Developmental Anomalies in Human Teeth: Odontoblastic Differentiation in Hamartomatous Calcifying Hyperplastic Dental Follicles Presenting with DSP, Nestin, and HES1
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Characteristics
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
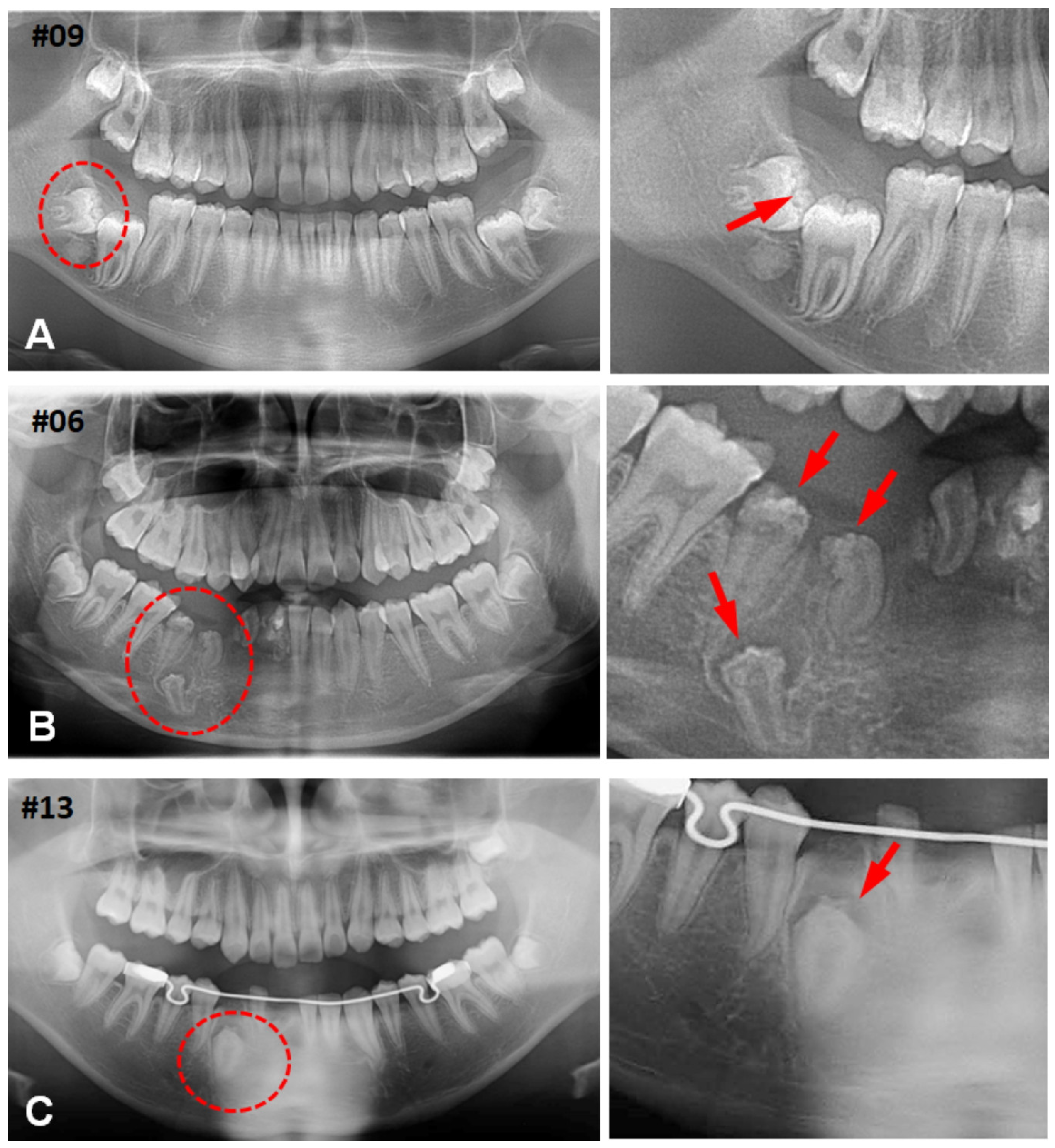
3.1. Clinical Characteristics of CHDFs
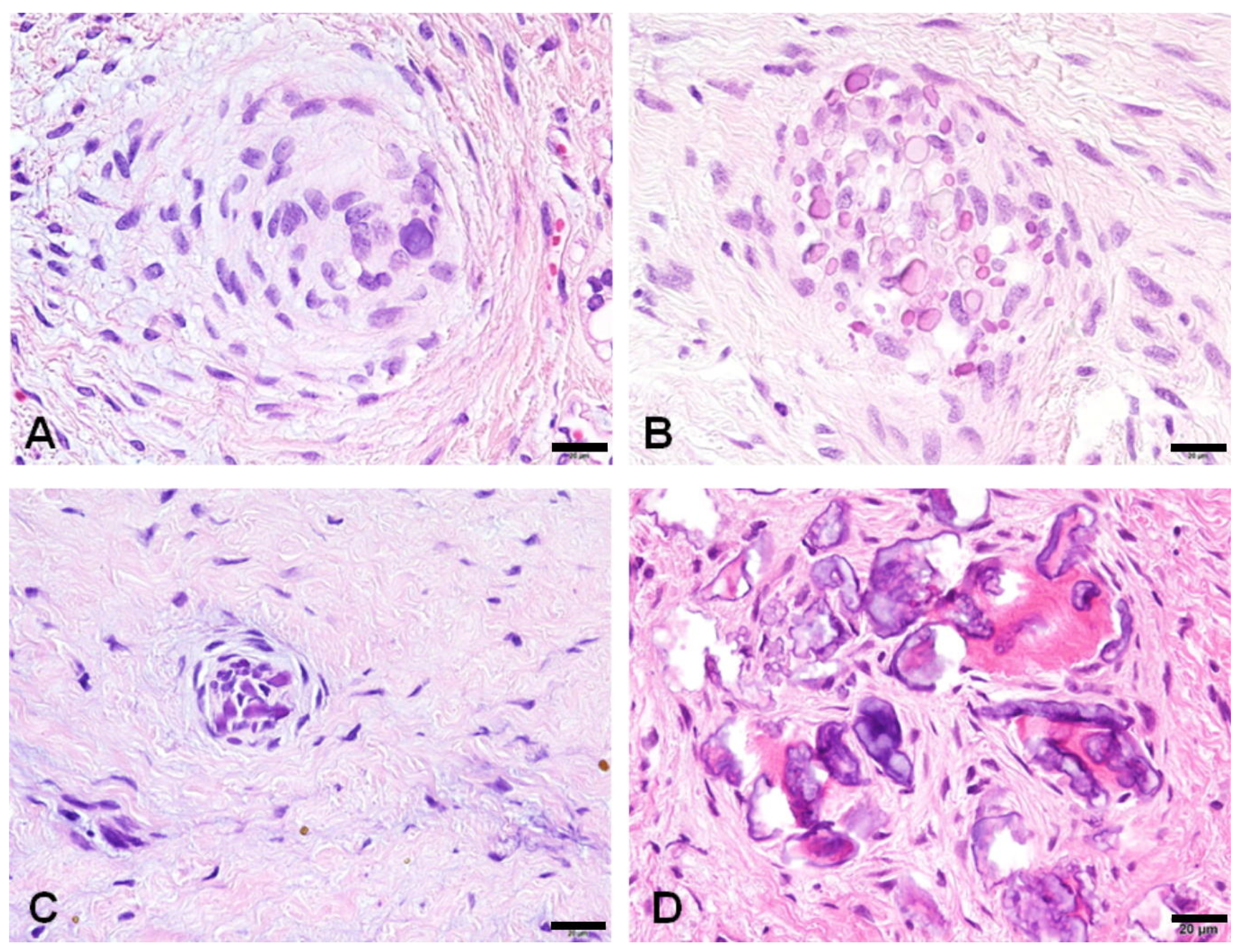
3.2. Histological Changes of HDFs
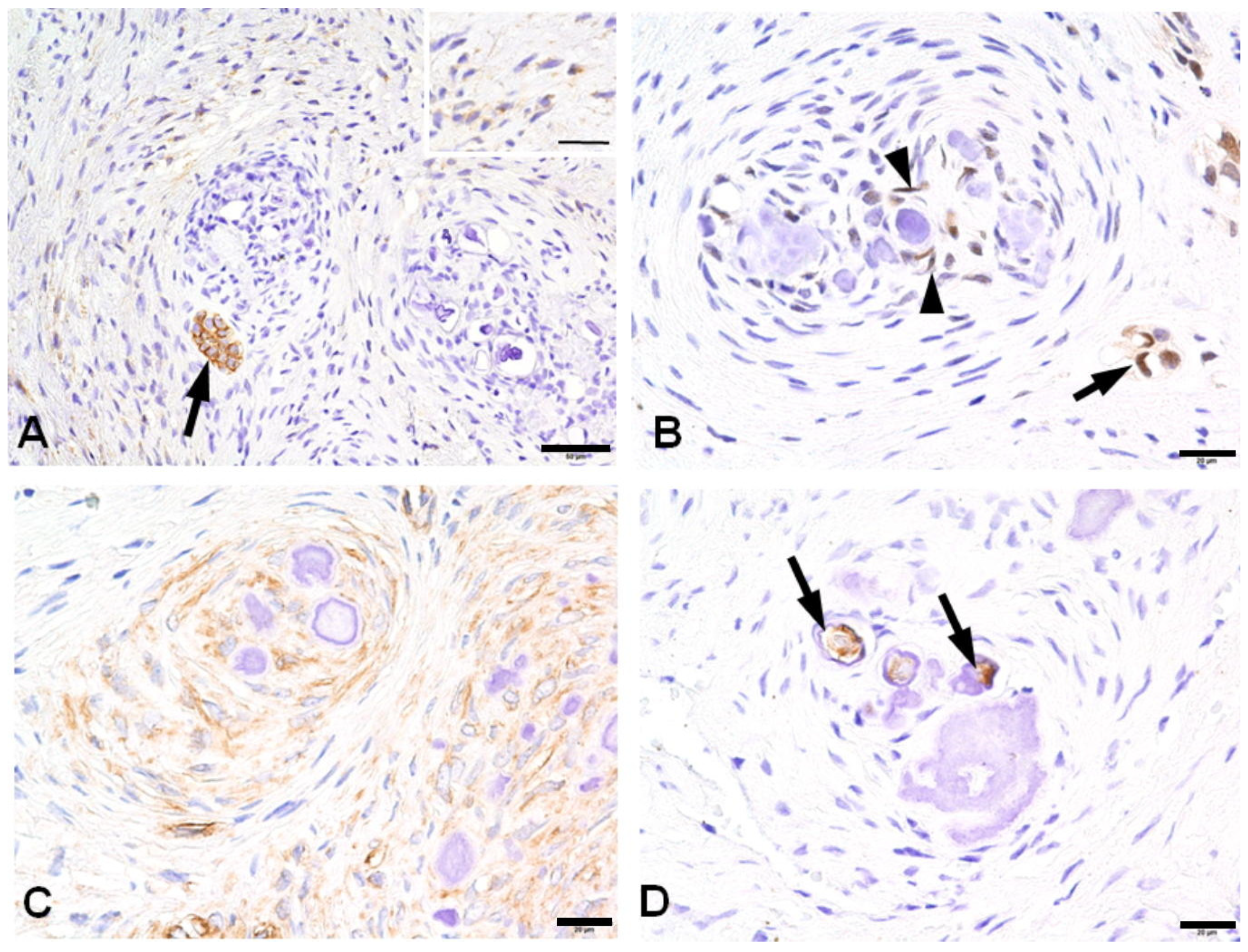
3.3. Immunohistochemical Profiles of CWNs in CHDFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karabas, H.C.; Ozcan, I.; Tekkesin, M.S.; Tasyapan, S.A.; Guray, B.; Atapek, M.M. Evaluation of Radiolucent Lesions Associated with Impacted Teeth: A Retrospective Study. Curr. Med. Imaging 2020, 16, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, A.; Buchner, A.; Dayan, D. The Central Odontogenic Fibroma and the Hyperplastic Dental Follicle: Study with Picrosirius Red and Polarizing Microscopy. J. Oral Pathol. Med. 1996, 25, 125–127. [Google Scholar] [CrossRef]
- Fukuta, Y.; Totsuka, M.; Takeda, Y.; Yamamoto, H. Pathological Study of the Hyperplastic Dental Follicle. J. Nihon Univ. Sch. Dent. 1991, 33, 166–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yonemochi, H.; Noda, T.; Saku, T. Pericoronal Hamartomatous Lesions in the Opercula of Teeth Delayed in Eruption: An Immunohistochemical Study of the Extracellular Matrix. J. Oral Pathol. Med. 1998, 27, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Ide, F.; Obara, K.; Yamada, H.; Mishima, K.; Saito, I.; Horie, N.; Shimoyama, T.; Kusama, K. Hamartomatous Proliferations of Odontogenic Epithelium within the Jaws: A Potential Histogenetic Source of Intraosseous Epithelial Odontogenic Tumors. J. Oral Pathol. Med. 2007, 36, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sandler, H.J.; Nersasian, R.R.; Cataldo, E.; Pochebit, S.; Dayal, Y. Multiple Dental Follicles with Odontogenic Fibroma-like Changes (WHO Type). Oral Surg. Oral Med. Oral Pathol. 1988, 66, 78–84. [Google Scholar] [CrossRef]
- Gardner, D.G.; Radden, B. Multiple Calcifying Hyperplastic Dental Follicles. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 603–606. [Google Scholar] [CrossRef]
- Gomez, R.S.; Silva, E.C.; Silva-Filho, E.C.; Castro, W.H. Multiple Calcifying Hyperplastic Dental Follicles. J. Oral Pathol. Med. 1998, 27, 333–334. [Google Scholar] [CrossRef]
- Cho, Y.-A.; Yoon, H.-J.; Hong, S.-P.; Lee, J.-I.; Hong, S.-D. Multiple Calcifying Hyperplastic Dental Follicles: Comparison with Hyperplastic Dental Follicles. J. Oral Pathol. Med. 2011, 40, 243–249. [Google Scholar] [CrossRef]
- Aydin, U.; Baykul, T.; Yildirim, B.; Yildirim, D.; Bozdemir, E.; Karaduman, A. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report. Imaging Sci. Dent. 2013, 43, 303–308. [Google Scholar] [CrossRef][Green Version]
- Jamshidi, S.; Zargaran, M.; Mohtasham, N. Multiple Calcifying Hyperplastic Dental Follicle (MCHDF): A Case Report. J. Dent. Res. Dent. Clin. Dent. Prospect. 2013, 7, 174–176. [Google Scholar] [CrossRef]
- Desai, R.S.; Momin, Y.N.A.; Bansal, S.; Karjodkar, F.R. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2017, 75, 1702–1705. [Google Scholar] [CrossRef]
- Davari, D.; Arzhang, E.; Soltani, P. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report. J. Oral Maxillofac. Surg. 2019, 77, 757–761. [Google Scholar] [CrossRef]
- Ulutürk, H.; Yücel, E.; Akinci, H.O.; Calisan, E.B.; Yildirim, B.; Gizli, A. Multiple Calcifying Hyperplastic Dental Follicles. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Jussila, M.; Thesleff, I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb. Perspect. Biol. 2012, 4, a008425. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Loreto, C.; Sava, A.; Mănoiu, V.; Didilescu, A.C. Human Adult Dental Pulp CD117/c-Kit-Positive Networks of Stromal Cells. Folia Morphol. 2014, 73, 68–72. [Google Scholar] [CrossRef][Green Version]
- Pisciotta, A.; Carnevale, G.; Meloni, S.; Riccio, M.; De Biasi, S.; Gibellini, L.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human Dental Pulp Stem Cells (hDPSCs): Isolation, Enrichment and Comparative Differentiation of Two Sub-Populations. BMC Dev. Biol. 2015, 15, 14. [Google Scholar] [CrossRef]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human Dental Follicle Cells Express Embryonic, Mesenchymal and Neural Stem Cells Markers. Arch. Oral Biol. 2017, 73, 121–128. [Google Scholar] [CrossRef]
- Kanao, S.; Ogura, N.; Takahashi, K.; Ito, K.; Suemitsu, M.; Kuyama, K.; Kondoh, T. Capacity of Human Dental Follicle Cells to Differentiate into Neural Cells In Vitro. Stem Cells Int. 2017, 2017, 8371326. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Nakatomi, M.; Ohshima, H. Expression Patterns of Nestin and Dentin Sialoprotein during Dentinogenesis in Mice. Biomed. Res. 2012, 33, 119–132. [Google Scholar] [CrossRef]
- Nakatomi, M.; Quispe-Salcedo, A.; Sakaguchi, M.; Ida-Yonemochi, H.; Okano, H.; Ohshima, H. Nestin Expression Is Differently Regulated between Odontoblasts and the Subodontoblastic Layer in Mice. Histochem. Cell Biol. 2018, 149, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Al-Tuwirqi, A.; Lambie, D.; Seow, W.K. Regional Odontodysplasia: Literature Review and Report of an Unusual Case Located in the Mandible. Pediatr. Dent. 2014, 36, 62–67. [Google Scholar] [PubMed]
- Schmitd, L.B.; Bravo-Calderón, D.M.; Soares, C.T.; Oliveira, D.T. Hyperplastic Dental Follicle: A Case Report and Literature Review. Case Rep. Dent. 2014, 2014, 251892. [Google Scholar] [CrossRef]
- Angiero, F.; Rossi, C.; Ferri, A.; Seramondi, R.; Magistro, S.; Farronato, D.; Benedicenti, S.; Farronato, G.; Fini, M.; Carpi, A.; et al. Stromal Phenotype of Dental Follicle Stem Cells. Front. Biosci. 2012, 4, 1009–1014. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-Expressing Progenitor Cells: Function, Identity and Therapeutic Implications. Cell Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef]
- Morsczeck, C.; Ernst, W.; Florian, C.; Reichert, T.E.; Proff, P.; Bauer, R.; Müller-Richter, U.; Driemel, O. Gene Expression of Nestin, Collagen Type I and Type III in Human Dental Follicle Cells after Cultivation in Serum-Free Medium. Oral Maxillofac. Surg. 2008, 12, 89–92. [Google Scholar] [CrossRef]
- Fujita, S.; Hideshima, K.; Ikeda, T. Nestin Expression in Odontoblasts and Odontogenic Ectomesenchymal Tissue of Odontogenic Tumours. J. Clin. Pathol. 2006, 59, 240–245. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Y.; Xu, H.; Jin, A.; Huang, X.; Gao, X.; Sun, S.; Liu, Y.; Liu, J.; Lu, T.; et al. The Odontoblastic Differentiation of Dental Mesenchymal Stem Cells: Molecular Regulation Mechanism and Related Genetic Syndromes. Front. Cell Dev. Biol. 2023, 11, 1174579. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Chen, Z.; Wu, L.; Feng, J.; Wang, F.; Shoff, L.; Li, X.; Donly, K.J.; MacDougall, M.; et al. Dentin Sialoprotein Facilitates Dental Mesenchymal Cell Differentiation and Dentin Formation. Sci. Rep. 2017, 7, 300. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Dai, X.-M.; Du, B. Hes1: A Key Role in Stemness, Metastasis and Multidrug Resistance. Cancer Biol. Ther. 2015, 16, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kageyama, R. Hes1 Regulates Embryonic Stem Cell Differentiation by Suppressing Notch Signaling. Genes. Cells 2010, 15, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, T.; Tümmers, M.; Mikami, T.; Itoh, N.; Zhang, N.; Gridley, T.; Thesleff, I. Lunatic Fringe, FGF, and BMP Regulate the Notch Pathway during Epithelial Morphogenesis of Teeth. Dev. Biol. 2002, 248, 281–293. [Google Scholar] [CrossRef]
- Felszeghy, S.; Suomalainen, M.; Thesleff, I. Notch Signalling Is Required for the Survival of Epithelial Stem Cells in the Continuously Growing Mouse Incisor. Differentiation 2010, 80, 241–248. [Google Scholar] [CrossRef]
- Sarica, I.; Derindag, G.; Kurtuldu, E.; Naralan, M.E.; Caglayan, F. A Retrospective Study: Do All. Impacted Teeth Cause Pathology? Niger. J. Clin. Pract. 2019, 22, 527–533. [Google Scholar] [CrossRef]

| Antibody | Product Number | Source | Clone | Treatment | Dilution |
|---|---|---|---|---|---|
| CD56/NCAM | M730429-2 | Agilent Technologies, Santa Clara, CA, USA | 123C3 | HIER | 1:100 |
| CD117/c-kit | A450229-2 | Agilent Technologies, Santa Clara, CA, USA | polyclonal | HIER | 1:50 |
| DSP | sc-73632 | Santa Cruz Biotechnology Inc., Dallas, TX, USA | polyclonal | Digestion | 1:4000 |
| HES1 | #11988 | Cell Signaling Technology Inc., Danvers, MA, USA | D6P2U | HIER * | 1:200 |
| Nestin | HPA007007 | Sigma-Aldrich, St. Louis, MO, USA | polyclonal | HIER * | 1:5000 |
| Components | CD56 | Nestin | HES1 | CD117 | DSP |
|---|---|---|---|---|---|
| Stroma | + | + | + | − | − |
| Epithelial islands | + | − | + | − | − |
| CWNs | − | + | + | − | + |
| Psammoma bodies | − | − | − | − | − |
| Dentinoid | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, H.; Shimada, K.; Ochiai, T.; Okada, Y. Developmental Anomalies in Human Teeth: Odontoblastic Differentiation in Hamartomatous Calcifying Hyperplastic Dental Follicles Presenting with DSP, Nestin, and HES1. J. Dev. Biol. 2024, 12, 7. https://doi.org/10.3390/jdb12010007
Hasegawa H, Shimada K, Ochiai T, Okada Y. Developmental Anomalies in Human Teeth: Odontoblastic Differentiation in Hamartomatous Calcifying Hyperplastic Dental Follicles Presenting with DSP, Nestin, and HES1. Journal of Developmental Biology. 2024; 12(1):7. https://doi.org/10.3390/jdb12010007
Chicago/Turabian StyleHasegawa, Hiromasa, Katsumitsu Shimada, Takanaga Ochiai, and Yasuo Okada. 2024. "Developmental Anomalies in Human Teeth: Odontoblastic Differentiation in Hamartomatous Calcifying Hyperplastic Dental Follicles Presenting with DSP, Nestin, and HES1" Journal of Developmental Biology 12, no. 1: 7. https://doi.org/10.3390/jdb12010007
APA StyleHasegawa, H., Shimada, K., Ochiai, T., & Okada, Y. (2024). Developmental Anomalies in Human Teeth: Odontoblastic Differentiation in Hamartomatous Calcifying Hyperplastic Dental Follicles Presenting with DSP, Nestin, and HES1. Journal of Developmental Biology, 12(1), 7. https://doi.org/10.3390/jdb12010007







