Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Cantley, T.C.; Day, B.N. Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol. Reprod. 1997, 57, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; Vos, M.D.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef]
- Aasen, T.; Johnstone, S.; Vidal-Brime, L.; Lynn, K.S.; Koval, M. Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. Int. J. Mol. Sci. 2018, 19, 1296. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Colley, D.; Barr, K.J.; Yee, S.P.; Kidder, G.M. Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. J. Cell Sci. 2007, 120, 4117–4125. [Google Scholar] [CrossRef]
- Norris, R.P.; Freudzon, M.; Mehlmann, L.M.; Cowan, A.E.; Simon, A.M.; Paul, D.L.; Lampe, P.D.; Jaffe, L.A. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development 2008, 135, 3229–3238. [Google Scholar] [CrossRef]
- Gittens, J.E.; Kidder, G.M. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J. Cell Sci. 2005, 118, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Sutton-McDowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.M.; Zeng, H.T.; Ren, Z.; Zhuang, G.L.; Liang, X.Y.; Shen, H.W.; Yao, S.Z.; Ke, P.Q.; Wang, N.N. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum. Reprod. 2008, 23, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Veitch, G.I.; Gittens, J.E.I.; Shao, Q.; Laird, D.W.; Kidder, G.M. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J. Cell Sci. 2004, 117, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Donfack, N.J.; Alves, K.A.; Alves, B.G.; Rocha, R.M.P.; Bruno, J.B.; Bertolini, M.; Santos, R.R.d.; Domingues, S.F.S.; Figueiredo, J.R.D.; Smitz, J.; et al. Stroma cell-derived factor 1 and connexins (37 and 43) are preserved after vitrification and in vitro culture of goat ovarian cortex. Theriogenology 2018, 116, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.M.; Goodenough, D.A.; Li, E.; Paul, D.L. Female infertility in mice lacking connexin 37. Nature 1997, 385, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Nuttinck, F.; Peynot, N.; Humblot, P.; Massip, A.; Dessy, F.; Fléchon, J.E. Comparative Immunohistochemical Distribution of Connexin 37 and Connexin 43 throughout Folliculogenesis in the Bovine Ovary; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; Volume 57. [Google Scholar]
- Grazul-Bilska, A.T.; Vonnahme, K.A.; Bilski, J.J.; Borowczyk, E.; Soni, D.; Mikkelson, B.; Johnson, M.L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S. Expression of gap junctional connexin proteins in ovine fetal ovaries: Effects of maternal diet. Domest. Anim. Endocrinol. 2011, 41, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, E.; Johnson, M.L.; Bilski, J.J.; Borowicz, P.; Grazul-Bilska, A.T. Gap Junctional Connexin 37 Is Expressed in Sheep Ovaries. Endocrine 2006, 30, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N.; Aberdam, E.; Sherizly, I. Spontaneous maturation in vitro of cumulus-enclosed rat oocytes is inhibited by forskolin. Biol. Reprod. 1984, 31, 244–250. [Google Scholar] [CrossRef]
- Francoise, U.; Herrmann, W.L.; Etienne-Emile, B.; Sabine, S.S. Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology 1983, 113, 1170–1172. [Google Scholar]
- Racowsky, C. Effect of forskolin on maintenance of meiotic arrest and stimulation of cumulus expansion, progesterone and cyclic AMP production by pig oocyte-cumulus complexes. Reproduction 1985, 74, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.D.; Gilchrist, R.B.; Kelly, J.M.; Thompson, J.G.; Sutton-McDowall, M.L. Regulation of sheep oocyte maturation using cAMP modulator. Theriogenology 2013, 79, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Albuz, F.K.; Sasseville, M.; Lane, M.; Armstrong, D.T.; Thompson, J.G.; Gilchrist, R.B. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef] [PubMed]
- Buell, M.; Chitwood, J.L.; Ross, P.J. cAMP modulation during sheep in vitro oocyte maturation delays progression of meiosis without affecting oocyte parthenogenetic developmental competence. Anim. Reprod. Sci. 2015, 154, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Paris, V.R.; Aflatounian, A.; Handelsman, D.J. Androgens and ovarian function: Translation from basic discovery research to clinical impact. J. Endocrinol. 2019, 242, R23–R50. [Google Scholar] [CrossRef]
- Sagit, S.-A.; Iris, E.; Dalia, G.; Nava, N.; Nava, D. Disruption of Gap Junctional Communication within the Ovarian Follicle Induces Oocyte Maturation. Endocrinology 2006, 147, 2280–2286. [Google Scholar]
- Sagar, G.V.; Larson, D.M. Carbenoxolone inhibits junctional transfer and upregulates Connexin43 expression by a protein kinase A-dependent pathway. J. Cell. Biochem. 2006, 98, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Ogawa, T.; Akiyama, M.; Ishida-Kitagawa, N.; Sasada, H.; Sato, E.; Takeya, T. PKA Implicated in the Phosphorylation of Cx43 Induced by Stimulation with FSH in Rat Granulosa Cells. Reprod. Dev. 2006, 52, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Eran, G.; Vicki, P.; Nava, D. Gap junctions in the ovary: Expression, localization and function. Mol. Cell. Endocrinol. 2008, 282, 18–25. [Google Scholar]
| Primer Name | Primer Sequences (5′–3′) | Fragment Length |
|---|---|---|
| GJA4 | F-CGACGAGCAGTCGGATTT R-AGATGACATGGCCCAGGTAG | 155 bp |
| β-actin | F-CCATCGGCAATGAGCGGT R-CGTGTTGGCGTAGAGGTC | 146 bp |
| Primary Antibodies | Firm | Host | Dilution Ratio | Protein Molecular Weight | Secondary Antibodies |
|---|---|---|---|---|---|
| Cx37 | Affinity | Rabbit | 1:500 | 37 KDa | IRDye®680CW (Donkey anti-Rabbit 1:10,000) |
| α-tubulin | Abcam | Mouse | 1:20,000 | 55 KDa | IRDye®800CW (Donkey anti-Mouse 1:10,000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Subudeng, G.; Zhao, Y.; Hao, S.; Li, H. Effect of Cyclic Adenosine Monophosphate on Connexin 37 Expression in Sheep Cumulus-Oocyte Complexes. J. Dev. Biol. 2024, 12, 10. https://doi.org/10.3390/jdb12020010
Zhao M, Subudeng G, Zhao Y, Hao S, Li H. Effect of Cyclic Adenosine Monophosphate on Connexin 37 Expression in Sheep Cumulus-Oocyte Complexes. Journal of Developmental Biology. 2024; 12(2):10. https://doi.org/10.3390/jdb12020010
Chicago/Turabian StyleZhao, Mengyao, Gerile Subudeng, Yufen Zhao, Shaoyu Hao, and Haijun Li. 2024. "Effect of Cyclic Adenosine Monophosphate on Connexin 37 Expression in Sheep Cumulus-Oocyte Complexes" Journal of Developmental Biology 12, no. 2: 10. https://doi.org/10.3390/jdb12020010
APA StyleZhao, M., Subudeng, G., Zhao, Y., Hao, S., & Li, H. (2024). Effect of Cyclic Adenosine Monophosphate on Connexin 37 Expression in Sheep Cumulus-Oocyte Complexes. Journal of Developmental Biology, 12(2), 10. https://doi.org/10.3390/jdb12020010






