Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine
Abstract
:1. Introduction
2. Synthesis of mRNA
3. Key Technologies for mRNA Therapeutics
4. mRNA-Based Protein Supplementation for Regenerative Medicine
5. mRNA for Cell Reprogramming
6. mRNA-Induced Cell Differentiation from iPSCs
7. mRNA for Cell Reprogramming and Differentiation Induction and Direct Reprogramming without Passage through Pluripotent Stem Cells
8. mRNA-Based Purification Methods of iPSCs and iPSC-Derived Cells
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Q.; Wu, M.; Zhou, C.; Lu, X.; Huang, B.; Zhang, N.; Zhao, H.; Chi, H.; Zhang, X.; Ling, D.; et al. Rational development of a combined mRNA vaccine against COVID-19 and influenza. NPJ Vaccines 2022, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ladak, R.J.; He, A.J.; Huang, Y.H.; Ding, Y. The Current Landscape of mRNA Vaccines Against Viruses and Cancer-A Mini Review. Front. Immunol. 2022, 13, 885371. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.; Haque, A.; Weidensee, B.; Weinmann, P.; Kormann, M.S.D. Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases. Mol. Ther. 2019, 27, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.J.; Huang, Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef] [PubMed]
- Muttach, F.; Muthmann, N.; Rentmeister, A. Synthetic mRNA capping. Beilstein J. Org. Chem. 2017, 13, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, Y.; Morgan, M.; Muthukrishnan, S.; Shatkin, A.J. Reovirus messenger RNA contains a methylated, blocked 5′-terminal structure: M-7G(5′)ppp(5′)G-MpCp. Proc. Natl. Acad. Sci. USA 1975, 72, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, Y.; Miura, K. A blocked structure at the 5′ terminus of mRNA from cytoplasmic polyhedrosis virus. Nature 1975, 253, 374–375. [Google Scholar] [CrossRef]
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, J.K. Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol. Cell. 2009, 101, 251–262. [Google Scholar] [CrossRef]
- Schuster, S.L.; Hsieh, A.C. The Untranslated Regions of mRNAs in Cancer. Trends Cancer 2019, 5, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Nobuta, R.; Machida, K.; Sato, M.; Hashimoto, S.; Toriumi, Y.; Nakajima, S.; Suto, D.; Imataka, H.; Inada, T. eIF4G-driven translation initiation of downstream ORFs in mammalian cells. Nucleic Acids Res. 2020, 48, 10441–10455. [Google Scholar] [CrossRef] [PubMed]
- Passmore, L.A.; Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 2022, 23, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Meet the authors: Katalin Kariko and Drew Weissman. Immunity 2021, 54, 2673–2675. [CrossRef] [PubMed]
- Nance, K.D.; Meier, J.L. Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent. Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.J.; Hurwitz, J. RNA capping by the vaccinia virus guanylyltransferase. Structure of enzyme-guanylate intermediate. J. Biol. Chem. 1984, 259, 13488–13494. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Akamine, S.; Mochizuki, M.; Hayashi, K.; Akichika, S.; Suzuki, T.; Saito, H. Versatile strategy using vaccinia virus-capping enzyme to synthesize functional 5′ cap-modified mRNAs. Nucleic Acids Res. 2023, 51, e34. [Google Scholar] [CrossRef] [PubMed]
- Grudzien-Nogalska, E.; Stepinski, J.; Jemielity, J.; Zuberek, J.; Stolarski, R.; Rhoads, R.E.; Darzynkiewicz, E. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007, 431, 203–227. [Google Scholar]
- Henderson, J.M.; Ujita, A.; Hill, E.; Yousif-Rosales, S.; Smith, C.; Ko, N.; McReynolds, T.; Cabral, C.R.; Escamilla-Powers, J.R.; Houston, M.E. Cap 1 Messenger RNA Synthesis with Co-transcriptional CleanCap((R)) Analog by In Vitro Transcription. Curr. Protoc. 2021, 1, e39. [Google Scholar] [CrossRef]
- Inagaki, M.; Abe, N.; Li, Z.; Nakashima, Y.; Acharyya, S.; Ogawa, K.; Kawaguchi, D.; Hiraoka, H.; Banno, A.; Meng, Z.; et al. Cap analogs with a hydrophobic photocleavable tag enable facile purification of fully capped mRNA with various cap structures. Nat. Commun. 2023, 14, 2657. [Google Scholar] [CrossRef]
- Melton, D.A.; Krieg, P.A.; Rebagliati, M.R.; Maniatis, T.; Zinn, K.; Green, M.R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984, 12, 7035–7056. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.F.; Lin, C.; Sung, H.C. A review of technological developments in lipid nanoparticle application for mRNA vaccination. Hum. Vaccin. Immunother. 2023, 19, 2256040. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.L.; Cullis, P.R. Modulation of membrane fusion by asymmetric transbilayer distributions of amino lipids. Biochemistry 1994, 33, 12573–12580. [Google Scholar] [CrossRef]
- Egli, M.; Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023, 51, 2529–2573. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stoter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Xu, X.; Xia, T. Recent Advances in Site-Specific Lipid Nanoparticles for mRNA Delivery. ACS Nanosci. Au 2023, 3, 192–203. [Google Scholar] [CrossRef]
- Kariko, K.; Kuo, A.; Barnathan, E.S.; Langer, D.J. Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochim. Biophys. Acta 1998, 1369, 320–334. [Google Scholar] [CrossRef]
- Kariko, K.; Kuo, A.; Barnathan, E. Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene. Ther. 1999, 6, 1092–1100. [Google Scholar] [CrossRef]
- Kariko, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef]
- Nelson, J.; Sorensen, E.W.; Mintri, S.; Rabideau, A.E.; Zheng, W.; Besin, G.; Khatwani, N.; Su, S.V.; Miracco, E.J.; Issa, W.J.; et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 2020, 6, eaaz6893. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Mir, F.F.; Jhunjhunwala, S.; Kaczmarek, J.C.; Hurtado, J.E.; Yang, J.H.; Webber, M.J.; Kowalski, P.S.; Heartlein, M.W.; DeRosa, F.; et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 2016, 109, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Liovic, M. Industry updates from the field of stem cell research and regenerative medicine in August 2023. Regen. Med. 2023; ahead of print. [Google Scholar]
- Qin, Y.; Ge, G.; Yang, P.; Wang, L.; Qiao, Y.; Pan, G.; Yang, H.; Bai, J.; Cui, W.; Geng, D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv. Sci. 2023, 10, e2207334. [Google Scholar] [CrossRef] [PubMed]
- Rizzino, A. A challenge for regenerative medicine: Proper genetic programming, not cellular mimicry. Dev. Dyn. 2007, 236, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Trommelmans, L. The challenge of regenerative medicine. Hastings Cent. Rep. 2010, 40, 24–26. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Okano, H.; Yamanaka, S. iPS cell technologies: Significance and applications to CNS regeneration and disease. Mol. Brain 2014, 7, 22. [Google Scholar] [CrossRef]
- Akiba, R.; Takahashi, M.; Baba, T.; Mandai, M. Progress of iPS cell-based transplantation therapy for retinal diseases. Jpn. J. Ophthalmol. 2023, 67, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wisitrasameewong, W.; Champaiboon, C.; Surisaeng, T.; Sa-Ard-Iam, N.; Freire, M.; Pardi, N.; Pichyangkul, S.; Mahanonda, R. The Impact of mRNA Technology in Regenerative Therapy: Lessons for Oral Tissue Regeneration. J. Dent. Res. 2022, 101, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Collen, A.; Bergenhem, N.; Carlsson, L.; Chien, K.R.; Hoge, S.; Gan, L.M.; Fritsche-Danielson, R. VEGFA mRNA for regenerative treatment of heart failure. Nat. Rev. Drug. Discov. 2022, 21, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Anttila, V.; Saraste, A.; Knuuti, J.; Hedman, M.; Jaakkola, P.; Laugwitz, K.L.; Krane, M.; Jeppsson, A.; Sillanmaki, S.; Rosenmeier, J.; et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol. Ther. 2023, 31, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Panos, J.A.; Coenen, M.J.; Nagelli, C.V.; McGlinch, E.B.; Atasoy-Zeybek, A.; De Padilla, C.L.; Coghlan, R.F.; Johnstone, B.; Ferreira, E.; Porter, R.M.; et al. IL-1Ra gene transfer potentiates BMP2-mediated bone healing by redirecting osteogenesis toward endochondral ossification. Mol. Ther. 2023, 31, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fukushima, Y.; Nozaki, K.; Nakanishi, H.; Deng, J.; Wakabayashi, N.; Itaka, K. Enhancement of bone regeneration by coadministration of angiogenic and osteogenic factors using messenger RNA. Inflamm. Regen. 2023, 43, 32. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Uchida, S.; Imai, H.; Nakatomi, H.; Kataoka, K.; Saito, N.; Itaka, K. Treatment of ischemic neuronal death by introducing brain-derived neurotrophic factor mRNA using polyplex nanomicelle. Biomaterials 2021, 270, 120681. [Google Scholar] [CrossRef]
- Lin, C.Y.; Crowley, S.T.; Uchida, S.; Komaki, Y.; Kataoka, K.; Itaka, K. Treatment of Intervertebral Disk Disease by the Administration of mRNA Encoding a Cartilage-Anabolic Transcription Factor. Mol. Ther. Nucleic Acids 2019, 16, 162–171. [Google Scholar] [CrossRef]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Zak, M.M.; Chepurko, E.; Sultana, N.; Sharkar, M.T.K.; Guo, N.; Komargodski, R.; Kurian, A.A.; et al. Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation 2020, 141, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Lui, K.O.; von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Spater, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S. Cardiovascular gene therapy with vascular endothelial growth factors. Gene 2013, 525, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Anttila, V.; Saraste, A.; Knuuti, J.; Jaakkola, P.; Hedman, M.; Svedlund, S.; Lagerstrom-Fermer, M.; Kjaer, M.; Jeppsson, A.; Gan, L.M. Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol. Ther. Methods Clin. Dev. 2020, 18, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.M.; Lagerstrom-Fermer, M.; Carlsson, L.G.; Arfvidsson, C.; Egnell, A.C.; Rudvik, A.; Kjaer, M.; Collen, A.; Thompson, J.D.; Joyal, J.; et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Poenisch, M.; Khanal, R.; Hu, Q.; Dai, Z.; Li, R.; Song, G.; Yuan, Q.; Yao, Q.; Shen, X.; et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 2021, 75, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Everton, E.; Smith, A.R.; Liu, H.; Osota, E.; Beattie, M.; Tam, Y.; Pardi, N.; Weissman, D.; Gouon-Evans, V. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat. Commun. 2021, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bonaguidi, M.; Muro, K.; Kessler, J.A. Generation of embryonic stem cells: Limitations of and alternatives to inner cell mass harvest. Neurosurg. Focus 2008, 24, E4. [Google Scholar] [CrossRef]
- Fung, R.K.; Kerridge, I.H. Uncertain translation, uncertain benefit and uncertain risk: Ethical challenges facing first-in-human trials of induced pluripotent stem (ips) cells. Bioethics 2013, 27, 89–96. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Osafune, K. Current status and future directions of clinical applications using iPS cells-focus on Japan. FEBS J. 2022, 289, 7274–7291. [Google Scholar] [CrossRef]
- Shao, L.; Wu, W.S. Gene-delivery systems for iPS cell generation. Expert. Opin. Biol. Ther. 2010, 10, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Hong, H.; Takahashi, K.; Yamanaka, S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat. Protoc. 2010, 5, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Saintcome, C.; Acker, G.R.; Strand, F.L. Development and Regeneration of Motor Systems under the Influence of Acth Peptides. Psychoneuroendocrino 1985, 10, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, L.; Hu, Y.; Lian, Z.M.; Hua, J.L. ALK Family Inhibitor A83-01 Promotes the Proliferation of Mouse Male Germline Stem Cells (mGSCs) Under Serum- and Feeder-Free Conditions. J. Integr. Agr. 2013, 12, 1839–1846. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, J.; Choi, D. Small-molecule-mediated reprogramming: A silver lining for regenerative medicine. Exp. Mol. Med. 2020, 52, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, Y.J.; Jung, H. Protein Kinases and Their Inhibitors in Pluripotent Stem Cell Fate Regulation. Stem. Cells Int. 2019, 2019, 1569740. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Huo, J.R.; Liang, A.; Chen, J.Z.; Chen, G.W.; Liu, D.Z. Gamma-Secretase Inhibitor (DAPT), a potential therapeutic target drug, caused neurotoxicity in planarian regeneration by inhibiting Notch signaling pathway. Sci. Total. Environ. 2021, 781, 146735. [Google Scholar] [CrossRef]
- Minato, Y.; Nakano-Doi, A.; Maeda, S.; Nakagomi, T.; Yagi, H. A Bone Morphogenetic Protein Signaling Inhibitor, LDN193189, Converts Ischemia-Induced Multipotent Stem Cells into Neural Stem/Progenitor Cell-Like Cells. Stem Cells Dev. 2022, 31, 756–765. [Google Scholar] [CrossRef]
- Suo, N.; Guo, Y.E.; He, B.Q.; Gu, H.F.; Xie, X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019, 67, 1320–1332. [Google Scholar] [CrossRef]
- Petkov, S.; Hyttel, P.; Niemann, H. The Small Molecule Inhibitors PD0325091 and CHIR99021 Reduce Expression of Pluripotency-Related Genes in Putative Porcine Induced Pluripotent Stem Cells. Cell. Reprogramming 2014, 16, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Gerbeth, L.; Gerling, M.; Rosenthal, R.; Steiger, K.; Weidinger, C.; Keye, J.; Wu, H.; Schmidt, F.; Weicheret, W.; et al. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFβ1 signalling in inflammation. Mucosal. Immunol. 2019, 12, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Titan, A.L.; Davitt, M.; Foster, D.; Salhotra, A.; Menon, S.; Chen, K.; Fahy, E.; Lopez, M.; Jones, R.E.; Baiu, I.; et al. Partial Tendon Injury at the Tendon-to-Bone Enthesis Activates Skeletal Stem Cells. Stem Cells Transl. Med. 2022, 11, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Fukui, L.; Henry, J.J. FGF Signaling Is Required for Lens Regeneration in Xenopus laevis. Biol. Bull. 2011, 221, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Shoae-Hassani, A.; Verdi, J. Reprogramming of endometrial adult stromal cells in the presence of a ROCK inhibitor, thiazovivin, could obtain more efficient iPSCs. Cell. Biol. Int. 2015, 39, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W.H. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug. Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Solanki, A.; Sasmal, P.K.; Lee, K.B. Single Vehicular Delivery of siRNA and Small Molecules to Control Stem Cell Differentiation. J. Am. Chem. Soc. 2013, 135, 15682–15685. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Z.; Dodge, M.E.; Tang, W.; Lu, J.M.; Ma, Z.Q.; Fan, C.W.; Wei, S.G.; Hao, W.N.; Kilgore, J.; Williams, N.S.; et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef]
- Lin, S.L.; Chang, D.C.; Chang-Lin, S.; Lin, C.H.; Wu, D.T.S.; Chen, D.T.; Ying, S.Y. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 2008, 14, 2115–2124. [Google Scholar] [CrossRef]
- Subramanyam, D.; Lamouille, S.; Judson, R.L.; Liu, J.Y.; Bucay, N.; Derynck, R.; Blelloch, R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 443–448. [Google Scholar] [CrossRef]
- Ishida, T.; Ueyama, T.; Ihara, D.; Harada, Y.; Nakagawa, S.; Saito, K.; Nakao, S.; Kawamura, T. c-Myc/microRNA-17-92 Axis Phase-Dependently Regulates PTEN and p21 Expression via ceRNA during Reprogramming to Mouse Pluripotent Stem Cells. Biomedicines 2023, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- He, X.P.; Cao, Y.; Wang, L.H.; Han, Y.L.; Zhong, X.Y.; Zhou, G.X.; Cai, Y.P.; Zhang, H.F.; Gao, P. Human Fibroblast Reprogramming to Pluripotent Stem Cells Regulated by the miR19a/b-PTEN Axis. PLoS ONE 2014, 9, e95213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.N.N.; Choo, K.B.; Huang, C.J.; Sugii, S.; Cheong, S.K.; Kamarul, T. miR-524-5p of the primate-specific C19MC miRNA cluster targets TP53IPN1-and EMT-associated genes to regulate cellular reprogramming. Stem. Cell Res. Ther. 2017, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Langroudi, L.; Jamshidi-Adegani, F.; Shafiee, A.; Rad, S.M.A.H.; Keramati, F.; Azadmanesh, K.; Arefian, E.; Soleimani, M. MiR-371-373 cluster acts as a tumor-suppressor-miR and promotes cell cycle arrest in unrestricted somatic stem cells. Tumor Biol. 2015, 36, 7765–7774. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Mantel, C.; Lee, S.A.; Moon, S.H.; Broxmeyer, H.E. MiR-31/SDHA Axis Regulates Reprogramming Efficiency through Mitochondrial Metabolism. Stem Cell Rep. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Milhavet, O.; Lemaitre, J.M. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics 2022, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Winger, Q.A.; Hill, J.R.; Shin, T.Y.; Watson, A.J.; Kraemer, D.C.; Westhusin, M.E. Genetic reprogramming of lactate dehydrogenase, citrate synthase, and phosphofructokinase mRNA in bovine nuclear transfer embryos produced using bovine fibroblast cell nuclei. Mol. Reprod. Dev. 2000, 56, 458–464. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Yakubov, E.; Rechavi, G.; Rozenblatt, S.; Givol, D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010, 394, 189–193. [Google Scholar] [CrossRef]
- Krug, C.; Birkholz, K.; Hombach, A.; Reuter, S.; Kershaw, M.; Kämpgen, E.; Schwenkert, M.; Fey, G.; Schule, G.; Abken, H.; et al. Reprogramming T cells with cancer specificity by non-viral transfer of mRNA encoding recombinant immunoreceptors. Hum. Gene Ther. 2009, 20, 1531. [Google Scholar]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, P.M.; Weissman, S.M. MRNA Mediated T Cell Reprogramming for Adoptive Immunotherapy. J. Immunother. 2012, 35, 727. [Google Scholar]
- Pandit, V.; Nesbitt, R.S.; Macione, J.; Kotha, S.P. Reprogramming of cells using modified mRNA. In Proceedings of the IEEE 37th Annual Northeast Bioengineering Conference (NEBEC), Troy, NY, USA, 1–3 April 2011. [Google Scholar]
- Mehta, A.; Verma, V.; Nandihalli, M.; Ramachandra, C.J.A.; Sequiera, G.L.; Sudibyo, Y.; Chung, Y.Y.; Sun, W.; Shim, W. A Systemic Evaluation of Cardiac Differentiation from mRNA Reprogrammed Human Induced Pluripotent Stem Cells. PLoS ONE 2014, 9, e103485. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ohlemacher, S.K.; Langer, K.B.; Meyer, J.S. Robust Differentiation of mRNA-Reprogrammed Human Induced Pluripotent Stem Cells Toward a Retinal Lineage. Stem Cells Transl. Med. 2016, 5, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yu, P.Z.; Lingampalli, N.; Kim, Y.J.; Tang, R.; Murthy, N. Peptide-enhanced mRNA transfection in cultured mouse cardiac fibroblasts and direct reprogramming towards cardiomyocyte-like cells. Int. J. Nanomed. 2015, 10, 1841–1854. [Google Scholar]
- Guo, X.R.; Wang, X.L.; Li, M.C.; Yuan, Y.H.; Chen, Y.; Zou, D.D.; Bian, L.J.; Li, D.S. PDX-1 mRNA-induced reprogramming of mouse pancreas-derived mesenchymal stem cells into insulin-producing cells in vitro. Clin. Exp. Med. 2015, 15, 501–509. [Google Scholar] [CrossRef]
- Carlsten, M.; Li, L.H.; Su, S.; Berg, M.; Reger, R.; Peshwa, M.V.; Childs, R. Clinical-Grade mRNA Electroporation of NK Cells: A Novel and Highly Efficient Method to Genetically Reprogram Human NK Cells for Cancer Immunotherapy. Blood 2014, 124, 2153. [Google Scholar] [CrossRef]
- Gaignerie, A.; Lefort, N.; Rousselle, M.; Forest-Choquet, V.; Flippe, L.; Francois-Campion, V.; Girardeau, A.; Caillaud, A.; Chariau, C.; Francheteau, Q.; et al. Urine-derived cells provide a readily accessible cell type for feeder-free mRNA reprogramming. Sci. Rep. 2018, 8, 14363. [Google Scholar] [CrossRef]
- Xu, Y.T.; Huang, L.; Kirschman, J.L.; Vanover, D.A.; Tiwari, P.M.; Santangelo, P.J.; Shen, X.L.; Russell, D.G. Exploitation of Synthetic mRNA To Drive Immune Effector Cell Recruitment and Functional Reprogramming In Vivo. J. Immunol. 2019, 202, 608–617. [Google Scholar] [CrossRef]
- Irene, S.; Ioannis, G.; Ioanna, V.; Anastasios, M.; Angeliki, K.; Marianna, T.; Myrto, P.; Anny, M.; Maria, R.G.; Kalliope, S.; et al. Reprogramming of bone marrow derived mesenchymal stromal cells to human induced pluripotent stem cells from pediatric patients with hematological diseases using a commercial mRNA kit. Blood Cell. Mol. Dis. 2019, 76, 32–39. [Google Scholar]
- Grace, H.E.; Galdun, P.; Lesnefsky, E.J.; West, F.D.; Lyer, S. mRNA Reprogramming of T8993G Leigh’s Syndrome Fibroblast Cells to Create Induced Pluripotent Stem Cell Models for Mitochondrial Disorders. Stem Cells Dev. 2019, 28, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, D.W.; Zureick, N.; Fernandez, N.; Beyersdorf, J.; Sheng, C.; Park, S.J.; Santangelo, P.; Cho, H. Synthetic TBX18 mRNA Induces Durable Reprogramming of Cardiac Myocytes to Pacemaker Cells. Circulation 2020, 142, A16575. [Google Scholar] [CrossRef]
- Harris, J.K.; Rohde, C.B.; Angel, M. Mesenchymal Stem Cells (MSCs) Generated Using mRNA Reprogramming Show Enhanced Growth Potential, Secretome, and Therapeutic Efficacy in a Demyelinating Disease Model. Mol. Ther. 2020, 28, 366–367. [Google Scholar]
- Wang, A.Y.L. Application of Modified mRNA in Somatic Reprogramming to Pluripotency and Directed Conversion of Cell Fate. Int. J. Mol. Sci. 2021, 22, 8148. [Google Scholar] [CrossRef] [PubMed]
- Chabanovska, O.; Galow, A.M.; David, R.; Lemcke, H. mRNA—A game changer in regenerative medicine, cell-based therapy and reprogramming strategies. Adv. Drug Deliver. Rev. 2021, 179, 114002. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Lin, C. mRNA-Based Genetic Reprogramming. Mol. Ther. 2019, 27, 729–734. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K.; Itaka, K. Screening of mRNA Chemical Modification to Maximize Protein Expression with Reduced Immunogenicity. Pharmaceutics 2015, 7, 137–151. [Google Scholar] [CrossRef]
- Connor, B.; Firmin, E.; McCaughey-Chapman, A.; Monk, R.; Lee, K.; Liot, S.; Geiger, J.; Rudolph, C.; Jones, K. Conversion of adult human fibroblasts into neural precursor cells using chemically modified mRNA. Heliyon 2018, 4, e00918. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Rayan, M.; Negmeldin, A.T.; Elhissi, A.; Khalil, A. Chemically modified mRNA beyond COVID-19: Potential preventive and therapeutic applications for targeting chronic diseases. Biomed. Pharmacother. 2022, 145, 112385. [Google Scholar] [CrossRef]
- McGrath, P.S.; McGarvey, S.S.; Kogut, I.; Bilousova, G. Efficient RNA-Based Reprogramming of Disease-Associated Primary Human Fibroblasts into Induced Pluripotent Stem Cells. Methods Mol. Biol. 2020, 2117, 271–284. [Google Scholar]
- Preskey, D.; Allison, T.F.; Jones, M.; Mamchaoui, K.; Unger, C. Synthetically modified mRNA for efficient and fast human iPS cell generation and direct transdifferentiation to myoblasts. Biochem. Biophys. Res. Commun. 2016, 473, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Rossi, D.J. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat. Protoc. 2013, 8, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Ni, Y.; Wang, J.; Guo, X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci. Rep. 2012, 2, 657. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Heinimann, K.; Miny, P.; Iezzi, G.; Glatz, K.; Scherberich, A.; Zulewski, H.; Fussenegger, M. mRNA transfection-based, feeder-free, induced pluripotent stem cells derived from adipose tissue of a 50-year-old patient. Metab. Eng. 2013, 18, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Varela, I.; Karagiannidou, A.; Oikonomakis, V.; Tzetis, M.; Tzanoudaki, M.; Siapati, E.K.; Vassilopoulos, G.; Graphakos, S.; Kanavakis, E.; Goussetis, E. Generation of human beta-thalassemia induced pluripotent cell lines by reprogramming of bone marrow-derived mesenchymal stromal cells using modified mRNA. Cell Reprogram. 2014, 16, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Velasquez-Mao, A.J.; Tsao, C.J.M.; Monroe, M.N.; Legras, X.; Bissig-Choisat, B.; Bissig, K.D.; Ruano, R.; Jacot, J.G. Differentiation of spontaneously contracting cardiomyocytes from non-virally reprogrammed human amniotic fluid stem cells. PLoS ONE 2017, 12, e0177824. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhang, L.G.; Zhu, W.H.; Guo, R.Q.; Sun, H.M.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther.-Meth. Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Polajzer, T.; Miklavcic, D. Immunogenic Cell Death in Electroporation-Based Therapies Depends on Pulse Waveform Characteristics. Vaccines 2023, 11, 1036. [Google Scholar] [CrossRef]
- Supakul, S.; Leventoux, N.; Tabuchi, H.; Mimura, M.; Ito, D.; Maeda, S.; Okano, H. Establishment of KEIOi005-A iPSC line from urine-derived cells (UDCs) of a mild Alzheimer’s disease (AD) donor with multiple risk SNPs for sporadic Alzheimer’s disease (sAD). Stem Cell Res. 2022, 62, 102802. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.H.; Li, X.Y.; Li, Y.H.; Guo, X.P.; Cao, G.K.; Chen, S.; Hao, L.L.; et al. Generation of Induced Pluripotent Stem Cells from Urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef]
- Riebeling, C.; Schlechter, K.; Buesen, R.; Spielmann, H.; Luch, A.; Seiler, A. Defined culture medium for stem cell differentiation: Applicability of serum-free conditions in the mouse embryonic stem cell test. Toxicol. Vitr. 2011, 25, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.H.; Zhao, J.; Baker, R.K.; Tran, E.; Zhan, L.S.; Kieffer, T.J. Differentiation of stem cell-derived pancreatic progenitors into insulin-secreting islet clusters in a multiwell-based static 3D culture system. Cell. Rep. Methods 2023, 3, 100466. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B-Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Kuraishy, A.I.; Deshpande, C.; Hong, J.S.; Cacalano, N.A.; Gatti, R.A.; Manis, J.P.; Damore, M.A.; Pellegrini, M.; Teitell, M.A. AID-Induced Genotoxic Stress Promotes B Cell Differentiation in the Germinal Center via ATM and LKB1 Signaling. Mol. Cell 2010, 39, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Meier-Stiegen, F.; Schwanbeck, R.; Bernoth, K.; Martini, S.; Hieronymus, T.; Ruau, D.; Zenke, M.; Just, U. Activated Notch1 Target Genes during Embryonic Cell Differentiation Depend on the Cellular Context and Include Lineage Determinants and Inhibitors. PLoS ONE 2010, 5, e11481. [Google Scholar] [CrossRef] [PubMed]
- Bories, J.C.; Cayuela, J.M.; Loiseau, P.; Sigaux, F. Expression of Human Recombination Activating Genes (Rag1 and Rag2) in Neoplastic Lymphoid-Cells—Correlation with Cell-Differentiation and Antigen Receptor Expression. Blood 1991, 78, 2053–2061. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.M.; Lee, S.; Cho, H.B.; Park, J.I.; Kim, J.H.; Park, J.S.; Park, K.H. Efficient CRISPR-Cas9-based knockdown of RUNX2 to induce chondrogenic differentiation of stem cells. Biomater. Sci. 2022, 10, 514–523. [Google Scholar] [CrossRef]
- Rubio, A.; Luoni, M.; Giannelli, S.G.; Radice, I.; Iannielli, A.; Cancellieri, C.; Di Berardino, C.; Regalia, G.; Lazzari, G.; Menegon, A.; et al. Rapid and efficient CRISPR/Cas9 gene inactivation in human neurons during human pluripotent stem cell differentiation and direct reprogramming. Sci. Rep. 2016, 6, 37540. [Google Scholar] [CrossRef]
- Goparaju, S.K.; Kohda, K.; Ibata, K.; Soma, A.; Nakatake, Y.; Akiyama, T.; Wakabayashi, S.; Matsushita, M.; Sakota, M.; Kimura, H.; et al. Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Sci. Rep. 2017, 7, 42367. [Google Scholar] [CrossRef]
- Akiyama, T.; Sato, S.; Ko, S.B.H.; Sano, O.; Sato, S.; Saito, M.; Nagai, H.; Ko, M.S.H.; Iwata, H. Synthetic mRNA-based differentiation method enables early detection of Parkinson’s phenotypes in neurons derived from Gaucher disease-induced pluripotent stem cells. Stem Cells Transl. Med. 2021, 10, 572–581. [Google Scholar] [CrossRef]
- Jmoudiak, M.; Futerman, A.H. Gaucher disease: Pathological mechanisms and modern management. Brit. J. Haematol. 2005, 129, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Kurolap, M.; del Toro, M.; Spiegel, R.; Gutstein, A.; Shafir, G.; Cohen, I.J.; Barrabés, J.A.; Feldman, H.B. Gaucher disease type 3c: New patients with unique presentations and review of the literature. Mol. Genet. Metab. 2019, 127, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Spitz, M.; Rozenberg, R.; Silveira, P.A.A.; Barbosa, E.R. Parkinsonism in type 1 Gaucher’s disease. J. Neurol. Neurosur. Ps 2006, 77, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Kubiura-Ichimaru, M.; Penfold, C.; Kojima, K.; Dollet, C.; Yabukami, H.; Semi, K.; Takashima, Y.; Boroviak, T.; Kawaji, H.; Woltjen, K.; et al. mRNA-based generation of marmoset PGCLCs capable of differentiation into gonocyte-like cells. Stem Cell Rep. 2023, 18, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Aakash, P.; Lee, S.; Yoon, Y.S. Endothelial cell direct reprogramming: Past, present, and future. J. Mol. Cell Cardiol. 2023, 180, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef]
- Zhao, Y.; Londono, P.; Cao, Y.; Sharpe, E.J.; Proenza, C.; O’Rourke, R.; Jones, K.L.; Jeong, M.Y.; Walker, L.A.; Buttrick, P.M.; et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 2015, 6, 8243. [Google Scholar] [CrossRef]
- Muraoka, N.; Nara, K.; Tamura, F.; Kojima, H.; Yamakawa, H.; Sadahiro, T.; Miyamoto, K.; Isomi, M.; Haginiwa, S.; Tani, H.; et al. Role of cyclooxygenase-2-mediated prostaglandin E2-prostaglandin E receptor 4 signaling in cardiac reprogramming. Nat. Commun. 2019, 10, 674. [Google Scholar] [CrossRef]
- Cao, N.; Huang, Y.; Zheng, J.; Spencer, C.I.; Zhang, Y.; Fu, J.D.; Nie, B.; Xie, M.; Zhang, M.; Wang, H.; et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 2016, 352, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, K.P.; Uppal, H. Direct reprogramming of human fibroblasts to hepatocyte-like cells by synthetic modified mRNAs. PLoS ONE 2014, 9, e100134. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, P.; Vu, N.B.; Dao, T.T.; Le, H.T.; Phi, L.T.; Phan, N.K. Production of endothelial progenitor cells from skin fibroblasts by direct reprogramming for clinical usages. Vitr. Cell Dev. Biol. Anim. 2017, 53, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Hadas, Y.; Kurian, A.A.; Zak, M.M.; Yoo, J.; Mahmood, A.; Girard, H.; Komargodski, R.; Io, T.; Santini, M.P.; et al. Direct reprogramming induces vascular regeneration post muscle ischemic injury. Mol. Ther. 2021, 29, 3042–3058. [Google Scholar] [CrossRef] [PubMed]
- Qabrati, X.; Kim, I.; Ghosh, A.; Bundschuh, N.; Noe, F.; Palmer, A.S.; Bar-Nur, O. Transgene-free direct conversion of murine fibroblasts into functional muscle stem cells. NPJ Regen. Med. 2023, 8, 43. [Google Scholar] [CrossRef]
- Pruszak, J.; Sonntag, K.C.; Aung, M.H.; Sanchez-Pernaute, R.; Isacson, O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell Populations. Stem Cells 2007, 25, 2257–2268. [Google Scholar] [CrossRef]
- Riordon, D.R.; Boheler, K.R. Immunophenotyping of Live Human Pluripotent Stem Cells by Flow Cytometry. Surfaceome Methods Protoc. 2018, 1722, 127–149. [Google Scholar]
- Fujita, Y.; Hirosawa, M.; Hayashi, K.; Hatani, T.; Yoshida, Y.; Yamamoto, T.; Saito, H. A versatile and robust cell purification system with an RNA-only circuit composed of microRNA-responsive ON and OFF switches. Sci. Adv. 2022, 8, eabj1793. [Google Scholar] [CrossRef]
- Okuzaki, A.; Tsuda, M.; Konagaya, K.; Tabei, Y. A novel strategy for promoting homoplasmic plastid transformant production using the barnase-barstar system. Plant Biotechnol. 2020, 37, 223–232. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Q.; Li, X.W.; Zhang, X.L. MicroRNA-21 regulates the proliferation and apoptosis of cervical cancer cells via tumor necrosis factor-α. Mol. Med. Rep. 2017, 16, 4659–4663. [Google Scholar] [CrossRef]
- Abe, N.; Imaeda, A.; Inagaki, M.; Li, Z.M.; Kawaguchi, D.; Onda, K.; Nakashima, Y.; Uchida, S.; Hashiya, F.; Kimura, Y.; et al. Complete Chemical Synthesis of Minimal Messenger RNA by Efficient Chemical Capping Reaction. ACS Chem. Biol. 2022, 17, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
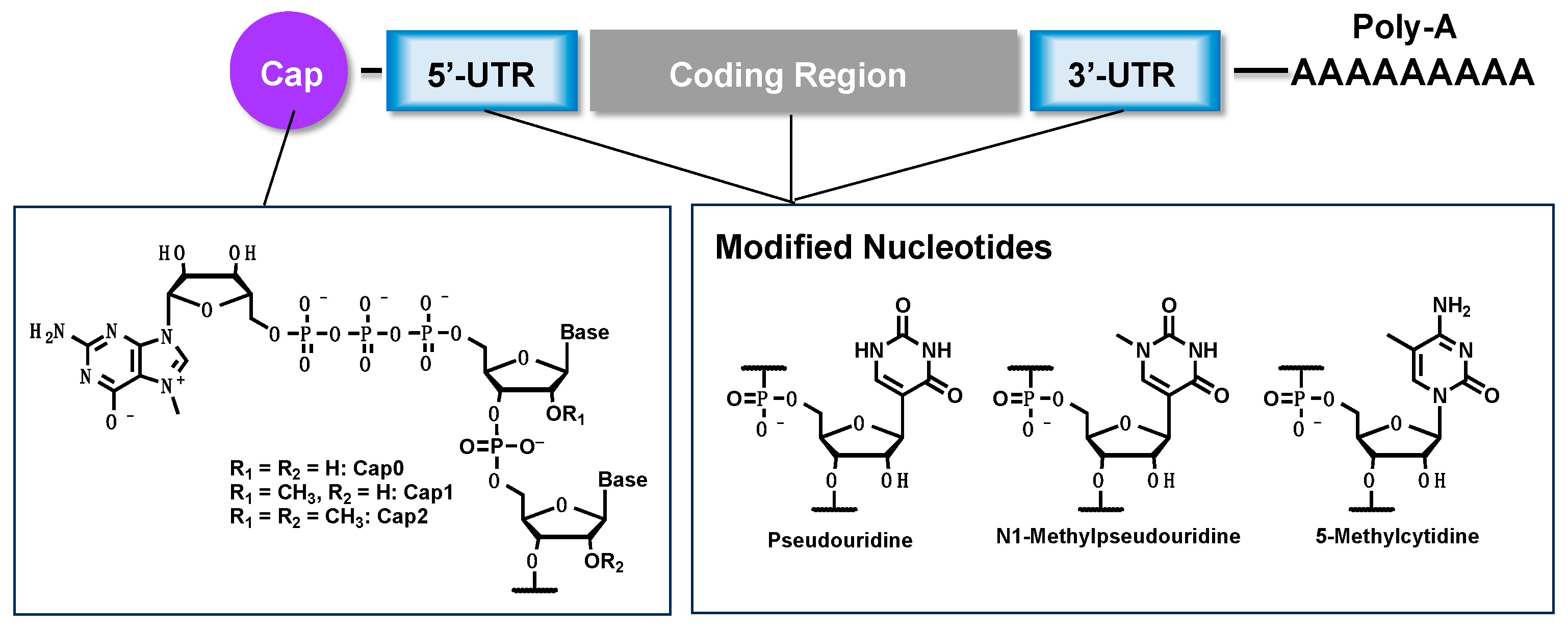
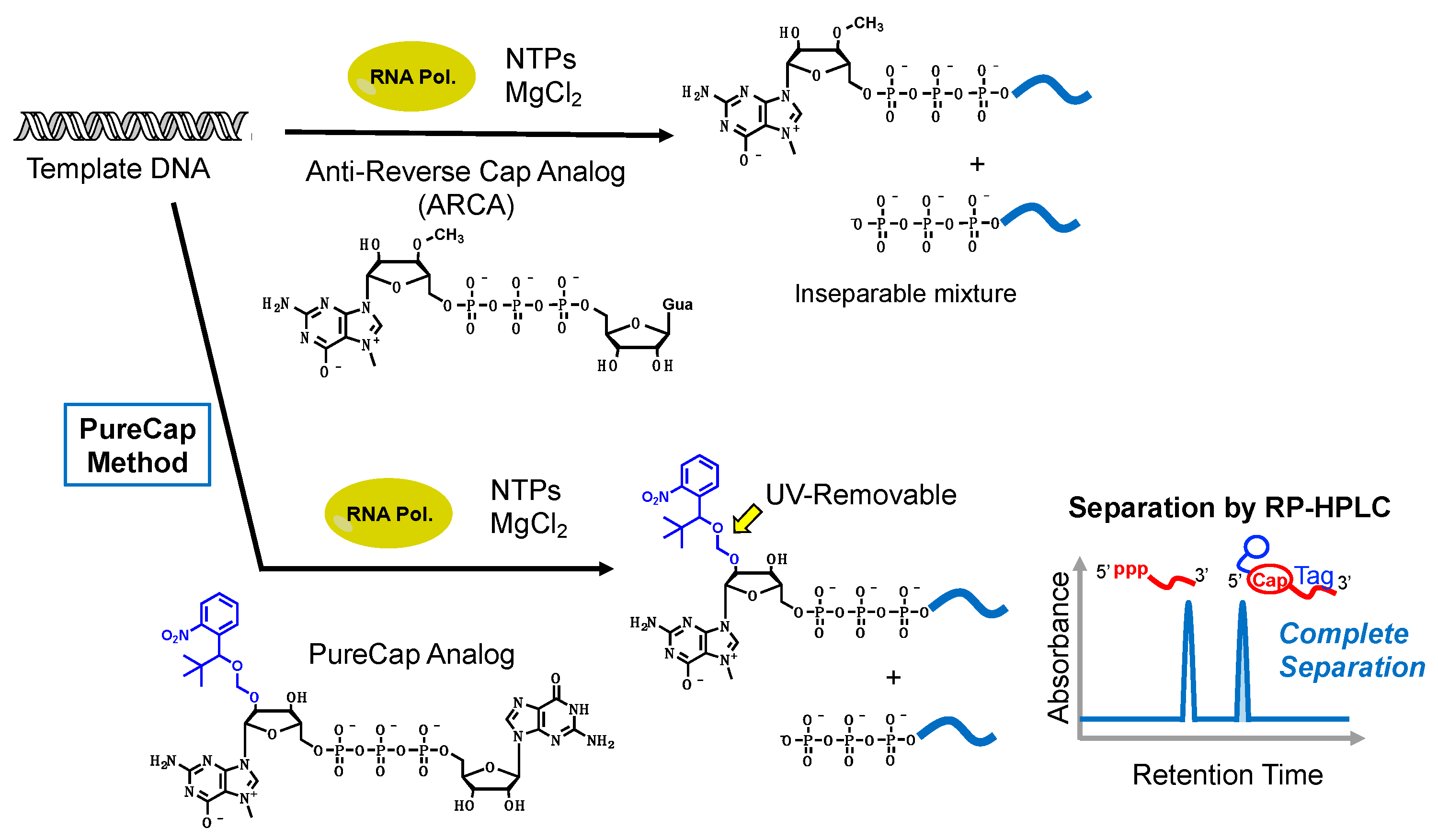
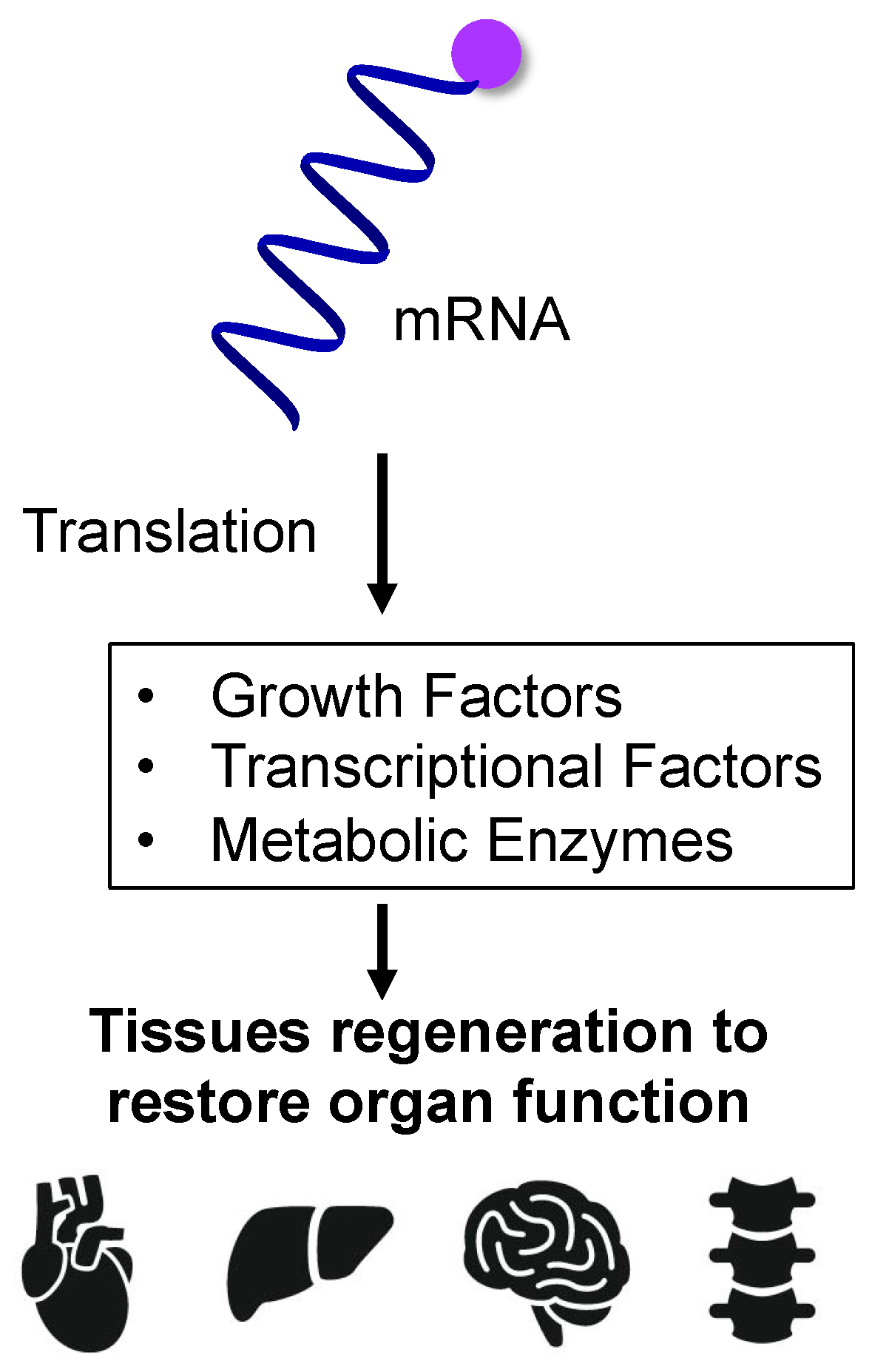

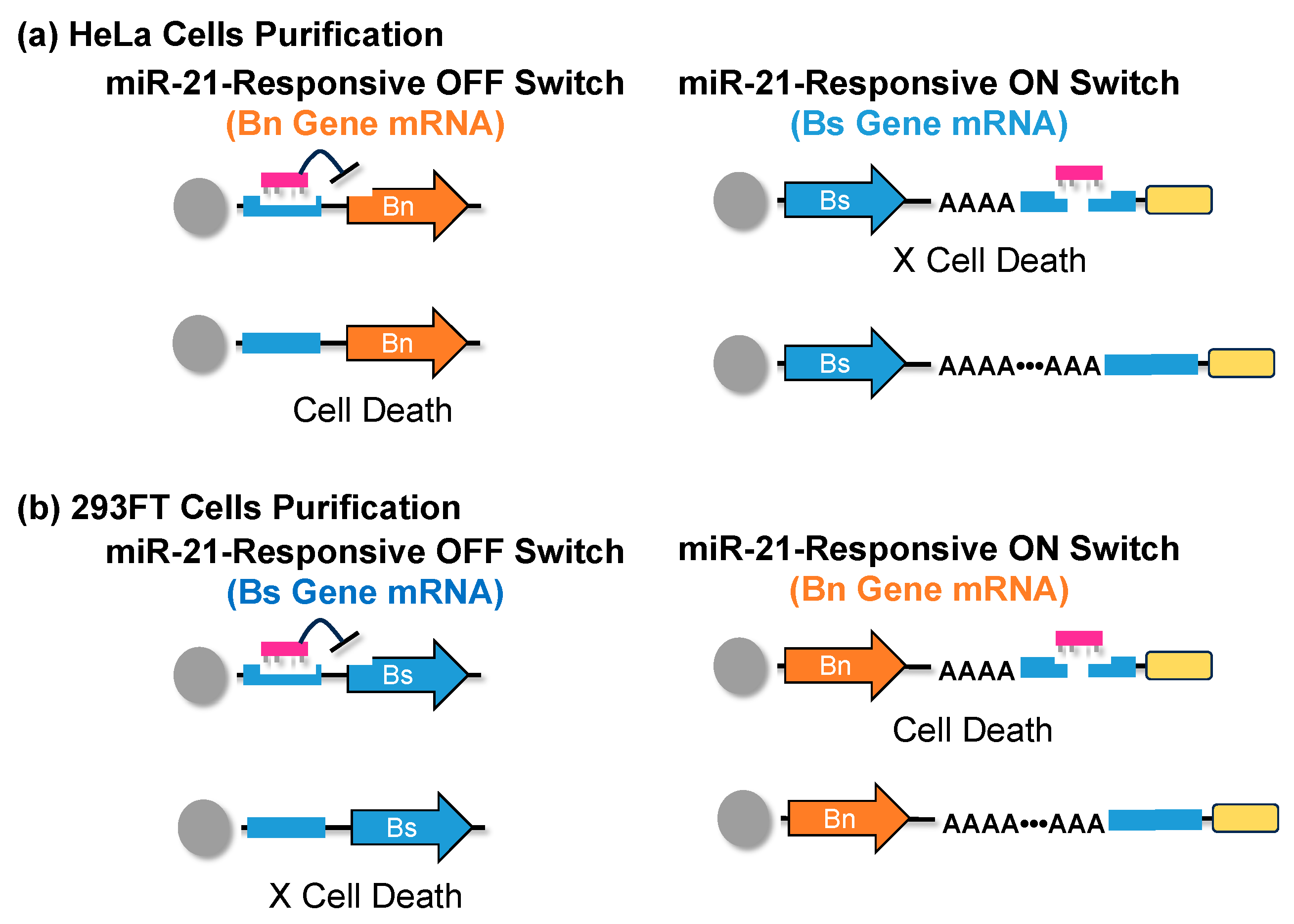
| Reprogramming Method | Advantages | Disadvantages |
|---|---|---|
| Retroviral vectors |
|
|
|
| |
| Plasmid vectors |
|
|
| Small molecules |
|
|
|
| |
|
| |
| microRNA |
|
|
|
| |
| mRNA |
|
|
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, M. Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine. J. Dev. Biol. 2024, 12, 1. https://doi.org/10.3390/jdb12010001
Inagaki M. Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine. Journal of Developmental Biology. 2024; 12(1):1. https://doi.org/10.3390/jdb12010001
Chicago/Turabian StyleInagaki, Masahito. 2024. "Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine" Journal of Developmental Biology 12, no. 1: 1. https://doi.org/10.3390/jdb12010001
APA StyleInagaki, M. (2024). Cell Reprogramming and Differentiation Utilizing Messenger RNA for Regenerative Medicine. Journal of Developmental Biology, 12(1), 1. https://doi.org/10.3390/jdb12010001






