Identification of a Chondrocyte-Specific Enhancer in the Hoxc8 Gene
Abstract
1. Background
2. Methods and Materials
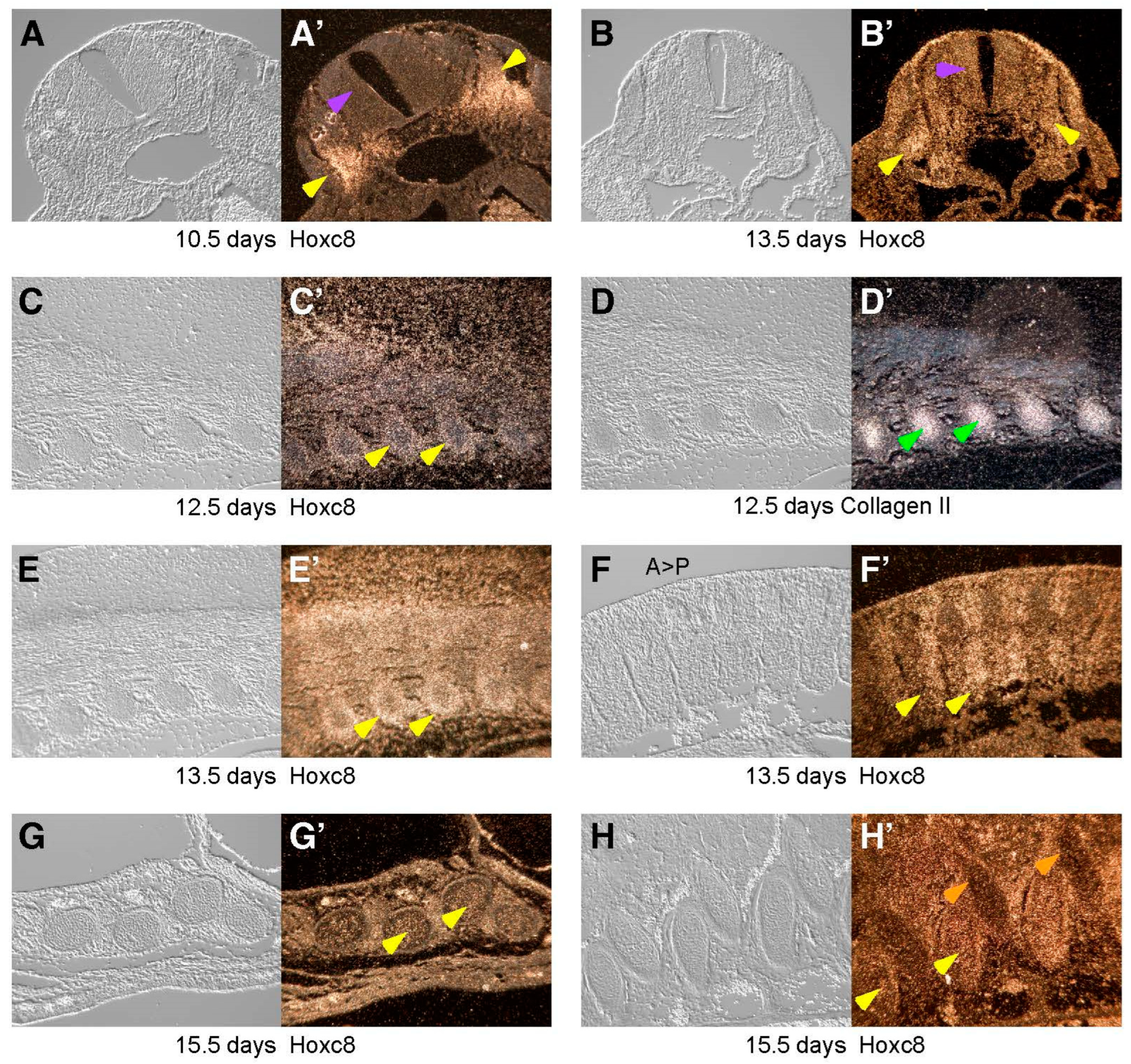
2.1. In Situ Hybridization
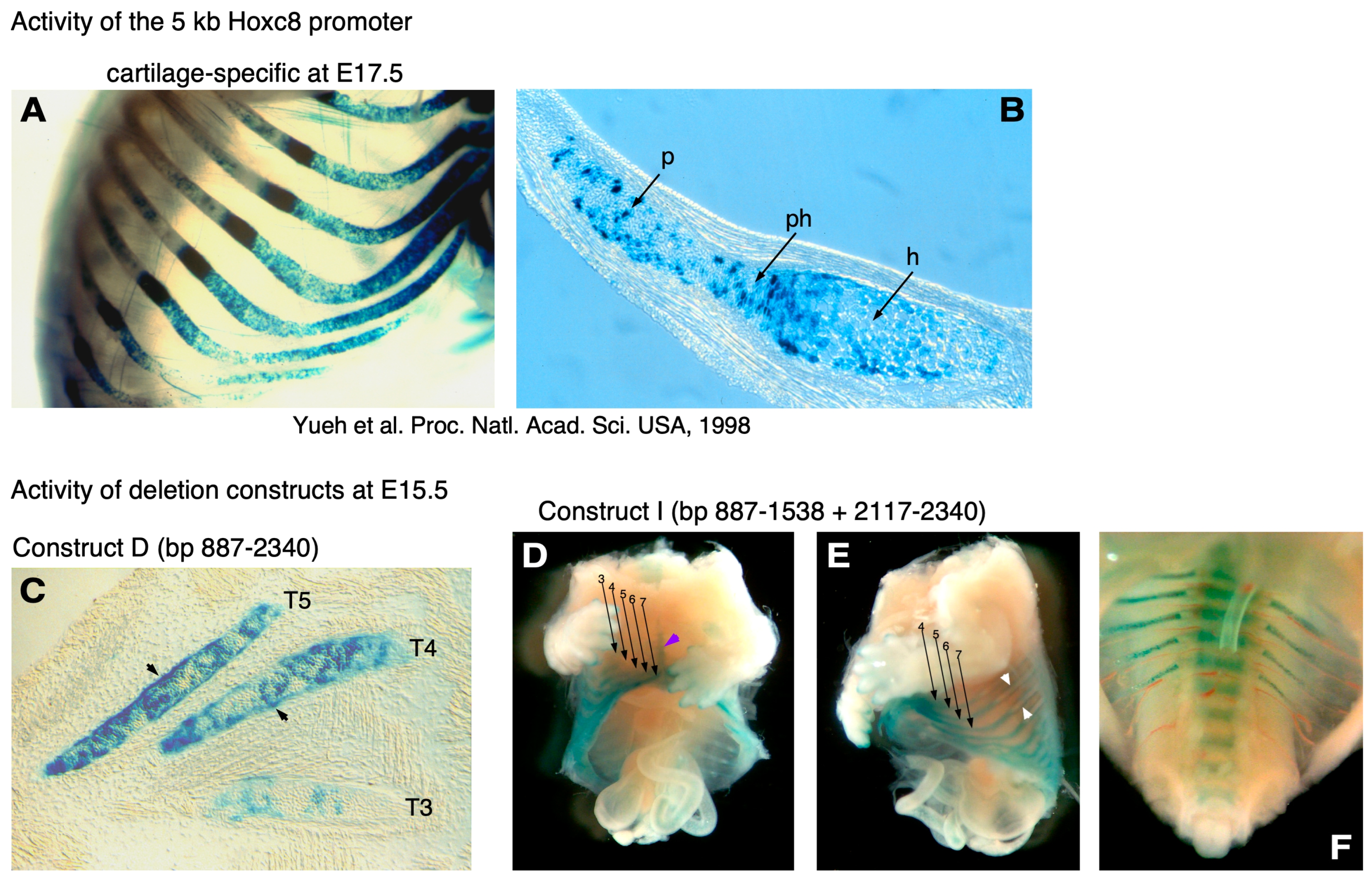
2.2. LacZ Transgene Constructs
2.3. Generation and Analysis of Transient Transgenic Embryos
2.4. Histology
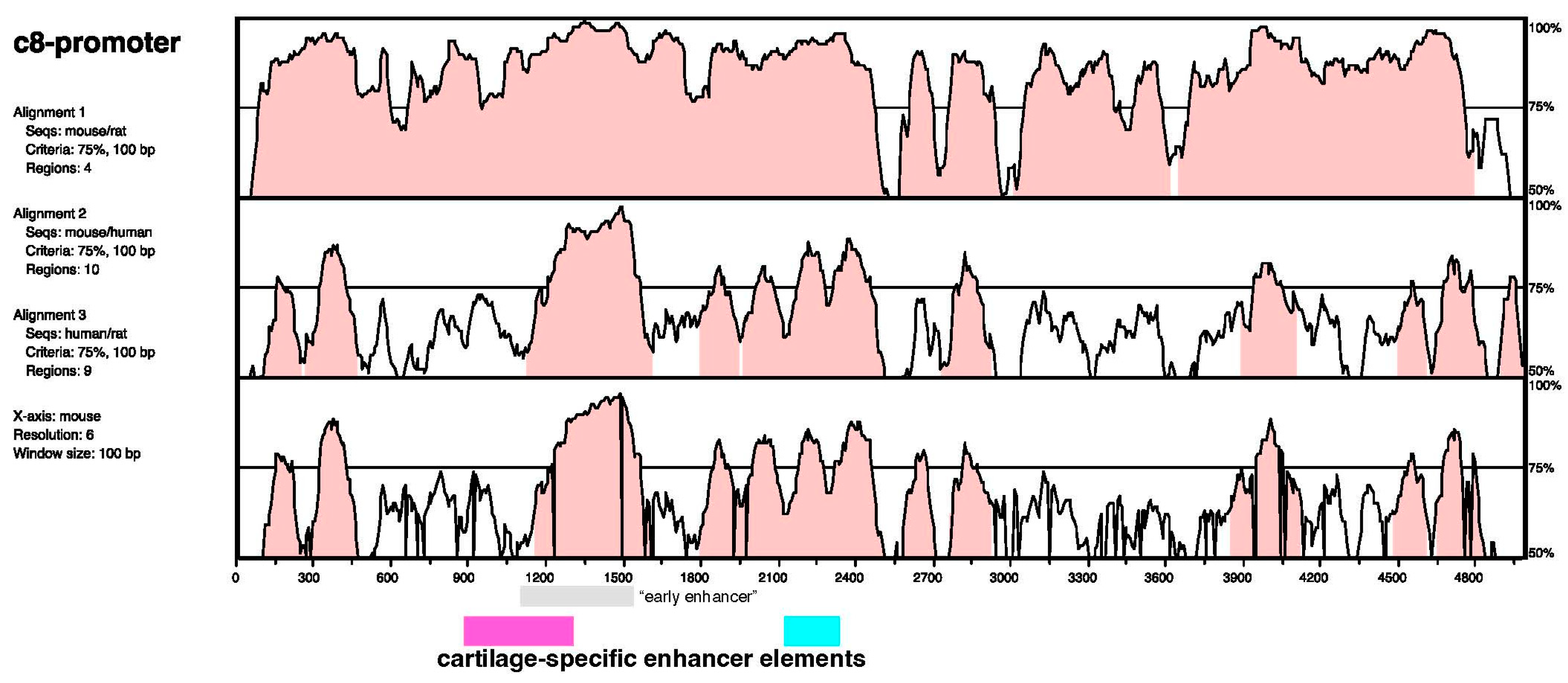
2.5. Sequence Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeMouellic, H.; Condamine, H.; Brulet, P. Pattern of transcription of the homeo gene Hox-3.1 in the mouse embryo. Genes. Dev. 1988, 2, 125–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breier, G.; Dressler, G.R.; Gruss, P. Primary structure and developmental expression pattern of Hox 3.1, a member of the murine Hox 3 homeobox gene cluster. EMBO J. 1988, 7, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Utset, M.F.; Awgulewitsch, A.; Ruddle, F.H.; McGinnis, W. Region-specific expression of two mouse homeo box genes. Science 1987, 235, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Belting, H.G.; Shashikant, C.S.; Ruddle, F.H. Multiple phases of expression and regulation of mouse Hoxc-8 during early embryogenesis. J. Exp. Zool. 1998, 282, 196–222. [Google Scholar] [CrossRef]
- Kwon, Y.; Shin, J.; Park, H.W.; Kim, M.H. Dynamic expression pattern of Hoxc8 during mouse early embryogenesis. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005, 283, 187–192. [Google Scholar] [CrossRef] [PubMed]
- LeMouellic, H.; Lallemand, Y.; Brulet, P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell 1992, 69, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Tiret, L.; Le Mouellic, H.; Maury, M.; Brulet, P. Increased apoptosis of motoneurons and altered somatotopic maps in the brachial spinal cord of Hoxc-8-deficient mice. Development 1998, 125, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, T.; Tang, Y.; Naot, Y.; Nardi, M.; Brulet, P.; Bieberich, C.J.; Takeshita, K. Hematopoietic progenitor cell abnormalities in Hoxc-8 null mutant mice. J. Exp. Zool. 1999, 283, 186–193. [Google Scholar] [CrossRef]
- Yueh, Y.G.; Gardner, D.P.; Kappen, C. Evidence for regulation of cartilage differentiation by the homeobox gene Hoxc-8. Proc. Natl. Acad. Sci. USA 1998, 95, 9956–9961. [Google Scholar] [CrossRef]
- Kamel, S.; Kruger, C.; Salbaum, J.M.; Kappen, C. Morpholino-mediated knockdown in primary chondrocytes implicates Hoxc8 in regulation of cell cycle progression. Bone 2009, 44, 708–716. [Google Scholar] [CrossRef]
- Bieberich, C.J.; Utset, M.F.; Awgulewitsch, A.; Ruddle, F.H. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc. Natl. Acad. Sci. USA 1990, 87, 8462–8466. [Google Scholar] [CrossRef] [PubMed]
- Shashikant, C.S.; Bieberich, C.J.; Belting, H.-G.; Wang, J.C.H.; Borbely, M.A.; Ruddle, F.H. Regulation of Hoxc-8 during mouse embryonic development: Identification and characterization of critical elements involved in early neural tube expression. Development 1995, 121, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J. In situ expression of collagen and proteoglycan genes in notochord and during skeletal development and growth. Microsc. Res. Technol. 1994, 28, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.W.; Ruddle, F.H. Multiplex gene regulation: A two-tiered approach to transgene regulation in transgenic mice. Proc. Natl. Acad. Sci. USA 1989, 86, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.P.; Byrne, G.W.; Ruddle, F.H.; Kappen, C. Spatial and temporal regulation of a LacZ reporter transgene in a binary transgenic mouse system. Transgenic Res. 1996, 5, 37–48. [Google Scholar] [CrossRef]
- Yaworsky, P.J.; Kappen, C. Heterogeneity of neural progenitor cells revealed by enhancers in the nestin gene. Dev. Biol. 1999, 205, 309–321. [Google Scholar] [CrossRef][Green Version]
- Yaworsky, P.J.; Gardner, D.P.; Kappen, C. Transgenic analyses reveal neuron and muscle specific elements in the murine neurofilament light chain gene promoter. J. Biol. Chem. 1997, 272, 25112–25120. [Google Scholar] [CrossRef]
- Awgulewitsch, A.; Bieberich, C.; Bogarad, L.; Shashikant, C.; Ruddle, F.H. Structural analysis of the Hox-3.1 transcription unit and the Hox-3.2--Hox-3.1 intergenic region. Proc. Natl. Acad. Sci. USA 1990, 87, 6428–6432. [Google Scholar] [CrossRef]
- Rundle, C.H.; Macias, M.P.; Gardner, D.P.; Yueh, Y.G.; Kappen, C. Transactivation of Hox Gene Expression in a VP16-Dependent Binary Transgenic Mouse System. Biochim. Biophys. Acta 1998, 1398, 164–178. [Google Scholar] [CrossRef]
- Shashikant, C.S.; Bolanowsky, S.A.; Anand, S.; Anderson, S.M. Comparison of diverged Hoxc8 early enhancer activities reveals modification of regulatory interactions at conserved cis-acting elements. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 242–249. [Google Scholar] [CrossRef]
- Puschel, A.W.; Balling, R.; Gruss, P. Separate elements cause lineage restriction and specify boundaries of Hox-1.1 expression. Development 1991, 112, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Whiting, J.; Marshall, H.; Cook, M.; Krumlauf, R.; Rigby, P.W.; Stott, D.; Allemann, R.K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991, 5, 2048–2059. [Google Scholar] [PubMed]
- Vogels, R.; Charite, J.; Degraaff, W.; Deschamps, J. Proximal cis-acting elements cooperate to set hoxb-7 (hox-2.3) expression boundaries in transgenic mice. Development 1993, 118, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Charite, J.; de Graaff, W.; Vogels, R.; Meijlink, F.; Deschamps, J. Regulation of the Hoxb-8 gene: Synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev. Biol. 1995, 171, 294–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerard, M.; Duboule, D.; Zakany, J. Structure and activity of regulatory elements involved in the activation of the hoxd-11 gene during late gastrulation. Embo J. 1993, 12, 3539–3550. [Google Scholar] [CrossRef]
- Shashikant, C.S.; Ruddle, F.H. Combinations of closely situated cis-acting elements determine tissue-specific patterns and anterior extent of early Hoxc8 expression. Proc. Natl. Acad. Sci. USA 1996, 93, 12364–12369. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.H.; Ruddle, F.H. Enhancer timing of Hox gene expression: Deletion of the endogenous Hoxc8 early enhancer. Development 2003, 130, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Samsa, W.E.; Zhou, G.; Lefebvre, V. Transcriptional control of chondrocyte specification and differentiation. Semin. Cell Dev. Biol. 2017, 62, 34–49. [Google Scholar] [CrossRef]
- Rosello-Diez, A.; Joyner, A.L. Regulation of Long Bone Growth in Vertebrates; It Is Time to Catch Up. Endocr. Rev. 2015, 36, 646–680. [Google Scholar] [CrossRef]
- Srour, M.K.; Fogel, J.L.; Yamaguchi, K.T.; Montgomery, A.P.; Izuhara, A.K.; Misakian, A.L.; Lam, S.; Lakeland, D.L.; Urata, M.M.; Lee, J.S.; et al. Natural large-scale regeneration of rib cartilage in a mouse model. J. Bone Miner. Res. 2015, 30, 297–308. [Google Scholar] [CrossRef]
- Bieberich, C.J.; Ruddle, F.H.; Stenn, K.S. Differential expression of the Hox 3.1 gene in adult mouse skin. Ann. N. Y. Acad. Sci. 1991, 642, 346–353, discussion in 353–344. [Google Scholar] [CrossRef] [PubMed]
- Brancaz, M.V.; Iratni, R.; Morrison, A.; Mancini, S.J.; Marche, P.; Sundberg, J.; Nonchev, S. A new allele of the mouse hairless gene interferes with Hox/LacZ transgene regulation in hair follicle primordia. Exp. Mol. Pathol. 2004, 76, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Pruett, N.D.; Visconti, R.P.; Jacobs, D.F.; Scholz, D.; McQuinn, T.; Sundberg, J.P.; Awgulewitsch, A. Evidence for Hox-specified positional identities in adult vasculature. BMC Dev. Biol. 2008, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Rux, D.R.; Song, J.Y.; Swinehart, I.T.; Pineault, K.M.; Schlientz, A.J.; Trulik, K.G.; Goldstein, S.A.; Kozloff, K.M.; Lucas, D.; Wellik, D.M. Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Dev. Cell 2016, 39, 653–666. [Google Scholar] [CrossRef]
- Song, J.Y.; Pineault, K.M.; Dones, J.M.; Raines, R.T.; Wellik, D.M. Hox genes maintain critical roles in the adult skeleton. Proc. Natl. Acad. Sci. USA 2020, 117, 7296–7304. [Google Scholar] [CrossRef]
- Taylor, H.S.; Vanden Heuvel, G.B.; Igarashi, P. A conserved Hox axis in the mouse and human female reproductive system: Late establishment and persistent adult expression of the Hoxa cluster genes. Biol. Reprod. 1997, 57, 1338–1345. [Google Scholar] [CrossRef]
- Abramovich, C.; Pineault, N.; Ohta, H.; Humphries, R.K. Hox genes: From leukemia to hematopoietic stem cell expansion. Ann. N. Y. Acad. Sci. 2005, 1044, 109–116. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. Role of HOX Genes in Stem Cell Differentiation and Cancer. Stem Cells Int. 2018, 2018, 3569493. [Google Scholar] [CrossRef]
- Chen, H.; Sukumar, S. HOX genes: Emerging stars in cancer. Cancer Biol. Ther. 2003, 2, 524–525. [Google Scholar] [CrossRef]
- Grier, D.G.; Thompson, A.; Kwasniewska, A.; McGonigle, G.J.; Halliday, H.L.; Lappin, T.R. The pathophysiology of HOX genes and their role in cancer. J. Pathol. 2005, 205, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Langley, S.E. Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int. 2014, 113, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Zyoud, A.; Allegrucci, C. A Case of Identity: HOX Genes in Normal and Cancer Stem Cells. Cancers 2019, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Awgulewitsch, A. Hox in hair growth and development. Naturwissenschaften 2003, 90, 193–211. [Google Scholar] [CrossRef]
- Tabaries, S.; Lapointe, J.; Besch, T.; Carter, M.; Woollard, J.; Tuggle, C.K.; Jeannotte, L. Cdx protein interaction with Hoxa5 regulatory sequences contributes to Hoxa5 regional expression along the axial skeleton. Mol. Cell Biol. 2005, 25, 1389–1401. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cormier, S.A.; Kappen, C. Identification of a Chondrocyte-Specific Enhancer in the Hoxc8 Gene. J. Dev. Biol. 2024, 12, 5. https://doi.org/10.3390/jdb12010005
Cormier SA, Kappen C. Identification of a Chondrocyte-Specific Enhancer in the Hoxc8 Gene. Journal of Developmental Biology. 2024; 12(1):5. https://doi.org/10.3390/jdb12010005
Chicago/Turabian StyleCormier, Stephania A., and Claudia Kappen. 2024. "Identification of a Chondrocyte-Specific Enhancer in the Hoxc8 Gene" Journal of Developmental Biology 12, no. 1: 5. https://doi.org/10.3390/jdb12010005
APA StyleCormier, S. A., & Kappen, C. (2024). Identification of a Chondrocyte-Specific Enhancer in the Hoxc8 Gene. Journal of Developmental Biology, 12(1), 5. https://doi.org/10.3390/jdb12010005





