Proteomic Approaches to Unravel the Molecular Dynamics of Early Pregnancy in Farm Animals: An In-Depth Review
Abstract
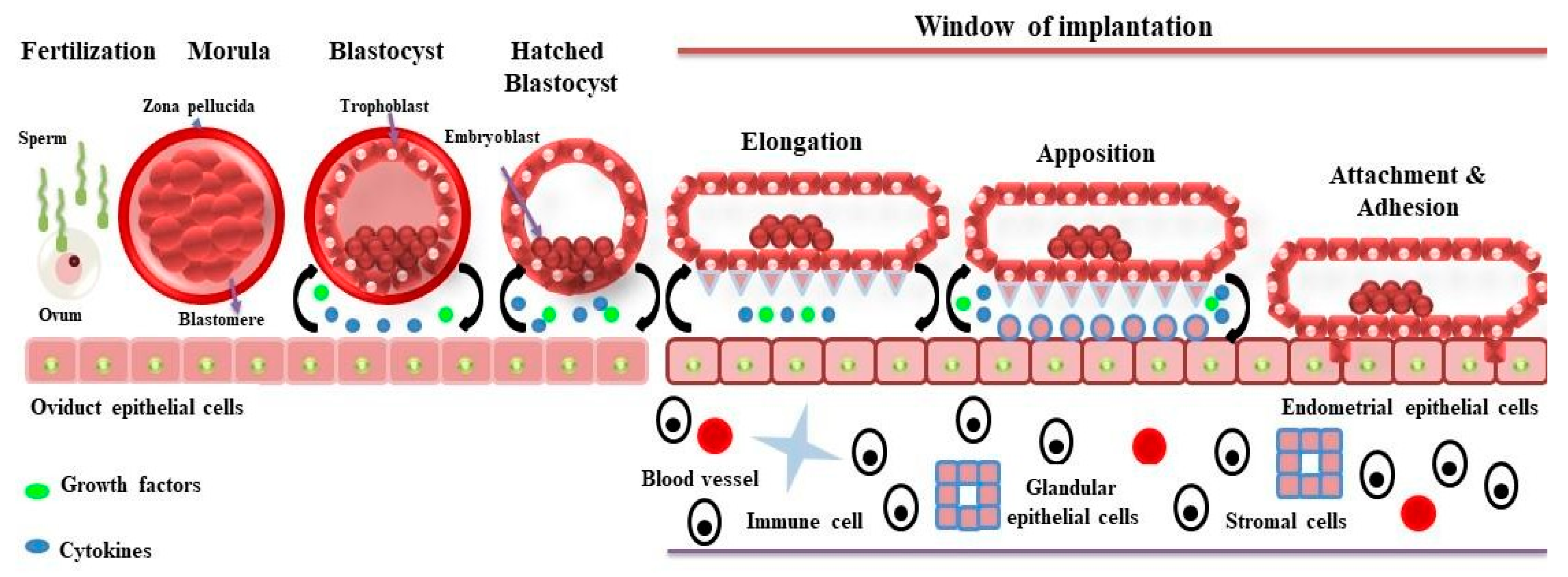
1. Introduction
2. Proteomics of Bovine Uterus Milieu to Decipher the Molecular Landscape of Successful Pregnancy
2.1. Proteome Profiling of Endometrial Tissue
2.2. Proteome Profiling of Uterine Lumen Fluid (ULF)
2.2.1. Proteomics of Uterine Lumen Fluid (ULF) in In Vivo Studies
2.2.2. Proteomics of Uterine Lumen Fluid (ULF) in In Vitro Studies
2.2.3. Proteomics of Exosomal microRNAs in Uterine Lumen Fluid (ULF)
2.3. Proteome Profiling of Oviductal Fluids (OF)
2.4. Proteome Profiling of Cows with Endometritis
3. Proteomics of the Sheep Uterine Milieu to Unfold the Molecular Landscape of Implantation
3.1. Proteome Profiling of Uterine Fluids (Pregnant vs. Non-Pregnant)
3.2. Proteome Profiling of Endometrial Tissues (Intercaruncular vs. Caruncular Areas)
3.3. Proteome Profiling of the Genital Tract (Day 0 vs. Day 10 of the Oestrous Cycle)
3.4. Proteome Profiling of Uterine Luminal Fluid (Days 12 to 16 of Pregnancy)
3.5. Proteome Profiling of Conceptuses and Endometrium
3.6. Proteome Profiling of Exosomes
4. Proteomics of Pigs Uterine Milieu to Understand the Mechanism of Implantation for Successful Pregnancy
4.1. Proteome Profiling of Oviductal Fluid and Conceptus
4.2. Proteome Profiling of Endometrial Tissues (Pregnant vs. Non-Pregnant)
4.3. Proteome Profiling of Endometrial Tissue (Preimplantation and Peri-Implantation Period)
4.4. Proteome Profiling of Luminal Fluid
4.5. Proteome Profiling of Serum
4.6. Proteome Profiling of Endometrial Tissue (High Prolific Breed vs. Low Prolific Breed)
5. Key Signaling Pathways Associated with Early Pregnancy in Farm Animals
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowen, J.A.; Burghardt, R.C. Cellular mechanisms of implantation in domestic farm animals. Semin. Cell Dev. Biol. 2000, 11, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; DeMayo, F.J. Animal models of implantation. Reproduction 2004, 128, 679–695. [Google Scholar] [CrossRef]
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Implantation mechanisms: Insights from the sheep. Reproduction 2004, 128, 657–668. [Google Scholar] [CrossRef]
- MacIntyre, D.M.; Lim, H.C.; Ryan, K.; Kimmins, S.; Small, J.A.; MacLaren, L.A. Implantation-associated changes in bovine uterine ex-pression of integrins and extracellular matrix. Biol. Reprod. 2002, 66, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.E.; Comizzoli, P. Endometrial receptivity and embryo implantation in carnivores-commonalities and differences with other mammalian species. Biol. Reprod. 2021, 104, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.K.; Sharma, N. Endocrine Causes of Early Embryonic Death: An Overview. Curr. Res. Dairy Sci. 2011, 3, 1–24. [Google Scholar] [CrossRef]
- Binelli, M.; Silva, F.A.C.C.; Rocha, C.C.; Martins, T.; Sponchiado, M.; Van Hoeck, V.; Cordeiro, A.; Campbell, M.; Leroy, J.L.M.R.; Peñagaricano, F.; et al. Endometrial receptivity in cattle: The mutual reprogramming paradigm. Anim. Reprod. 2022, 19, e20220097. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.T.; Franco, G.A.; Poole, R.K.; Hood, R.; Fernadez Montero, L.; Oliveira Filho, R.V.; Cooke, R.F.; Pohler, K.G. Pregnancy loss in beef cattle: A meta-analysis. Anim. Reprod. Sci. 2020, 212, 106251. [Google Scholar] [CrossRef]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 260–267. [Google Scholar] [CrossRef]
- Spencer, T.E. Early pregnancy: Concepts, challenges, and potential solutions. Anim. Front. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- La, Y.; Tang, J.; Guo, X.; Zhang, L.; Gan, S.; Zhang, X.; Zhang, J.; Hu, W.; Chu, M. Proteomic analysis of sheep uterus reveals its role in prolificacy. J. Proteom. 2020, 210, 103526. [Google Scholar] [CrossRef] [PubMed]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Agar, J.N.; Chamot-Rooke, J.; Danis, P.O.; Ge, Y.; Loo, J.A.; Paša-Tolić, L.; Tsybin, Y.O.; Kelleher, N.L. Consortium for Top-Down Proteomics. The Human Proteoform Project: Defining the human proteome. Sci. Adv. 2021, 7, eabk0734. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. Is proteomics the new genomics? Cell 2007, 130, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, K.; Andonovski, M.; Coorssen, J.R. Proteomes Are of Proteoforms: Embracing the Complexity. Proteomes 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kelleher, N.L.; Consortium for Top Down Proteomics. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef]
- Ercan, H.; Resch, U.; Hsu, F.; Mitulovic, G.; Bileck, A.; Gerner, C.; Yang, J.W.; Geiger, M.; Miller, I.; Zellner, M. A Practical and Analytical Comparative Study of Gel-Based Top-Down and Gel-Free Bottom-Up Proteomics Including Unbiased Proteoform Detection. Cells 2023, 12, 747. [Google Scholar] [CrossRef]
- Haider, S.; Pal, R. Integrated analysis of transcriptomic and proteomic data. Curr. Genom. 2013, 14, 91–110. [Google Scholar] [CrossRef]
- Bludau, I.; Frank, M.; Dörig, C.; Cai, Y.; Heusel, M.; Rosenberger, G.; Picotti, P.; Collins, B.C.; Röst, H.; Aebersold, R. Systematic detection of functional proteoform groups from bottom-up proteomic datasets. Nat. Commun. 2021, 12, 3810. [Google Scholar] [CrossRef]
- Thasmi, C.N.; Siregar, T.N.; Wahyuni, S.; Aliza, D.; Panjaitan, B.; Nazaruddin, N.; Sabila, F.N.; Fallatanza, M. Anatomical and histological changes of uterine horn of Aceh cattle with repeat breeding. J. Adv. Vet. Anim. Res. 2018, 5, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yoshioka, S.; Tasaki, Y.; Okuda, K. Remodeling of bovine endometrium throughout the estrous cycle. Anim. Reprod. Sci. 2013, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Forde, N. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal 2014, 8 (Suppl. S1), 64–69. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Hölker, M.; Rings, F.; Ghanem, N.; Ulas-Cinar, M.; Peippo, J.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Bovine pretransfer endometrium and embryo transcriptome fingerprints as predictors of pregnancy success after embryo transfer. Physiol. Genom. 2010, 42, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, X.; Xie, H.; Zhang, G.; Deng, M.; Deng, K.; Gao, X.; Bao, Y.; Li, K.; Wang, F. PPP2R2A affects embryonic implantation by regulating the proliferation and apoptosis of Hu sheep endometrial stromal cells. Theriogenology 2021, 176, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.R.; Fröhlich, T.; Arnold, G.J. Proteomics of bovine endometrium, oocytes and early embryos. Biosci. Proc. 2019, 8. [Google Scholar] [CrossRef][Green Version]
- Ledgard, A.M.; Lee, R.S.; Peterson, A.J. Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol. Reprod. Dev. 2009, 76, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ledgard, A.M.; Smolenski, G.A.; Henderson, H.; Lee, R.S. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod. Fertil. Dev. 2015, 27, 395–406. [Google Scholar] [CrossRef]
- Mullen, M.P.; Elia, G.; Hilliard, M.; Parr, M.H.; Diskin, M.G.; Evans, A.C.; Crowe, M.A. Proteomic characterization of histotroph during the preimplantation phase of the estrous cycle in cattle. J. Proteome Res. 2012, 11, 3004–3018. [Google Scholar] [CrossRef]
- Faulkner, S.; Elia, G.; Mullen, M.P.; O’Boyle, P.; Dunn, M.J.; Morris, D. A comparison of the bovine uterine and plasma proteome using iTRAQ proteomics. Proteomics 2012, 12, 2014–2023. [Google Scholar] [CrossRef]
- Faulkner, S.; Elia, G.; O’Boyle, P.; Dunn, M.; Morris, D. Composition of the bovine uterine proteome is associated with stage of cycle and concentration of systemic progesterone. Proteomics 2013, 13, 3333–3353. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; McGettigan, P.A.; Mehta, J.P.; O’Hara, L.; Mamo, S.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 2014, 147, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Aranciaga, N.; Morton, J.D.; Maes, E.; Gathercole, J.L.; Berg, D.K. Proteomic determinants of uterine receptivity for pregnancy in early and mid-postpartum dairy cows. Biol. Reprod. 2021, 105, 1458–1473. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.L.; Gasparrini, B.; Neglia, G.; Vecchio, D.; Strazzullo, M.; Giovane, A.; Servillo, L.; Zicarelli, L.; D’Occhio, M.J.; Campanile, G. Proteomic Profiles of the Embryonic Chorioamnion and Uterine Caruncles in Buffaloes (Bubalus bubalis) with Normal and Retarded Embryonic Development. Bio. Reprod. 2013, 88, 1–14. [Google Scholar] [CrossRef]
- Berendt, F.J.; Fröhlich, T.; Schmidt, S.E.; Reichenbach, H.D.; Wolf, E.; Arnold, G.J. Holistic differential analysis of embryo-induced alter-ations in the proteome of bovine endometrium in the preattachment period. Proteomics 2005, 5, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Hou, Z.; Miao, K.; Zhao, H.; Wang, R.; Guo, M.; Wu, Z.; Tian, J.; An, L. Comparative analysis of proteomic profiles between endometrial caruncular and intercaruncular areas in ewes during the peri-implantation period. J. Anim. Sci. Biotechnol. 2013, 4, 39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Gubory, K.H.; Arianmanesh, M.; Garrel, C.; Bhattacharya, S.; Cash, P.; Fowler, P.A. Proteomic analysis of the sheep caruncular and intercaruncular endometrium reveals changes in functional proteins crucial for the establishment of pregnancy. Reproduction 2014, 147, 599–614. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Sui, L.; Miao, K.; An, L.; Wang, D.; Hou, Z.; Wang, R.; Guo, M.; Wang, Z.; Xu, J.; et al. Comparative analysis between endometrial proteomes of pregnant and non-pregnant ewes during the peri-implantation period. J. Anim. Sci. Biotechnol. 2015, 6, 18. [Google Scholar] [CrossRef]
- Arianmanesh, M.; Fowler, P.A.; Al-Gubory, K.H. The sheep conceptus modulates proteome profiles in caruncular endometrium during early pregnancy. Anim. Reprod. Sci. 2016, 175, 48–56. [Google Scholar] [CrossRef]
- Koch, J.M.; Ramadoss, J.; Magness, R.R. Proteomic profile of uterine luminal fluid from early pregnant ewes. J. Proteome Res. 2010, 9, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Burns, G.W.; Moraes, J.G.; Spencer, T.E. Analysis of the Uterine Epithelial and Conceptus Transcriptome and Luminal Fluid Proteome During the Peri-Implantation Period of Pregnancy in Sheep. Biol. Reprod. 2016, 95, 88. [Google Scholar] [CrossRef]
- Romero, J.J.; Liebig, B.E.; Broeckling, C.D.; Prenni, J.E.; Hansen, T.R. Pregnancy-induced changes in metabolome and proteome in ovine uterine flushings. Biol. Reprod. 2017, 97, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.I.; Kim, J.; Lee, S.G.; Jeon, Y.J.; Kim, D.W.; Soh, Y.; Seo, K.S.; Lee, H.K.; Choi, N.J.; Ryu, J.; et al. Proteomic analysis of pregnancy-related proteins from pig uterus endometrium during pregnancy. Proteome Sci. 2011, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Jalali, B.M.; Bogacki, M.; Dietrich, M.; Likszo, P.; Wasielak, M. Proteomic analysis of porcine endometrial tissue during peri-implan-tation period reveals altered protein abundance. J. Proteom. 2015, 125, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Jalali, B.; Likszo, P.; Skarzynski, D.J. Proteomic and network analysis of pregnancy-induced changes in the porcine endometrium on Day 12 of gestation. Mol. Reprod. Dev. 2016, 83, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, D.; Liput, K.; Korwin-Kossakowska, A.; Ogłuszka, M.; Poławska, E.; Nawrocka, A.; Urbański, P.; Ciepłoch, A.; Juszczuk-Kubiak, E.; Lepczyński, A.; et al. Molecular Characterisation of Uterine Endometrial Proteins during Early Stages of Pregnancy in Pigs by MALDI TOF/TOF. Int. J. Mol. Sci. 2021, 22, 6720. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, K.; Xu, Q.; Liu, Y.; Li, W.; Bai, Y.; Wang, J.; Ding, C.; Liu, X.; Tang, Q.; et al. Protein expression profiles in Meishan and Duroc sows during mid-gestation reveal differences affecting uterine capacity, endometrial receptivity, and the maternal-fetal Interface. BMC Genom. 2019, 20, 991. [Google Scholar] [CrossRef]
- Liu, S.; Cui, H.; Li, Q.; Zhang, L.; Na, Q.; Liu, C. RhoGDI2 is expressed in human trophoblasts and involved in their migration by inhibiting the activation of RAC1. Biol. Reprod. 2014, 90, 88. [Google Scholar] [CrossRef]
- Landrock, D.; Atshaves, B.P.; McIntosh, A.L.; Landrock, K.K.; Schroeder, F.; Kier, A.B. Acyl-CoA binding protein gene ablation induces pre-implantation embryonic lethality in mice. Lipids 2010, 45, 567–580. [Google Scholar] [CrossRef]
- Choi, J.H.; Ishida, M.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M. Involvement of 20α-hydroxysteroid dehydrogenase in the maintenance of pregnancy in mice. J. Reprod. Dev. 2008, 54, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Campanile, G.; Neglia, G.; Gasparrini, B.; Galiero, G.; Prandi, A.; Di Palo, R.; D’Occhio, M.J.; Zicarelli, L. Embryonic mortality in buffaloes synchronized and mated by AI during the seasonal decline in reproductive function. Theriogenology 2005, 63, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Vecchio, D.; Neglia, G.; Pacelli, C.; Prandi, A.; Gasparrini, B.; Zicarelli, L.; D’Occhio, M.J.; Campanile, G. Corpus luteum function and pregnancy outcome in buffaloes during the transition period from breeding to non-breeding season. Reprod. Domest. Anim. 2010, 45, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, C.M.; Kavelaars, A.; Eijkemans, M.J.; Amarouchi, K.; Teklenburg, G.; Gutknecht, D.; Fauser, B.J.; Heijnen, C.J.; Macklon, N.S. Cytokine profiling in endometrial secretions: A non-invasive window on endometrial receptivity. Reprod. Biomed. Online 2009, 18, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, T.C.; Chen, X. The endometrial proteomic profile around the time of embryo implantation. Biol. Reprod. 2021, 104, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Wu, G.; Johnson, G.A.; Kim, J.; Song, G. Uterine histotroph and conceptus development: Select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol. Reprod. 2011, 85, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Simintiras, C.A.; Drum, J.N.; Liu, H.; Sofia Ortega, M.; Spencer, T.E. Uterine lumen fluid is metabolically semi-autonomous. Commun. Biol. 2022, 5, 191. [Google Scholar] [CrossRef]
- Itze-Mayrhofer, C.; Brem, G. Quantitative proteomic strategies to study reproduction in farm animals: Female reproductive flu-ids. J. Proteom. 2020, 225, 103884. [Google Scholar] [CrossRef]
- Ledgard, A.M.; Berg, M.C.; McMillan, W.H.; Smolenski, G.; Peterson, A.J. Effect of asynchronous transfer on bovine embryonic devel-opment and relationship with early cycle uterine proteome profiles. Reprod. Fertil. Dev. 2012, 24, 962–972. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Nair, R.R.; Khanna, A.; Singh, K. Role of inflammatory proteins S100A8 and S100A9 in pathophysiology of recurrent early preg-nancy loss. Placenta 2013, 34, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Beltman, M.E.; Mullen, M.P.; Elia, G.; Hilliard, M.; Diskin, M.G.; Evans, A.C.; Crowe, M.A. Global proteomic characterization of uterine histotroph recovered from beef heifers yielding good quality and degenerate day 7 embryos. Domest. Anim. Endocrinol. 2014, 46, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Minhas, B.S.; Ripps, B.A.; Zhu, Y.P.; Kim, H.N.; Burwinkel, T.H.; Gleicher, N. Platelet activating factor and conception. Am. J. Reprod. Immunol. 1996, 35, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ni, J.; Chen, B.; Sun, F.; Huang, J.; Ni, S.; Tang, Z. PAFAH1B3 predicts poor prognosis and promotes progression in lung adenocarcinoma. BMC Cancer 2022, 22, 525. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, X.; Liu, J.; Chen, Q.; Huang, X.; Huang, K.; Liu, H.; Zhu, W.; Zhang, X. Identification of PAFAH1B3 as Candidate Prognosis Marker and Potential Therapeutic Target for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 700. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Qin, Y. S100A4 promotes the development of lipopolysaccharide-induced mouse endometritis. Biol. Reprod. 2018, 99, 960–967. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of metastasis-induced protein S100A4 in human non-tumor pathophysiologies. Cell Biosci. 2017, 7, 64. [Google Scholar] [CrossRef]
- Fortes, M.R.S.; Zacchi, L.F.; Nguyen, L.T.; Raidan, F.; Weller, M.M.D.C.A.; Choo, J.J.Y.; Reverter, A.; Rego, J.P.A.; Boe-Hansen, G.B.; Porto-Neto, L.R.; et al. Pre- and post-puberty expression of genes and proteins in the uterus of Bos indicus heifers: The luteal phase effect post-puberty. Anim. Genet. 2018, 49, 539–549. [Google Scholar] [CrossRef]
- Rottmayer, R.; Ulbrich, S.E.; Kölle, S.; Prelle, K.; Neumueller, C.; Sinowatz, F.; Meyer, H.H.; Wolf, E.; Hiendleder, S. A bovine oviduct epithelial cell suspension culture system suitable for studying embryo-maternal interactions: Morphological and functional characterization. Reproduction 2006, 132, 637–648. [Google Scholar] [CrossRef]
- Avilés, M.; Gutiérrez-Adán, A.; Coy, P. Oviductal secretions: Will they be key factors for the future ARTs? Mol. Hum. Reprod. 2010, 16, 896–906. [Google Scholar] [CrossRef]
- Algarra, B.; Han, L.; Soriano-Úbeda, C.; Avilés, M.; Coy, P.; Jovine, L.; Jiménez-Movilla, M. The C-terminal region of OVGP1 remodels the zona pellucida and modifies fertility parameters. Sci. Rep. 2016, 6, 32556. [Google Scholar] [CrossRef] [PubMed]
- Laheri, S.; Ashary, N.; Bhatt, P.; Modi, D. Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion. J. Assist. Reprod. Genet. 2018, 35, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Bertling, E.; Hotulainen, P.; Mattila, P.K.; Matilainen, T.; Salminen, M.; Lappalainen, P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol. Biol. Cell 2004, 15, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Budipitoj, T.; Matsuzaki, S.; Cruzana, M.B.; Baltazar, E.T.; Hondo, E.; Sunaryo, S.; Kitamura, N.; Yamada, J. Immunolocalization of gastrin-releasing peptide in the bovine uterus and placenta. J. Vet. Med. Sci. 2001, 63, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.G.N.; Behura, S.K.; Bishop, J.V.; Hansen, T.R.; Geary, T.W.; Spencer, T.E. Analysis of the uterine lumen in fertility-classified heifers: II. Proteins and metabolites †. Biol. Reprod. 2020, 102, 571–587. [Google Scholar] [CrossRef]
- Berry, D.P.; Wall, E.; Pryce, J.E. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 2014, 8, 105–121. [Google Scholar] [CrossRef]
- Gegenfurtner, K.; Fröhlich, T.; Flenkenthaler, F.; Kösters, M.; Fritz, S.; Desnoës, O.; Le Bourhis, D.; Salvetti, P.; Sandra, O.; Charpigny, G.; et al. Genetic merit for fertility alters the bovine uterine luminal fluid proteome †. Biol. Reprod. 2020, 102, 730–739. [Google Scholar] [CrossRef]
- Steinhauser, C.B.; Landers, M.; Myatt, L.; Burghardt, R.C.; Vallet, J.L.; Bazer, F.W.; Johnson, G.A. Fructose Synthesis and Transport at the Uterine-Placental Interface of Pigs: Cell-Specific Localization of SLC2A5, SLC2A8, and Components of the Polyol Pathway. Biol. Reprod. 2016, 95, 108. [Google Scholar] [CrossRef]
- Gumen, A.; Keskin, A.; Yilmazbas-Mecitoglu, G.; Karakaya, E.; Wiltbank, M. Dry period management and optimization of post-partum reproductive management in dairy cattle. Reprod. Domest. Anim. 2011, 46, 11–17. [Google Scholar] [CrossRef]
- Jhamat, N.; Niazi, A.; Guo, Y.; Chanrot, M.; Ivanova, E.; Kelsey, G.; Bongcam-Rudloff, E.; Andersson, G.; Humblot, P. LPS-treatment of bovine endometrial epithelial cells causes differential DNA methylation of genes associated with inflammation and endometrial function. BMC Genom. 2020, 21, 385. [Google Scholar] [CrossRef]
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268. [Google Scholar] [CrossRef] [PubMed]
- De Bem, T.H.C.; Tinning, H.; Vasconcelos, E.J.R.; Wang, D.; Forde, N. Endometrium On-a-Chip Reveals Insulin-and Glucose-induced Alterations in the Transcriptome and Proteomic Secretome. Endocrinology 2021, 162, bqab054. [Google Scholar] [CrossRef] [PubMed]
- Goharitaban, S.; Abedelahi, A.; Hamdi, K.; Khazaei, M.; Esmaeilivand, M.; Niknafs, B. Role of endometrial microRNAs in repeated implantation failure (mini-review). Front. Cell Dev. Biol. 2022, 10, 936173. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, S.; Wang, Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017, 15, 90. [Google Scholar]
- Kusama, K.; Rashid, M.B.; Kowsar, R.; Marey, M.A.; Talukder, A.K.; Nagaoka, K.; Shimada, M.; Khatib, H.; Imakawa, K.; Miyamoto, A. Day 7 Embryos Change the Proteomics and Exosomal Micro-RNAs Content of Bovine Uterine Fluid: Involvement of Innate Immune Functions. Front. Genet. 2021, 12, 676791. [Google Scholar] [CrossRef]
- Niikura, Y.; Kitagawa, K. Functions of SGT1, a Co-chaperone. In Heat Shock Protein 90 in Human Diseases and Disorders; Asea, A., Kaur, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 317–370. [Google Scholar]
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Almughlliq, F.B.; Meier, S.; Burke, C.R.; Roche, J.R.; Reed, C.B.; Arachchige, B.J.; Reed, S.; et al. Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. J. Dairy Sci. 2018, 101, 6462–6473. [Google Scholar] [CrossRef]
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the oviduct in mammalian fertilization. Reproduction 2012, 144, 649–660. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrin. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Locatelli, Y.; Zlotkowska, A.M.; Almiñana, C.; Tsikis, G.; Mermillod, P.; Schoen, J.; et al. Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation. Sci. Rep. 2022, 12, 4135. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2019, 21, 223. [Google Scholar] [CrossRef]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Gerke, V. Annexins–unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117 Pt 13, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.; Salker, M.S.; Umbach, A.T.; Rajaxavier, J.; Okumura, T.; Singh, Y.; Wagner, A.; Brucker, S.Y.; Wallwiener, D.; Brosens, J.J.; et al. Annexin A7 Regulates Endometrial Receptivity. Front. Cell Dev. Biol. 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shao, Y. Annexin A2 acts as an adherent molecule under the regulation of steroids during embryo implantation. Mol. Hum. Reprod. 2020, 26, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, T.M.; Lee, K.F.; Chiu, P.C.; Pang, R.T.; Ng, E.H.; Yeung, W.S. Annexin A2 Acts as an Adhesion Molecule on the Endometrial Epithelium during Implantation in Mice. PLoS ONE 2015, 10, e0139506. [Google Scholar] [CrossRef]
- Pillai, V.V.; Weber, D.M.; Phinney, B.S.; Selvaraj, V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE 2017, 12, e0188105. [Google Scholar] [CrossRef]
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implica-tions for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef]
- Papp, S.M.; Fröhlich, T.; Radefeld, K.; Havlicek, V.; Kösters, M.; Yu, H.; Mayrhofer, C.; Brem, G.; Arnold, G.J.; Besenfelder, U. A novel approach to study the bovine oviductal fluid proteome using transvaginal endoscopy. Theriogenology 2019, 132, 53–61. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, B.; Maillo, V.; Acuña, O.S.; López-Úbeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Avilés, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Car-bohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681. [Google Scholar] [CrossRef]
- Gegenfurtner, K.; Fröhlich, T.; Kösters, M.; Mermillod, P.; Locatelli, Y.; Fritz, S.; Salvetti, P.; Forde, N.; Lonergan, P.; Wolf, E.; et al. Influence of metabolic status and genetic merit for fertility on proteomic composition of bovine oviduct fluid. Biol. Reprod. 2019, 101, 893–905. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of uterine microbiota in postpartum dairy cows with clinical or subclinical endometritis. Sci. Rep. 2020, 10, 12353. [Google Scholar] [CrossRef]
- Choe, C.; Park, J.W.; Kim, E.S.; Lee, S.G.; Park, S.Y.; Lee, J.S.; Cho, M.J.; Kang, K.R.; Han, J.; Kang, D. Proteomic analysis of differentially expressed proteins in bovine endometrium with endome-tritis. Korean J. Physiol. Pharmacol. 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465. [Google Scholar] [CrossRef]
- Almeida, A.M.; Bassols, A.; Bendixen, E.; Bhide, M.; Ceciliani, F.; Cristobal, S.; Eckersall, P.D.; Hollung, K.; Lisacek, F.; Mazzucchelli, G.; et al. Animal board invited review: Advances in proteomics for animal and food sciences. Animal 2015, 9, 1–17. [Google Scholar] [CrossRef]
- Cairoli, F.; Battocchio, M.; Veronesi, M.C.; Brambilla, D.; Conserva, F.; Eberini, I.; Wait, R.; Gianazza, E. Serum protein pattern during cow pregnancy: Acute-phase proteins increase in the peripartum period. Electrophoresis 2006, 27, 1617–1625. [Google Scholar] [CrossRef]
- Brown, W.E.; Garcia, M.; Mamedova, L.K.; Christman, K.R.; Zenobi, M.G.; Staples, C.R.; Leno, B.M.; Overton, T.R.; Whitlock, B.K.; Daniel, J.A.; et al. Acute-phase protein α-1-acid glycoprotein is negatively associated with feed intake in postpartum dairy cows. J. Dairy Sci. 2021, 104, 806–817. [Google Scholar] [CrossRef]
- Tóthová, C.; Nagy, O.; Kovác, G. Changes in the concentrations of selected acute phase proteins and variables of energetic profile in dairy cows after parturition. J. Appl. Anim. Res. 2014, 42, 278–283. [Google Scholar] [CrossRef]
- Fazio, E.; Bionda, A.; Liotta, L.; Amato, A.; Chiofalo, V.; Crepaldi, P.; Satué, K.; Lopreiato, V. Changes of acute-phase proteins, glucose, and lipid metabolism during pregnancy in lactating dairy cows. Arch. Anim. Breed. 2022, 65, 329–339. [Google Scholar] [CrossRef]
- Piras, C.; Guo, Y.; Soggiu, A.; Chanrot, M.; Greco, V.; Urbani, A.; Charpigny, G.; Bonizzi, L.; Roncada, P.; Humblot, P. Changes in protein expression profiles in bovine endometrial epithelial cells exposed to E. coli LPS challenge. Mol. BioSystems 2017, 13, 392–405. [Google Scholar] [CrossRef]
- Zhang, S.D.; Dong, S.W.; Wang, D.S.; Oguejiofor, C.F.; Fouladi-Nashta, A.A.; Yang, Z.Q.; Yan, Z.T. Differential proteomic profiling of endometrium and plasma indicate the importance of hydrolysis in bovine endometritis. J. Dairy Sci. 2017, 100, 9324–9337. [Google Scholar] [CrossRef]
- Ren, Z.; Bai, L.; Shen, L.; Luo, Z.Z.; Zhou, Z.H.; Zuo, Z.C.; Ma, X.P.; Deng, J.L.; Wang, Y.; Xu, S.Y.; et al. Comparative iTRAQ Proteomics Reveals Multiple Effects of Selenium Yeast on Dairy Cows in Parturition. Biol. Trace Element Res. 2020, 197, 464–474. [Google Scholar] [CrossRef]
- Miller, B.A.; Brewer, A.; Nanni, P.; Lim, J.J.; Callanan, J.J.; Grossmann, J.; Kunz, L.; de Almeida, A.M.; Meade, K.G.; Chapwanya, A. Characterization of circulating plasma proteins in dairy cows with cytological endometritis. J. Proteom. 2019, 205, 103421. [Google Scholar] [CrossRef]
- Helfrich, A.L.; Reichenbach, H.D.; Meyerholz, M.M.; Schoon, H.A.; Arnold, G.J.; Fröhlich, T.; Weber, F.; Zerbe, H. Novel sampling procedure to characterize bovine subclinical endometritis by uterine secretions and tissue. Theriogenology 2020, 141, 186–196. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; McDougall, S.; Graham, E.; Burke, C.R.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zou, H.; Dai, Z.; Feng, S.; Zhang, M.; Xiao, G.; Liu, Z.; Cheng, Q. The Basic Characteristics of the Pentraxin Family and Their Functions in Tumor Progression. Front. Immunol. 2020, 11, 1757. [Google Scholar] [CrossRef]
- Bottazzi, B.; Garlanda, C.; Teixeira, M.M. Editorial: The Role of Pentraxins: From Inflammation, Tissue Repair and Immunity to Biomarkers. Front. Immunol. 2019, 10, 2817. [Google Scholar] [CrossRef]
- Johnson, G.A.; Bazer, F.W.; Burghardt, R.C.; Wu, G.; Seo, H.; Kramer, A.C.; McLendon, B.A. Cellular events during ovine implantation and impact for gestation. Anim. Reprod. 2018, 15, 843–855. [Google Scholar] [CrossRef]
- Miao, X.; Luo, Q.; Zhao, H.; Qin, X. Ovarian proteomic study reveals the possible molecular mechanism for hyperprolificacy of Small Tail Han sheep. Sci. Rep. 2016, 6, 27606. [Google Scholar] [CrossRef]
- Tang, J.; Hu, W.; Chen, S.; Di, R.; Liu, Q.; Wang, X.; He, X.; Gan, S.; Zhang, X.; Zhang, J.; et al. The genetic mechanism of high prolificacy in small tail han sheep by comparative proteomics of ovaries in the follicular and luteal stages. J. Proteom. 2019, 204, 103394. [Google Scholar] [CrossRef]
- Soleilhavoup, C.; Riou, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.; Reynaud, K.; de Graaf, S.P.; Gerard, N.; Druart, X. Proteomes of the Female Genital Tract During the Oestrous Cycle. Mol. Cell Proteom. 2016, 15, 93–108. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, W.; Wang, Y.; Miao, K.; Zhao, H.; Wang, R.; Guo, M.; Wang, Z.; Tian, J.; An, L. The proteome of IVF-induced aberrant embryo-maternal crosstalk by implantation stage in ewes. J. Animal Sci. Biotechnol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Ptak, G.E.; D’Agostino, A.; Toschi, P.; Fidanza, A.; Zacchini, F.; Czernik, M.; Monaco, F.; Loi, P. Post-implantation mortality of in vitro produced embryos is associated with DNA me-thyltransferase 1 dysfunction in sheep placenta. Hum. Reprod. 2013, 28, 298–305. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Wang, Y.; Zhao, W.; Wang, W.; Cui, J.; Yang, J.; Yue, Y.; Zhang, S.; Chu, M.; et al. A proteomic atlas of ligand-receptor interactions at the ovine maternal-fetal interface reveals the role of histone lactylation in uterine remodeling. J. Biol. Chem. 2022, 298, 101456. [Google Scholar] [CrossRef]
- O’Neil, E.V.; Burns, G.W.; Ferreira, C.R.; Spencer, T.E. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterus†. Biol. Reprod. 2020, 102, 1020–1032. [Google Scholar] [CrossRef]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular Vesicles Originate from the Conceptus and Uterus During Early Pregnancy in Sheep. Biol. Reprod. 2016, 94, 56. [Google Scholar] [CrossRef]
- Kaczynski, P.; Goryszewska-Szczurek, E.; Baryla, M.; Waclawik, A. Novel insights into conceptus-maternal signaling during preg-nancy establishment in pigs. Mol. Reprod. Dev. 2023, 90, 658–672. [Google Scholar] [CrossRef]
- Bidarimath, M.; Tayade, C. Pregnancy and spontaneous fetal loss: A pig perspective. Mol. Reprod. Dev. 2017, 84, 856–869. [Google Scholar] [CrossRef]
- Roberts, R.M. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor. Rev. 2007, 18, 403–408. [Google Scholar] [CrossRef]
- Degrelle, S.A.; Blomberg Le Ann Garrett, W.M.; Li, R.W.; Talbot, N.C. Comparative proteomic and regulatory network analyses of the elongating pig conceptus. Proteomics 2009, 9, 2678–2694. [Google Scholar] [CrossRef]
- Kolakowska, J.; Franczak, A.; Saini, R.K.R.; Souchelnytskyi, S. Progress and challenges in the proteomics of domestic pig in research on the female reproductive system. J. Element. 2016, 21, 1055–1069. [Google Scholar]
- Georgiou, A.S.; Sostaric, E.; Wong, C.H.; Snijders, A.P.; Wright, P.C.; Moore, H.D.; Fazeli, A. Gametes alter the oviductal secretory proteome. Mol. Cell Proteom. 2005, 4, 1785–1796. [Google Scholar] [CrossRef]
- He, Y.; Zang, X.; Kuang, J.; Yang, H.; Gu, T.; Yang, J.; Li, Z.; Zheng, E.; Xu, Z.; Cai, G.; et al. iTRAQ-based quantitative proteomic analysis of porcine uterine fluid during pre-implantation period of pregnancy. J. Proteom. 2022, 261, 104570. [Google Scholar] [CrossRef]
- Padua, M.B.; Lynch, V.J.; Alvarez, N.V.; Garthwaite, M.A.; Golos, T.G.; Bazer, F.W.; Kalkunte, S.; Sharma, S.; Wagner, G.P.; Hansen, P.J. ACP5 (Uteroferrin): Phylogeny of an ancient and conserved gene expressed in the endometrium of mammals. Biol. Reprod. 2012, 86, 123. [Google Scholar] [CrossRef] [PubMed]
- Ducsay, C.A.; Buhi, W.C.; Bazer, F.W.; Roberts, R.M. Role of uteroferrin in iron transport and macromolecular uptake by allantoic epithelium of the porcine conceptus. Biol. Reprod. 1982, 26, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Meng, F.; Li, B.; Wang, S.; Hu, B.; Li, J. Global proteomic analysis of serum during early pregnancy in the pig using data-independent acquisition mass spectrometry with verification by parallel reaction monitoring. Reprod. Fertil. Dev. 2022, 34, 1115–1127. [Google Scholar] [CrossRef]
- Sell-Kubiak, E. Selection for litter size and litter birthweight in Large White pigs: Maximum, mean and variability of reproduc-tion traits. Animal 2021, 15, 100352. [Google Scholar] [CrossRef] [PubMed]
- Palencia, J.Y.P.; Lemes, M.A.G.; Garbossa, C.A.P.; Abreu, M.L.T.; Pereira, L.J.; Zangeronimo, M.G. Arginine for gestating sows and foetal development: A systematic review. J. Anim. Physiol. Anim. Nutr. 2018, 102, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Lim, H.; Das, S.K.; Reese, J.; Dey, S.K. Molecular signaling in uterine receptivity for implantation. Semin Cell Dev Biol. 2000, 11, 67–76. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Dowland, S.N.; Murphy, C.R. Uterine focal adhesions are retained at implantation after rat ovarian hyperstimu-lation. Reproduction 2016, 152, 753–763. [Google Scholar] [CrossRef]
- Kaneko, Y.; Lecce, L.; Day, M.L.; Murphy, C.R. Focal Adhesion Kinase Localizes to Sites of Cell-to-Cell Contact In Vivo and Increases Apically in Rat Uterine Luminal Epithelium and the Blastocyst at the Time of Implantation. J. Morphol. 2012, 273, 639–650. [Google Scholar] [CrossRef]


| Animal Species | Biological Sources | Gestation Days | Proteomic Approach | Key Findings | References |
|---|---|---|---|---|---|
| Bovine | Uterine fluids | Days 16 and 18 | 2-D gel | 9 protein spots were identified in pregnant | [27] |
| electrophoresis | compared to non-pregnant | ||||
| Days 5 and 9 | 2-D gel | 10 proteins were differently abundant | [28] | ||
| electrophoresis | between Days 5 and 9 | ||||
| Days 7 and 13 | Label-free | 5 proteins were more abundant on Day 7 | [29] | ||
| LC−MS/MS | compared with Day 13, and 29 proteins were | ||||
| more abundant on Day 13 compared with | |||||
| Day 7 | |||||
| Day 7 of the | iTRAQ proteomics | 35 proteins were up-regulated and 18 were | [30] | ||
| oestrous cycle | down-regulated | ||||
| Days 7 and 15 | iTRAQ proteomics | 20 proteins were up-regulated and 20 were | [31] | ||
| down-regulated | |||||
| Days 10, 13, 16 | iTRAQ proteomics | 1652 peptides were identified on day 16 | [32] | ||
| and 19 | |||||
| Day 7 | LC–MS/MS | 1563 proteins were detected, of which 472 had not | [33] | ||
| been previously reported | |||||
| Day 7 | iTRAQ proteomics | 336 proteins were identified, of which 260 | [34] | ||
| were more than 2-fold higher in AI | |||||
| cows than Ctrl cows | |||||
| Endometrial | Day 27 after AI | 2D-gel | 93 protein spots were differentially expressed | [35] | |
| tissue | electrophoresis | between normal caruncles (NC) and retarded | |||
| and MALDI- | caruncles | ||||
| ToF/ToF | |||||
| Day 18 | 2-D DIGE | 4 proteins with significantly higher | [36] | ||
| abundances in each sample derived from the | |||||
| pregnant animals | |||||
| Sheep | Endometrial | Day 17 | LC-MS/MS | 170 differentially expressed proteins (DEPs) | [37] |
| tissue | were identified. 60 proteins were up- | ||||
| regulated in caruncular areas, and 110 proteins were up-regulated in intercaruncular areas. | |||||
| Days 12, 16, and 20 | 2DE gel electrophoresis | 11 proteins in the caruncular endometrium and six proteins in the intercaruncular endometrium were identified | [38] | ||
| Day 17 | LC-MS/MS | 94 and 257 differentially expressed proteins (DEPs) were identified in the endometrial caruncular and intercaruncular areas, respectively | [39] | ||
| Days 16 and 20 | Two-dimensional gel electrophoresis and mass spectrometry | 57 protein spots were up-regulated in the gravid horn at day 16, and 27 protein spots were up-regulated in the gravid horn at day 20 | [40] | ||
| Uterine fluids | Day 16 | LC-MS/MS | 100 of the most abundant uterine luminal proteins were identified, of which 15 were significantly altered in early pregnancy | [41] | |
| Day 10, 12, 14, 16, and 20 | LC-MS/MS | more than 1400 proteins were detected | [42] | ||
| Day 14–16 | LC-MS/MS | 783 proteins were present by Days 14–16 | [43] | ||
| Pig | Endometrial tissue | Days 40, 70, and 93 | 2-DE gel electrophoresis | 63 of the 98 proteins are regulated differentially among non-pregnant and pregnant tissues | [44] |
| Day 9 to Day 12 | 2D-DIGE | From a total of 1280 matched spots, 85 spots significantly changed in abundance with the progression of pregnancy from Days 9 to 12 | [45] | ||
| Days 11 to 12 | 2-DE gel electrophoresis | Forty-four differentially abundant proteins in the pregnant endometrium were identified by mass spectrometry | [46] | ||
| 9 days (9D), 12 days (12D), and 16 days | 2DE-MALDI-TOF/TOF | A total of sixteen differentially expressed proteins (DEPs) were identified | [47] | ||
| Mid-gestation day 49, day 72 | iTRAQ | A total of 2170 proteins were identified, and 114 differentially expressed proteins (DEPs) were identified in Meishan and Duroc sows, respectively | [48] |
| Pregnant vs. Non Pregnant; Days of Pregnancy (Days 12, 16, 18) | References | ||
| Bovine | Endometrial Tissue | Luminal Fluid | |
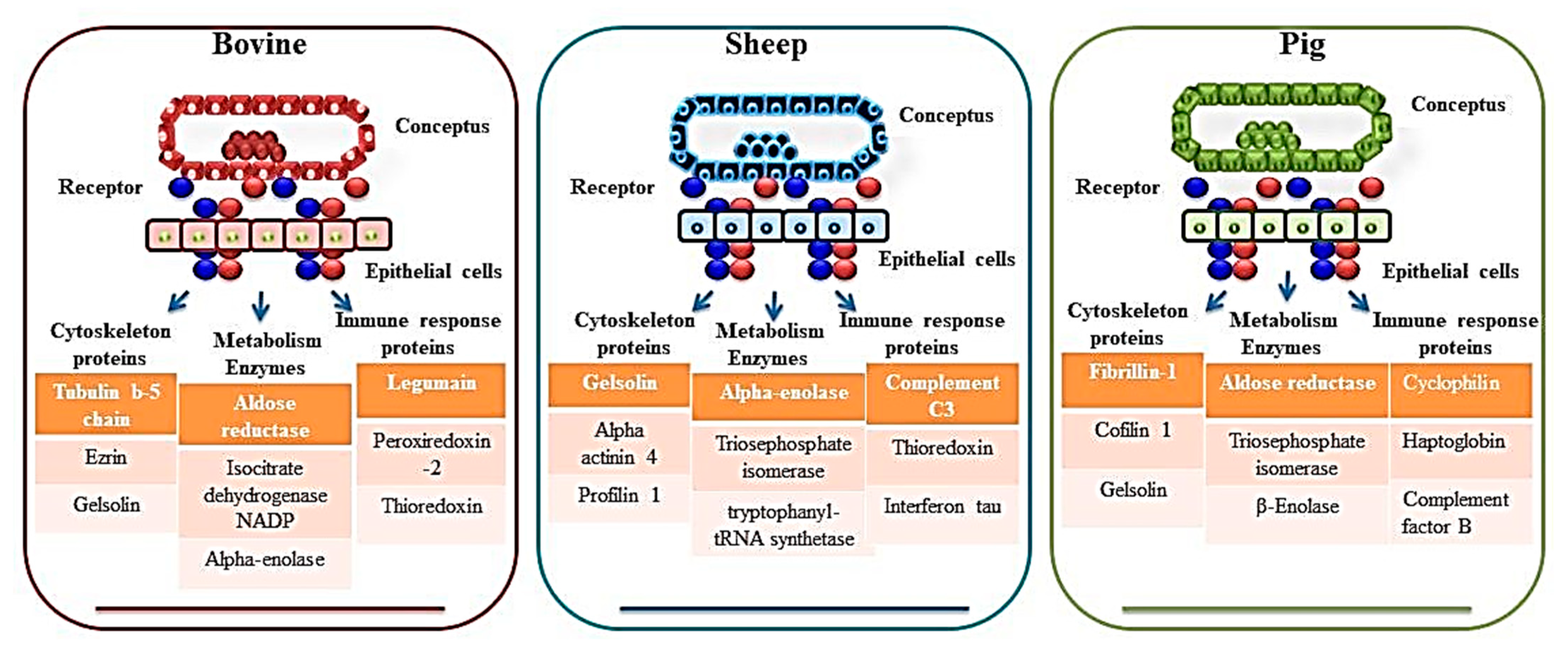
| Rho GDP dissociation inhibitor beta, 20 alpha-hydroxysteroid dehydrogenase (20 alpha-HSD), NADP1-dependent isocitrate dehydrogenase 1, acyl-CoA-binding protein, HSP 90-alpha, calreticulin, annexin A1, annexin A2, fibrinogen alphachain, alpha-1-antiproteinase, serpin H1, serpin A3-8), nuclear ribonucleoproteins A2/B1 and K, serine/threonineprotein phosphatase 2A, ezrin, amine oxidase-A, haptoglobin, albumin, serotransferrin precursor (TF), ovalbumin, isocitrate dehydrogenase, cytoplasmic (IDHA), purine nucleoside phosphorylase (PNP), cystatin-M precursor (CST6), retinol-binding protein 4, aldose reductase (ALDR), cathepsin D (CATD), heat-shock cognate 71 kDa protein (HSP7C), actin, cytoplasmic 1 (ACTB), prepro complement component C3 (CO3), cathepsin B precursor (CATB), transitional endoplasmic reticulum ATPase isoform 3 (Canis lupus familiaris), heat-shock protein, HSP 90-alpha, fructose-bisphosphate aldolase A (ALDOA), guanine deaminase (GDA), rab GDP dissociation inhibitor beta (GD1B), legumain precursor, metalloproteinase inhibitor 2 precursor (TIMP2), CAP1 protein (CAP1), gelsolin isoform b (GELS), serpin A3-1 precursor (SERPINA31) | Carbonic anhydrase, ezrin, heat shock protein 70, isocitrate dehydrogenase, nucleoside diphosphate kinase, peroxiredoxin 1, purine nucleoside phosphorylase, thioredoxin, triosephosphate isomerise, cystatin, legumain, retinol-binding protein, and tissue inhibitor of matrix metalloproteinase 2. | [32,35,36] | |
| Sheep | Gelsolin isoform b (GSN), Transferrin (TF), Adenosylhomocysteinase (AHCY), Carbonic anhydrase 2 (CA-II), Heat shock 60 kDa protein 1 (HSP60), Apolipoprotein A-1 (APOA1), Galectin 15 (LGALS15/OVGAL11), Ceruloplasmin, Cystatin E/M, Complement C3-like, Cystatin C, Cathepsin L1 isoform 1, Insulin-like growth factor-binding protein-1, Retinol-binding protein 4, Extracellular superoxide dismutase 3, Phosphoglycerate kinase 1, Heat Shock 70kDa Protein 8, Alpha-enolase isoform 1 | Transgelin, placental proteins like PP9, component 4 (CC4), immunoglobulin, heavy constant mu (IGHM), adenosylhomocysteinase, glucose 6 phosphate isomerase (GPI), apolipoprotein AI (APO-AI), Ceruloplasmin, A1B glycoprotein (A1BG), actinin 4 (ACTN4), BCL2-like 15 (BCL2-L15), carbonic anhydrase II (CA II), and A2 macroglobulin | [41,42] |
| Pig | Transferrin, protein DJ-1, transgelin, galectin-1, septin 2, stathmin 1, actin smooth muscle gamma-actin, cofilin 1, fascin 1, heat shock protein (HSP) 90b, HSP 27, serpins, cofilin, annexin A2 (ANXA2), aldose reductase, Annexin A2, cyclophilin, protein disulphide isomerase A3, peroxiredoxin1, haptoglobin, transthyretin, ceruloplasmin Apolipoprotein A-1 (APOA1), F actin capping protein subunit beta (CAPZB), L-Lactate dehydrogenase B chain (LDHB), annexins (ANXA4 and 5), Complement factor B (CFB), B Actin (ACTB), SMS, Actin related protein 3 (ACTR3), Beta enolase (ENO3), Ornithine oxo-acid aminotransferase (OAT), Transthyretin (TTR) | Cathepsin C and B (CTSC, and CTSB), Acid Phosphatase 5 (ACP5) | [44,45,47] |
| Species | Protein Name | Function | Change in Expression (References) |
|---|---|---|---|
| Bovine | Ezrin | Involved in cytoskeletal rearrangements that facilitate uterine receptivity and embryo-endometrium attachment. | Up (Uterine fluids vs. plasma) [30] Up (Pregnant vs. non-pregnant) [27] Down (Pregnant vs. open) [75] |
| Interferon tau | Interferon tau is the signal for maternal recognition of pregnancy in ruminants. | Up (Pregnant vs. open) [75] | |
| S100-A4 | Expressed in the endometrium and play a role in embryo adhesion. | Up (Pregnant with viable embryo vs. Pregnant with degenerate embryo) [62] Up (Day 7 vs. Day 13post estrus) [29] | |
| Peroxiredoxin-2 | PRDX2 is an antioxidant protein that helps in trophoblast proliferation and migration. | Up (Pregnant with viable embryo vs. Pregnant with degenerate embryo) [62] Up (Uterine fluids vs. plasma) [30] Down (Day 15 vs. Day 7) [31] | |
| Metalloproteinase inhibitor 2 precursor | TIMP-2 is a proteinase inhibitor and functions as a regulator of extracellular matrix integrity by controlling the activity of matrix metalloproteinases. | Up (Day 15 vs. Day 7) [31] Up (Day 16 vs. Days 10,13,19) [32] Down (Pregnant vs. non-pregnant) [27] Up (Day 14 vs. Days 9, 5) [59] Up (Day 7 vs. Day 13post estrus) [29] | |
| Legumain | LGMN is a lysosomal cysteine protease that plays an important role in implantation and placentation. | Up (Day 15 vs. Day 7) [31] Up (Day 16 vs. Days 10,13,19) [32] Down (Pregnant vs. non-pregnant) [27] Up (Day 14 vs. Days 9, 5) [59] Up (Day 7 vs. Day 13 post estrus) [29] | |
| Annexin A1 and A2 | Annexins are phospholipid binding proteins. Annexin A1 and A2 help in the maintenance of placentation. | Down (normal chorioamnions vs. retarded chorioamnions) [35] Up (Uterine fluids vs. plasma) [30] Down (Day 15 vs. Day 7) [31] Up (Day 9 vs. Days 5) [59] | |
| Sheep | Alpha actinin 4 | ACTN4 may act as an important regulator of trophoblast proliferation and differentiation during early pregnancy. | Up (Pregnant vs. Non-pregnant) [41] |
| Transgelin | It helps in the formation of podosomes and is involved in tissue invasion and matrix remodeling. | Up (Pregnant vs. Non-pregnant) [41] Up (C vs. IC) [37] | |
| Integrin, alpha 1 | It helps in the attachment of the embryo to endometrial cells. | Up (C vs. IC) [37] Up (IC vs. C areas in the pregnant) [39] | |
| Annexin | ANXA4 promotes trophoblast cell proliferation and invasion. | Up (implantation (P16) vs. pre-attachment (P12) [38] | |
| Signal transducer and activator of transcription 1-alpha/beta | Depletion or absence of STAT1 abolishes cell proliferation and invasion of trophoblast cells. | Up (IC of pregnant vs. IC non-pregnant) [39] | |
| Gelsolin | GSN is involved in the regulation of actin polymerization during early pregnancy. | Down (implantation (P16) vs. oestrous cycle (C16) [38] Up (Non Gravid horn vs. Gravid Horn at day 20) [40] | |
| Profilin 1 | PFN1 is expressed both in the embryo and in the endometrial epithelium and regulates the cytoskeletal organization. | Days 14,16 of pregnancy [43] | |
| Pig | Transgelin, | It helps in the formation of podosomes and is involved in tissue invasion and matrix remodeling. | Up (Non-pregnant vs. pregnant) [44] |
| Aldose reductase | ALDR plays an important role in the biosynthesis of prostaglandins and takes part in pregnancy recognition and conceptus development. | Up (day 12 vs. day 9 of pregnant) [45] Up (Pregnant vs. non-pregnant) [46] | |
| Annexin A2 | Annexin A2 helps in the maintenance of placentation. | Up (Pregnant vs. non-pregnant) [46] | |
| Cyclophilin | CYPA helps in transforming the uterus from a non-receptive state into a receptive state to receive blastocyst. | Up (Pregnant vs. non-pregnant) [46] | |
| Haptoglobin | HP might contribute to endometrial receptivity for conceptus attachment. | Up (Pregnant vs. non-pregnant) [46] Up (day 12 vs. day 9 of pregnant) [45] Up (9D vs. 16D of pregnancy) [47] | |
| Annexin A4 | ANXA4 promotes trophoblast cell proliferation and invasion. | Up (day 12 vs. day 9 of pregnant) [45] Up (9D vs. 16D of pregnancy) [47] | |
| Fibrillin-1 | These proteins are components of the extracellular matrix and are significantly increased in the endometrium at the time of implantation, suggesting a role in blastocyst attachment. | Up (Day 72 vs. Day 49) [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamwal, S.; Jena, M.K.; Tyagi, N.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Kumar, S.; Mohanty, A.K. Proteomic Approaches to Unravel the Molecular Dynamics of Early Pregnancy in Farm Animals: An In-Depth Review. J. Dev. Biol. 2024, 12, 2. https://doi.org/10.3390/jdb12010002
Jamwal S, Jena MK, Tyagi N, Kancharla S, Kolli P, Mandadapu G, Kumar S, Mohanty AK. Proteomic Approaches to Unravel the Molecular Dynamics of Early Pregnancy in Farm Animals: An In-Depth Review. Journal of Developmental Biology. 2024; 12(1):2. https://doi.org/10.3390/jdb12010002
Chicago/Turabian StyleJamwal, Shradha, Manoj Kumar Jena, Nikunj Tyagi, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu, Sudarshan Kumar, and Ashok Kumar Mohanty. 2024. "Proteomic Approaches to Unravel the Molecular Dynamics of Early Pregnancy in Farm Animals: An In-Depth Review" Journal of Developmental Biology 12, no. 1: 2. https://doi.org/10.3390/jdb12010002
APA StyleJamwal, S., Jena, M. K., Tyagi, N., Kancharla, S., Kolli, P., Mandadapu, G., Kumar, S., & Mohanty, A. K. (2024). Proteomic Approaches to Unravel the Molecular Dynamics of Early Pregnancy in Farm Animals: An In-Depth Review. Journal of Developmental Biology, 12(1), 2. https://doi.org/10.3390/jdb12010002








