Crucial Convolution: Genetic and Molecular Mechanisms of Coiling during Epididymis Formation and Development in Embryogenesis
Abstract
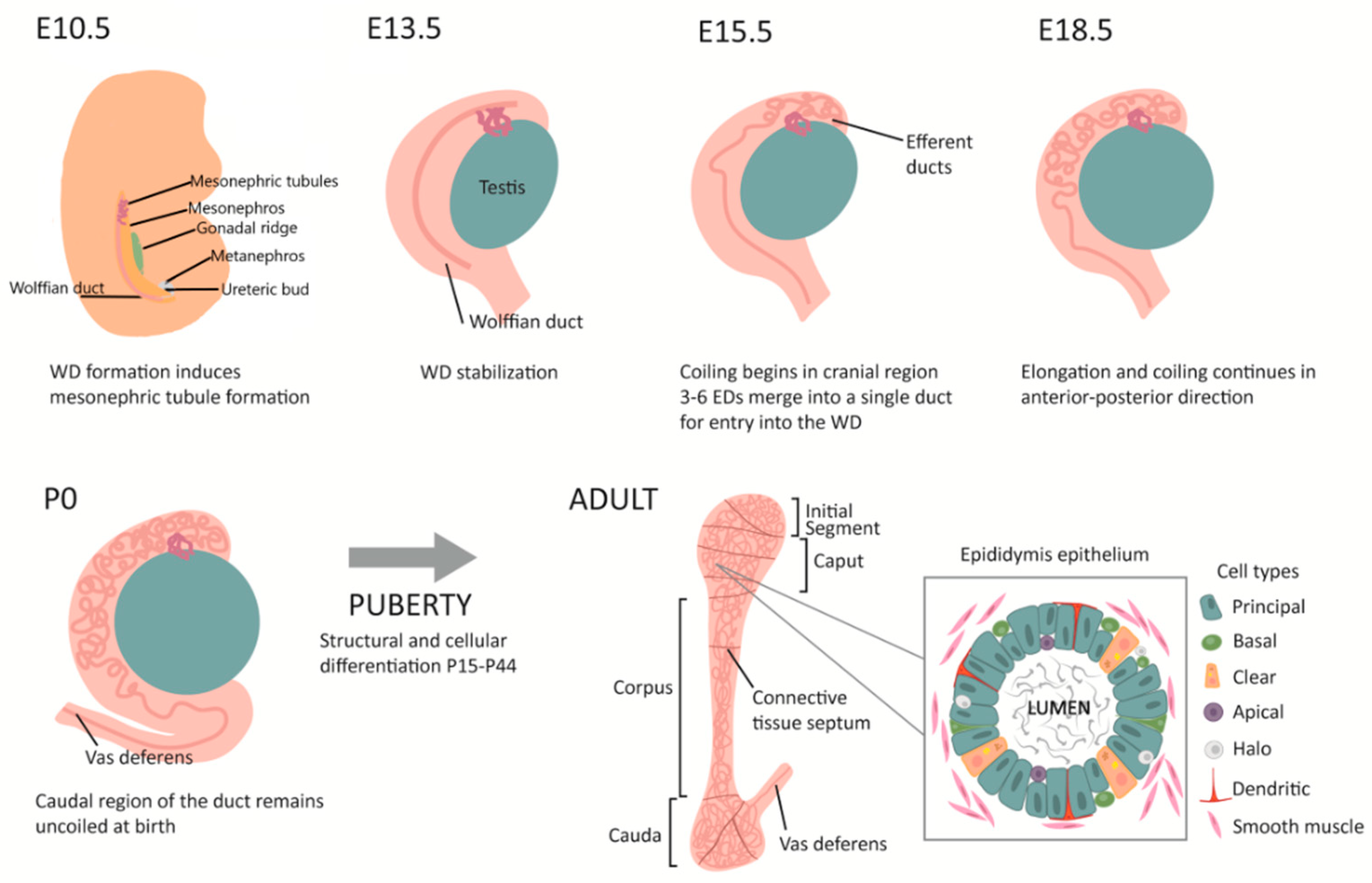
1. Introduction
1.1. The Need for and the Mechanisms of Coiling and Folding across Development
1.1.1. The Epididymis as a Model for Understanding Tissue Coiling
1.1.2. Androgen Signalling
1.1.3. WNT Signalling
1.1.4. Activin A
1.1.5. A Role for the Extracellular Matrix?
1.2. Seminiferous Tubules
Intestine
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinzel, B.; Pikiolek, M.; Orsini, V.; Sprunger, J.; Isken, A.; Zietzling, S.; Desplanches, M.; Dubost, V.; Breustedt, D.; Valdez, R.; et al. Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev. Biol. 2014, 390, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.T.; Galdamez, M.M.; Sutherland, A.; Bomgardner, D.; Xu, B.; Abdel-Fattah, R.; Yang, L. How Do You Get Six Meters of Epididymis Inside a Human Scrotum? J. Androl. 2011, 32, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.N.; Lesieur, E.; Harley, V.R.; Sinclair, A.H.; Little, M.H.; Wilhelm, D.; Koopman, P. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev. Dyn. 2009, 238, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Ritsche, I.S.; Fahlke, J.M.; Wieder, F.; Hilger, A.; Manke, I.; Hampe, O. Relationships of cochlear coiling shape and hearing frequencies in cetaceans, and the occurrence of infrasonic hearing in Miocene Mysticeti. Foss. Rec. 2018, 21, 33–45. [Google Scholar] [CrossRef]
- Dworkin, S.; Jane, S.M. Novel mechanisms that pattern and shape the midbrain-hindbrain boundary. CMLS 2013, 70, 3365–3374. [Google Scholar] [CrossRef]
- Männer, J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin. Anat. 2009, 22, 21–35. [Google Scholar] [CrossRef]
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Updat. 2016, 22, 574–587. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Mahadevan, L.; Tabin, C.J. BMP signaling controls buckling forces to modulate looping morphogenesis of the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 2277–2282. [Google Scholar] [CrossRef]
- Sela-Donenfeld, D.; Kayam, G.; Wilkinson, D.G. Boundary cells regulate a switch in the expression of FGF3 in hindbrain rhombomeres. BMC Dev. Biol. 2009, 9, 16. [Google Scholar] [CrossRef]
- Nakata, H. Morphology of mouse seminiferous tubules. Anat. Sci. Int. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Lewis, S.; Chen, L.; Raghuram, V.; Khundmiri, S.J.; Chou, C.-L.; Yang, C.-R.; Knepper, M.A. “SLC-omics” of the kidney: Solute transporters along the nephron. Am. J. Physiol. Cell Physiol. 2021, 321, C507–C518. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.J.; Ewald, A.J. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev. Biol. 2010, 341, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Lubarsky, B.; Krasnow, M.A. Tube Morphogenesis: Making and Shaping Biological Tubes. Cell 2003, 112, 19–28. [Google Scholar] [CrossRef]
- Iruela-Arispe, M.L.; Beitel, G.J. Tubulogenesis. Development 2013, 140, 2851–2855. [Google Scholar] [CrossRef]
- Soffers, J.H.; Hikspoors, J.P.; Mekonen, H.K.; Koehler, S.E.; Lamers, W.H. The growth pattern of the human intestine and its mesentery. BMC Dev. Biol. 2015, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Kikuchi, K.; Hochgreb, T.; Poss, K.D.; Stainier, D.Y. Hand2 Regulates Extracellular Matrix Remodeling Essential for Gut-Looping Morphogenesis in Zebrafish. Dev. Cell 2010, 18, 973–984. [Google Scholar] [CrossRef]
- Onouchi, S.; Ichii, O.; Nakamura, T.; Elewa, Y.H.A.; Kon, Y. Spatiotemporal distribution of extracellular matrix changes during mouse duodenojejunal flexure formation. Cell Tissue Res. 2016, 365, 367–379. [Google Scholar] [CrossRef]
- Savin, T.; Kurpios, N.A.; Shyer, A.E.; Florescu, P.; Liang, H.; Mahadevan, L.; Tabin, C.J. On the growth and form of the gut. Nature 2011, 476, 57–61. [Google Scholar] [CrossRef]
- Campione, M.; Steinbeisser, H.; Schweickert, A.; Deissler, K.; van Bebber, F.; Lowe, L.; Nowotschin, S.; Viebahn, C.; Haffter, P.; Kuehn, M.; et al. The homeobox gene Pitx2: Mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 1999, 126, 1225–1234. [Google Scholar] [CrossRef]
- Tsuda, T.; Majumder, K.; Linask, K.K. Differential expression of flectin in the extracellular matrix and left-right asymmetry in mouse embryonic heart during looping stages. Genesis 1998, 23, 203–214. [Google Scholar] [CrossRef]
- Eshi, Y.; Eyao, J.; Young, J.M.; Fee, J.A.; Eperucchio, R.; Taber, L.A. Bending and twisting the embryonic heart: A computational model for c-looping based on realistic geometry. Front. Physiol. 2014, 5, 297. [Google Scholar]
- Le Garrec, J.-F.; Domínguez, J.N.; Desgrange, A.; Ivanovitch, K.D.; Raphaël, E.; Bangham, J.A.; Torres, M.; Coen, E.; Mohun, T.J.; Meilhac, S.M. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 2017, 6, e28951. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, O.H.; Coskun, H.; Minguillón, C.; Murawala, P.; Tanaka, E.M.; Galceran, J.; Muñoz-Chápuli, R.; Nieto, M.A. A right-handed signalling pathway drives heart looping in vertebrates. Nature 2017, 549, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; Han, M.; Cai, D.H.; Brauer, P.R.; Maisastry, S.M. Cardiac morphogenesis: Matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev. Dyn. 2005, 233, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; VanAuker, M. A Role for the Cytoskeleton in Heart Looping. Sci. World J. 2007, 7, 280–298. [Google Scholar] [CrossRef]
- Hinton, B.T.; Murashima, A.; Xu, B. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J. Androl. 2015, 17, 749–755. [Google Scholar] [CrossRef]
- França, L.R.; Avelar, G.F.; Almeida, F.F. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 2005, 63, 300–318. [Google Scholar] [CrossRef]
- Dacheux, J.-L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014, 147, R27–R42. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Dacheux, F.C.; Dacheux, J.; Gatti, J.-L. The Epididymal Transcriptome and Proteome Provide Some Insights Into New Epididymal Regulations. J. Androl. 2011, 32, 651–664. [Google Scholar] [CrossRef]
- Joseph, A.; Yao, H.; Hinton, B.T. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev. Biol. 2009, 325, 6–14. [Google Scholar] [CrossRef]
- McArdle, C.A.; Roberson, M.S.; Plant, T.M.; Zeleznik, A.J. Orgebin-Crist, The epididymis. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1071–1148. [Google Scholar]
- Ferreira, L.G.; Nishino, F.A.; Fernandes, S.G.; Ribeiro, C.M.; Hinton, B.T.; Avellar, M.C.W. Epididymal embryonic development harbors TLR4/NFKB signaling pathway as a morphogenetic player. J. Reprod. Immunol. 2022, 149, 103456. [Google Scholar] [CrossRef] [PubMed]
- Jelinsky, S.A.; Turner, T.T.; Bang, H.J.; Finger, J.N.; Solarz, M.K.; Wilson, E.; Brown, E.L.; Kopf, G.S.; Johnston, D.S. The Rat Epididymal Transcriptome: Comparison of Segmental Gene Expression in the Rat and Mouse Epididymides1. Biol. Reprod. 2007, 76, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Damdimopoulos, A.; Damdimopoulou, P.; Gasperoni, J.G.; Tran, S.C.; Grommen, S.V.; De Groef, B.; Dworkin, S. Transcriptome analysis of the epididymis from Plag1 deficient mice suggests dysregulation of sperm maturation and extracellular matrix genes. Dev. Dyn. 2020, 249, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Juma, A.R.; Tran, S.C.; Gasperoni, J.G.; Grommen, S.V.H.; De Groef, B. Deficiency of the transcription factor PLAG1 results in aberrant coiling and morphology of the epididymis. Asian J. Androl. 2020, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Murashima, A.; Kishigami, S.; Thomson, A.; Yamada, G. Androgens and mammalian male reproductive tract development. Biochim. Biophys. Acta 2015, 1849, 163–170. [Google Scholar] [CrossRef]
- Hannema, S.E.; Hughes, I.A. Regulation of Wolffian Duct Development. Horm. Res. Paediatr. 2007, 67, 142–151. [Google Scholar] [CrossRef]
- Shaw, G.; Renfree, M.B. Wolffian Duct Development. Sex. Dev. 2014, 8, 273–280. [Google Scholar] [CrossRef]
- Menad, R.; Fernini, M.; Lakabi, L.; Smaï, S.; Gernigon-Spychalowicz, T.; Farida, K.; Bonnet, X.; Moudilou, E.; Exbrayat, J.-M. Androgen and estrogen receptors immunolocalization in the sand rat (Psammomys obesus) cauda epididymis. Acta Histochem. 2021, 123, 151683. [Google Scholar] [CrossRef]
- Welsh, M.; Saunders, P.; Marchetti, N.I.; Sharpe, R.M. Androgen-Dependent Mechanisms of Wolffian Duct Development and Their Perturbation by Flutamide. Endocrinology 2006, 147, 4820–4830. [Google Scholar] [CrossRef][Green Version]
- Welsh, M.; Sharpe, R.M.; Walker, M.; Smith, L.B.; Saunders, P.T.K. New Insights into the Role of Androgens in Wolffian Duct Stabilization in Male and Female Rodents. Endocrinology 2009, 150, 2472–2480. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Ferreira, L.G.; Thimoteo, D.S.; Smith, L.B.; Hinton, B.T.; Avellar, M.C.W. Novel androgen-induced activity of an antimicrobial β-defensin: Regulation of Wolffian duct morphogenesis. Mol. Cell. Endocrinol. 2017, 442, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Alvarez, L.; Blank, N.; Balbach, M.; Turunen, H.; Laajala, T.D.; Toivanen, J.; Krutskikh, A.; Wahlberg, N.; Huhtaniemi, I.; et al. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol. Cell. Endocrinol. 2016, 427, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.; Fok, K.L.; Chen, H.; Yu, M.K.; Duan, Y.; Chung, C.M.; Li, Z.; Wu, H.; Li, Z.; Zhang, H.; et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014, 6, 108–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Webb, S.; Lettice, L.; Tardif, S.; Kilanowski, F.; Tyrrell, C.; MacPherson, H.; Semple, F.; Tennant, P.; Baker, T.; et al. Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice. PLoS Genet. 2013, 9, e1003826. [Google Scholar] [CrossRef]
- Zhou, C.X.; Zhang, Y.-L.; Xiao, L.; Zheng, M.; Leung, K.M.; Chan, M.Y.; Lo, P.S.; Tsang, L.L.; Wong, H.Y.; Ho, L.S.; et al. An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 2004, 6, 458–464. [Google Scholar] [CrossRef]
- Osterhoff, C.; Kirchhoff, C.; Krull, N.; Ivell, R. Molecular Cloning and Characterization of a Novel Human Sperm Antigen (HE2) Specifically Expressed in the Proximal Epididymis. Biol. Reprod. 1994, 50, 516–525. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Queiróz, D.B.; Patrão, M.T.; Denadai-Souza, A.; Romano, R.M.; Silva, E.J.; Avellar, M.C.W. Dynamic changes in the spatio-temporal expression of the β-defensin SPAG11C in the developing rat epididymis and its regulation by androgens. Mol. Cell. Endocrinol. 2015, 404, 141–150. [Google Scholar] [CrossRef]
- Murashima, A.; Miyagawa, S.; Ogino, Y.; Nishida-Fukuda, H.; Araki, K.; Matsumoto, T.; Kaneko, T.; Yoshinaga, K.; Yamamura, K.-I.; Kurita, T.; et al. Essential Roles of Androgen Signaling in Wolffian Duct Stabilization and Epididymal Cell Differentiation. Endocrinology 2011, 152, 1640–1651. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Prog. Mol. Biol. Transl. Sci. 2017, 153, 181–207. [Google Scholar]
- Carroll, T.J.; Park, J.-S.; Hayashi, S.; Majumdar, A.; McMahon, A.P. Wnt9b Plays a Central Role in the Regulation of Mesenchymal to Epithelial Transitions Underlying Organogenesis of the Mammalian Urogenital System. Dev. Cell 2005, 9, 283–292. [Google Scholar] [CrossRef]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Syed, S.M.; Taketo, M.M.; Tanwar, P.S. Epithelial Wnt/βcatenin signalling is essential for epididymal coiling. Dev. Biol. 2016, 412, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, M.; Murashima, A.; Harada, M.; Nakagata, N.; Noguchi, M.; Morimoto, M.; Kimura, T.; Ornitz, D.M.; Yamada, G. Region-specific regulation of cell proliferation by FGF receptor signaling during the Wolffian duct development. Dev. Biol. 2015, 400, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Warr, N.; Siggers, P.; Bogani, D.; Brixey, R.; Pastorelli, L.; Yates, L.; Dean, C.H.; Wells, S.; Satoh, W.; Shimono, A.; et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev. Biol. 2009, 326, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.; Ajima, R.; Sharma, N.; Costantini, F.; Mackem, S.; Lewandoski, M.; Yamaguchi, T.P.; Perantoni, A.O. Non-canonical Wnt5a/Ror2 signaling regulates kidney morphogenesis by controlling intermediate mesoderm extension. Hum. Mol. Genet. 2014, 23, 6807–6814. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Washington, A.M.; Domeniconi, R.F.; Souza, A.C.F.; Lu, X.; Sutherland, A.; Hinton, B.T. Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct. Dev. Biol. 2016, 412, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.; Wharton, K.A.; Carroll, T.J. Planar cell polarity and vertebrate organogenesis. Semin. Cell Dev. Biol. 2006, 17, 194–203. [Google Scholar] [CrossRef]
- Lee, J.; Andreeva, A.; Sipe, C.W.; Liu, L.; Cheng, A.; Lu, X. PTK7 Regulates Myosin II Activity to Orient Planar Polarity in the Mammalian Auditory Epithelium. Curr. Biol. 2012, 22, 956–966. [Google Scholar] [CrossRef][Green Version]
- Andreeva, A.; Lee, J.; Lohia, M.; Wu, X.; Macara, I.G.; Lu, X. PTK7-Src Signaling at Epithelial Cell Contacts Mediates Spatial Organization of Actomyosin and Planar Cell Polarity. Dev. Cell 2014, 29, 20–33. [Google Scholar] [CrossRef]
- Mendis, S.H.; Meachem, S.J.; Sarraj, M.A.; Loveland, K.L. Activin A Balances Sertoli and Germ Cell Proliferation in the Fetal Mouse Testis. Biol. Reprod. 2010, 84, 379–391. [Google Scholar] [CrossRef]
- Wijayarathna, R.; de Kretser, D.M.; Meinhardt, A.; Middendorff, R.; Ludlow, H.; Groome, N.P.; Loveland, K.A.; Hedger, M.P. Activin over-expression in the testis of mice lacking the inhibin α-subunit gene is associated with androgen deficiency and regression of the male reproductive tract. Mol. Cell. Endocrinol. 2017, 470, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.C.; Wakeling, S.I.; Stringer, J.M.; Bergen, J.A.V.D.; Wilhelm, D.; Sinclair, A.H.; Western, P.S. Signaling through the TGF Beta-Activin Receptors ALK4/5/7 Regulates Testis Formation and Male Germ Cell Development. PLoS ONE 2013, 8, e54606. [Google Scholar] [CrossRef] [PubMed]
- Meehan, T.; Schlatt, S.; O’Bryan, M.; de Kretser, D.M.; Loveland, K. Regulation of Germ Cell and Sertoli Cell Development by Activin, Follistatin, and FSH. Dev. Biol. 2000, 220, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bilezikjian, L.M.; Blount, A.L.; Donaldson, C.J.; Vale, W.W. Pituitary actions of ligands of the TGF-β family: Activins and inhibins. Reproduction 2006, 132, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cui, X.; Ge, J.; Li, J.; Niu, L.; Liu, H.; Qi, Y.; Liu, Z.; Wang, Y. Activin A inhibits activities of lipopolysaccharide-activated macrophages via TLR4, not of TLR2. Biochem. Biophys. Res. Commun. 2013, 435, 222–228. [Google Scholar] [CrossRef]
- Nicolas, N.; Muir, J.A.; Hayward, S.; Chen, J.L.; Stanton, P.G.; Gregorevic, P.; de Kretser, D.M.; Loveland, K.L.; Bhushan, S.; Meinhardt, A.; et al. Induction of experimental autoimmune orchitis in mice: Responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction 2017, 154, 293–305. [Google Scholar] [CrossRef]
- Hedger, M.P.; Winnall, W.R. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol. 2012, 359, 30–42. [Google Scholar] [CrossRef]
- Muttukrishna, S.; Farouk, A.; Sharma, S.K.; Evans, L.; Groome, N.; Ledger, W.; Sathanandan, M. Serum activin A and follistatin in disorders of spermatogenesis in men. Eur. J. Endocrinol. 2001, 144, 425–429. [Google Scholar] [CrossRef][Green Version]
- Ritvos, O.; Tuuri, T.; Erämaa, M.; Sainio, K.; Hilden, K.; Saxén, L.; Gilbert, S.F. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech. Dev. 1995, 50, 229–245. [Google Scholar] [CrossRef]
- Cancilla, B.; Jarred, R.A.; Wang, H.; Mellor, S.L.; Cunha, G.R.; Risbridger, G. Regulation of Prostate Branching Morphogenesis by Activin A and Follistatin. Dev. Biol. 2001, 237, 145–158. [Google Scholar] [CrossRef]
- Tomaszewski, J.; Joseph, A.; Archambeault, D.; Yao, H.H. Essential roles of inhibin beta A in mouse epididymal coiling. Proc. Natl. Acad. Sci. USA 2007, 104, 11322–11327. [Google Scholar] [CrossRef] [PubMed]
- Wijayarathna, R.; Sarraj, M.A.; Genovese, R.; Girling, J.; Michel, V.; Ludlow, H.; Loveland, K.L.; Meinhardt, A.; De Kretser, D.M.; Hedger, M.P. Activin and follistatin interactions in the male reproductive tract: Activin expression and morphological abnormalities in mice lacking follistatin 288. Andrology 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Wu, H.; Sarraj, M.; Rogers, P.A.W.; De Kretser, D.M.; Girling, J.; Hedger, M. Expression patterns of activin, inhibin and follistatin variants in the adult male mouse reproductive tract suggest important roles in the epididymis and vas deferens. Reprod. Fertil. Dev. 2013, 25, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Daley, W.P.; Yamada, K.M. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr. Opin. Genet. Dev. 2013, 23, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Mayor, R. Collective cell migration in development. J. Cell Biol. 2016, 212, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N.; Potter-Perigo, S. The extracellular matrix: An active or passive player in fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, 950–955. [Google Scholar] [CrossRef]
- Long, K.B.; Artlett, C.M.; Blankenhorn, E.P. Tight skin 2 mice exhibit a novel time line of events leading to increased extracellular matrix deposition and dermal fibrosis. Matrix Biol. 2014, 38, 91–100. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Manon-Jensen, T.; Genovese, F.; Kristensen, J.H.; Nielsen, M.J.; Sand, J.M.B.; Hansen, N.-U.B.; Bay-Jensen, A.-C.; Bager, C.L.; Krag, A.; et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Liver Physiol. 2015, 308, G807–G830. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, X.; Hu, X.; Liu, Q.; Man, Z.; Huang, H.; Meng, Q.; Zhou, C.; Ao, Y. Silencing of miR-101 Prevents Cartilage Degradation by Regulating Extracellular Matrix–related Genes in a Rat Model of Osteoarthritis. Mol. Ther. 2015, 23, 1331–1340. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Dai, L.; Hu, X.; Zhu, J.; Li, L.; Zhou, C.; Ao, Y. Long Noncoding RNA Related to Cartilage Injury Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Arthritis Rheumatol. 2014, 66, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Hoshii, T.; Takeo, T.; Nakagata, N.; Takeya, M.; Araki, K.; Yamamura, K.-I. LGR4 Regulates the Postnatal Development and Integrity of Male Reproductive Tracts in Mice1. Biol. Reprod. 2007, 76, 303–313. [Google Scholar] [CrossRef]
- Obara-Ishihara, T.; Kuhlman, J.; Niswander, L.; Herzlinger, D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 1999, 126, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Arend, L.J. Pkd1 is required for male reproductive tract development. Mech. Dev. 2013, 130, 567–576. [Google Scholar] [CrossRef]
- Chauvet, V.; Qian, F.; Boute, N.; Cai, Y.; Phakdeekitacharoen, B.; Onuchic, L.F.; Attié-Bitach, T.; Guicharnaud, L.; Devuyst, O.; Germino, G.G.; et al. Expression of PKD1 and PKD2 Transcripts and Proteins in Human Embryo and during Normal Kidney Development. Am. J. Pathol. 2002, 160, 973–983. [Google Scholar] [CrossRef]
- Nie, X.; Arend, L.J. Novel roles of Pkd2 in male reproductive system development. Differentiation 2014, 87, 161–171. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.-X.; Wang, D.; Qi, X.; Li, T.-G.; Hao, J.; Mishina, Y.; Garbers, D.L.; Zhao, G.-Q. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev. Biol. 2004, 276, 158–171. [Google Scholar] [CrossRef]
- Zhao, G.; Liaw, L.; Hogan, B. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 1998, 125, 1103–1112. [Google Scholar] [CrossRef]
- Zhao, G.-Q.; Chenb, Y.X.; Liub, X.M.; Xuab, Z.; Qiab, X. Mutation in Bmp7 Exacerbates the Phenotype of Bmp8a Mutants in Spermatogenesis and Epididymis. Dev. Biol. 2001, 240, 212–222. [Google Scholar] [CrossRef]
- Kato, S.; Matsubara, M.; Matsuo, T.; Mohri, Y.; Kazama, I.; Hatano, R.; Umezawa, A.; Nishimori, K. Leucine-Rich Repeat-Containing G Protein-Coupled Receptor-4 (LGR4, Gpr48) Is Essential for Renal Development in Mice. Nephron Exp. Nephrol. 2006, 104, e63–e75. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Y.; Oyama, K.; Akamatsu, A.; Kato, S.; Nishimori, K. Lgr4-deficient mice showed premature differentiation of ureteric bud with reduced expression of Wnt effector Lef1 and Gata3. Dev. Dyn. 2011, 240, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Mendive, F.; Laurent, P.; van Schoore, G.; Skarnes, W.; Pochet, R.; Vassart, G. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev. Biol. 2006, 290, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Cool, J.; Carmona, F.; Szucsik, J.; Capel, B. Peritubular Myoid Cells Are Not the Migrating Population Required for Testis Cord Formation in the XY Gonad. Sex. Dev. 2008, 2, 128–133. [Google Scholar] [CrossRef]
- Coveney, D.; Cool, J.; Oliver, T.; Capel, B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc. Natl. Acad. Sci. USA 2008, 105, 7212–7217. [Google Scholar] [CrossRef]
- Svingen, T.; Koopman, P. Building the mammalian testis: Origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar] [CrossRef]
- Chen, S.-R.; Liu, Y.-X. Testis Cord Maintenance in Mouse Embryos: Genes and Signaling1. Biol. Reprod. 2016, 94, 42. [Google Scholar] [CrossRef]
- Nel-Themaat, L.; González, G.; Akiyama, H.; Behringer, R.R. Illuminating Testis Morphogenesis in the Mouse. J. Androl. 2010, 31, 5–10. [Google Scholar] [CrossRef]
- Archambeault, D.R.; Tomaszewski, J.; Joseph, A.; Hinton, B.T.; Yao, H.H.-C. Epithelial-mesenchymal crosstalk in Wolffian duct and fetal testis cord development. Genesis 2009, 47, 40–48. [Google Scholar] [CrossRef][Green Version]
- Cool, J.; DeFalco, T.; Capel, B. Testis formation in the fetal mouse: Dynamic and complex de novo tubulogenesis. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 847–859. [Google Scholar] [CrossRef]
- Archambeault, D.R.; Yao, H.H.-C. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 10526–10531. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Intestinal atresia and stenosis: A review comparing its etiopathogenesis. Veter. Res. Commun. 1986, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Shaw-Smith, C. Review of genetic factors in intestinal malrotation. Pediatr. Surg. Int. 2010, 26, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Iwuagwu, O.; Deans, G.T. Small bowel volvulus: A review. J. R. Coll. Surg. Edinb. 1999, 44, 150–155. [Google Scholar]
- Chinya, A.; Naranje, K.; Mandelia, A. Situs inversus abdominalis, polysplenia, complex jejunal atresia and malrotation in a neonate: A rare association. Int. J. Surg. Case Rep. 2019, 56, 93–95. [Google Scholar] [CrossRef]
- Davis, N.M.; Kurpios, N.A.; Sun, X.; Gros, J.; Martin, J.F.; Tabin, C.J. The Chirality of Gut Rotation Derives from Left-Right Asymmetric Changes in the Architecture of the Dorsal Mesentery. Dev. Cell 2008, 15, 134–145. [Google Scholar] [CrossRef]
- Shiratori, H.; Sakuma, R.; Watanabe, M.; Hashiguchi, H.; Mochida, K.; Sakai, Y.; Nishino, J.; Saijoh, Y.; Whitman, M.; Hamada, H. Two-Step Regulation of Left–Right Asymmetric Expression of Pitx2: Initiation by Nodal Signaling and Maintenance by Nkx2. Mol. Cell 2001, 7, 137–149. [Google Scholar] [CrossRef]
- Welsh, I.C.; Thomsen, M.; Gludish, D.W.; Alfonso-Parra, C.; Bai, Y.; Martin, J.F.; Kurpios, N.A. Integration of Left-Right Pitx2 Transcription and Wnt Signaling Drives Asymmetric Gut Morphogenesis via Daam2. Dev. Cell 2013, 26, 629–644. [Google Scholar] [CrossRef]
- Shiratori, H.; Yashiro, K.; Shen, M.M.; Hamada, H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development 2006, 133, 3015–3025. [Google Scholar] [CrossRef]
- Cervantes, S.; Yamaguchi, T.P.; Hebrok, M. Wnt5a is essential for intestinal elongation in mice. Dev. Biol. 2009, 326, 285–294. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, J.; Gasperoni, J.; Fuller, J.; Grommen, S.V.H.; De Groef, B.; Hogarth, C.; Dworkin, S. Crucial Convolution: Genetic and Molecular Mechanisms of Coiling during Epididymis Formation and Development in Embryogenesis. J. Dev. Biol. 2022, 10, 25. https://doi.org/10.3390/jdb10020025
Wong J, Gasperoni J, Fuller J, Grommen SVH, De Groef B, Hogarth C, Dworkin S. Crucial Convolution: Genetic and Molecular Mechanisms of Coiling during Epididymis Formation and Development in Embryogenesis. Journal of Developmental Biology. 2022; 10(2):25. https://doi.org/10.3390/jdb10020025
Chicago/Turabian StyleWong, Joanne, Jemma Gasperoni, Jarrad Fuller, Sylvia V. H. Grommen, Bert De Groef, Cathryn Hogarth, and Sebastian Dworkin. 2022. "Crucial Convolution: Genetic and Molecular Mechanisms of Coiling during Epididymis Formation and Development in Embryogenesis" Journal of Developmental Biology 10, no. 2: 25. https://doi.org/10.3390/jdb10020025
APA StyleWong, J., Gasperoni, J., Fuller, J., Grommen, S. V. H., De Groef, B., Hogarth, C., & Dworkin, S. (2022). Crucial Convolution: Genetic and Molecular Mechanisms of Coiling during Epididymis Formation and Development in Embryogenesis. Journal of Developmental Biology, 10(2), 25. https://doi.org/10.3390/jdb10020025





