FES Cycling and Closed-Loop Feedback Control for Rehabilitative Human–Robot Interaction
Abstract
1. Introduction
2. Materials and Methods
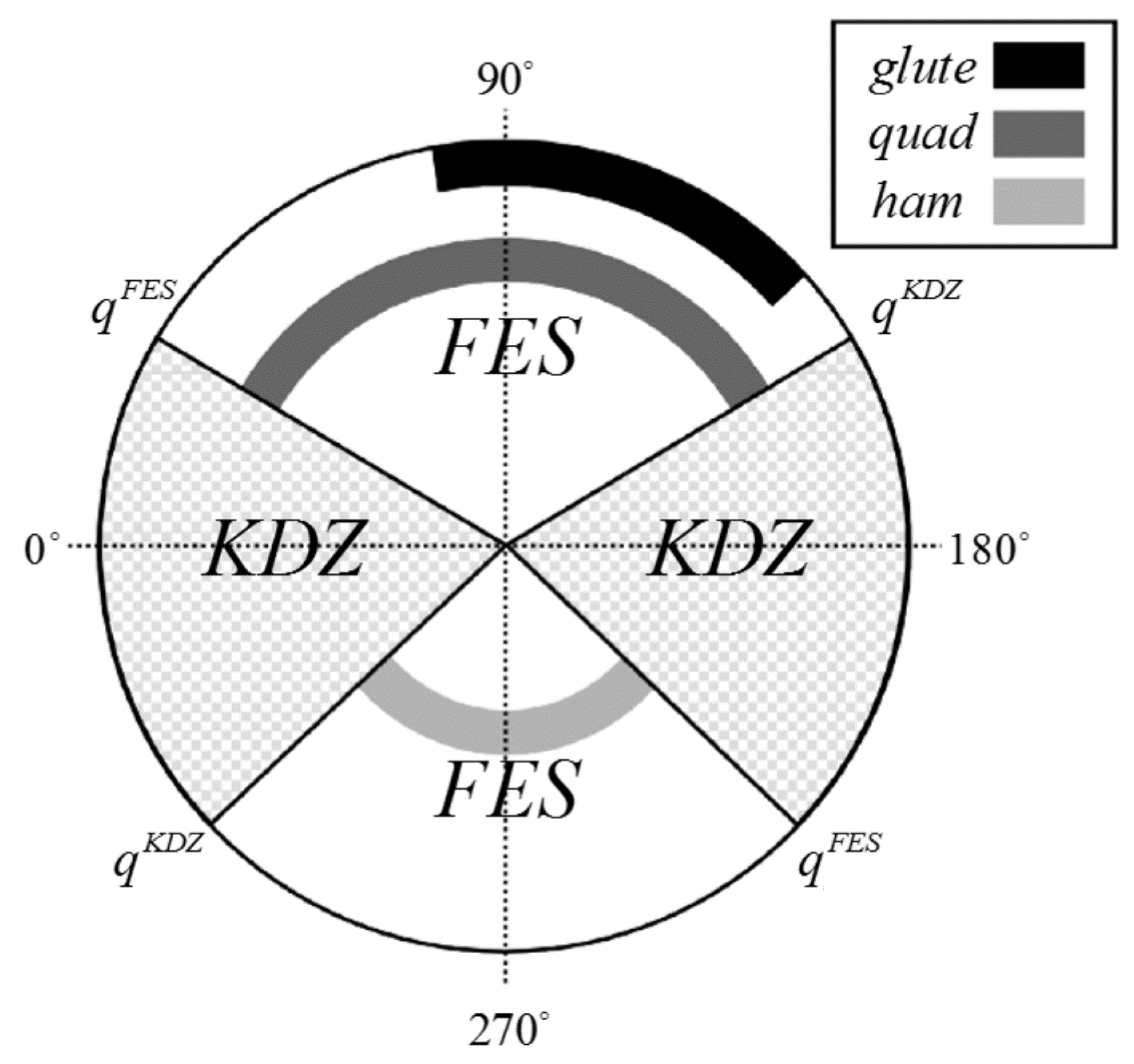
2.1. Functional Electrical Stimulation
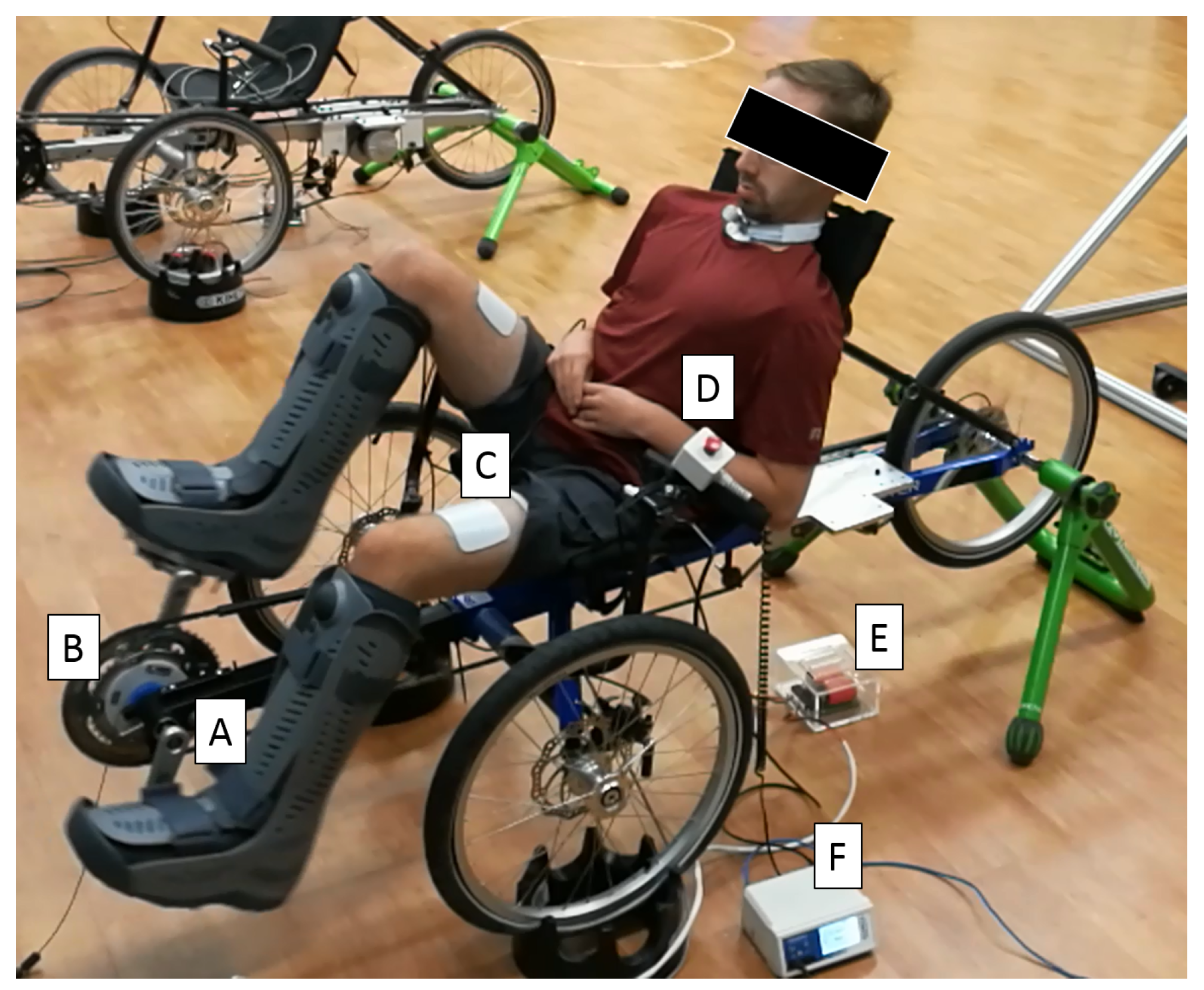
2.2. Single-Crank FES Cycle
2.3. Split-Crank FES Cycle
2.4. Safety Measures
2.5. Dynamics
3. Results
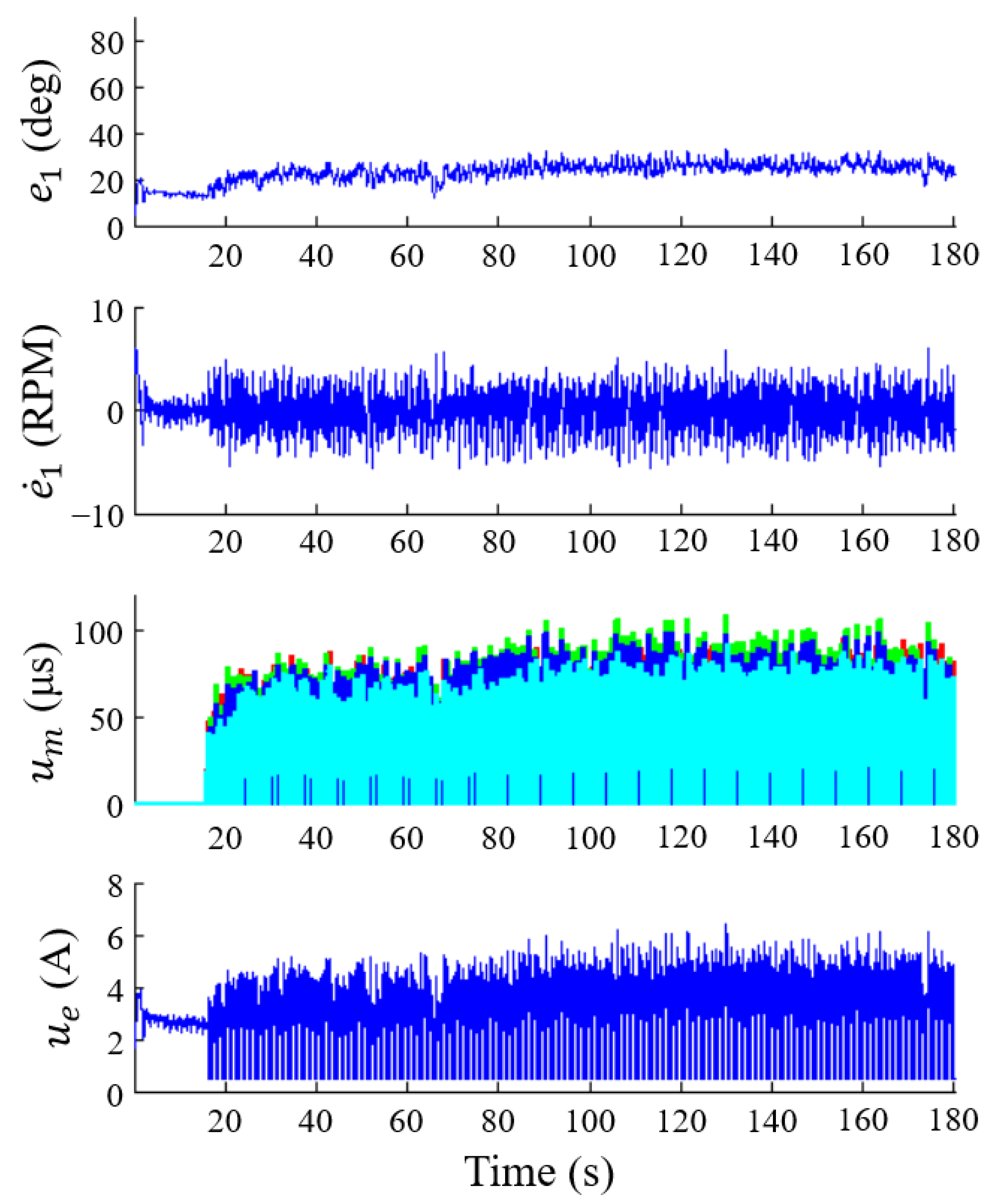
3.1. Cadence Control
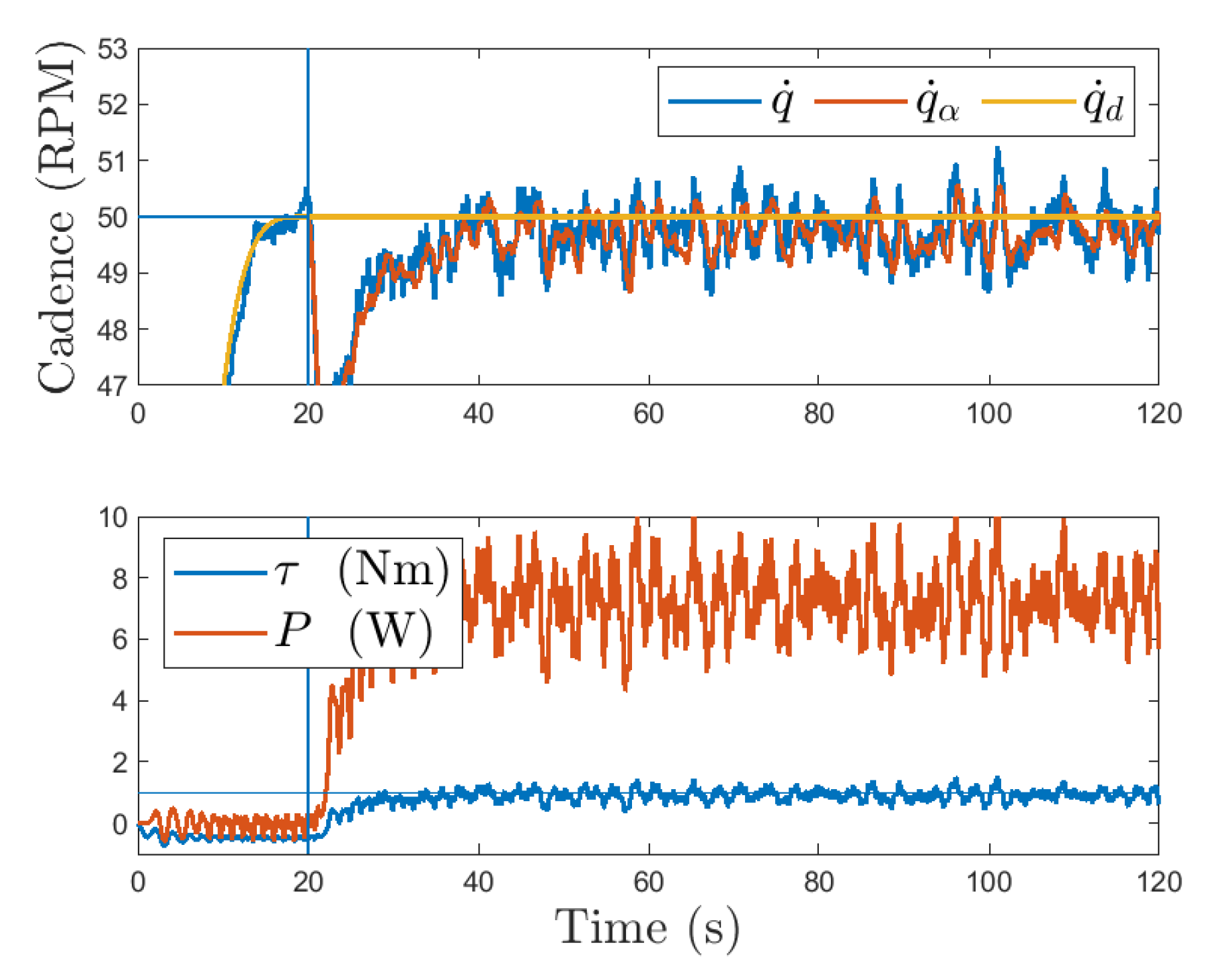
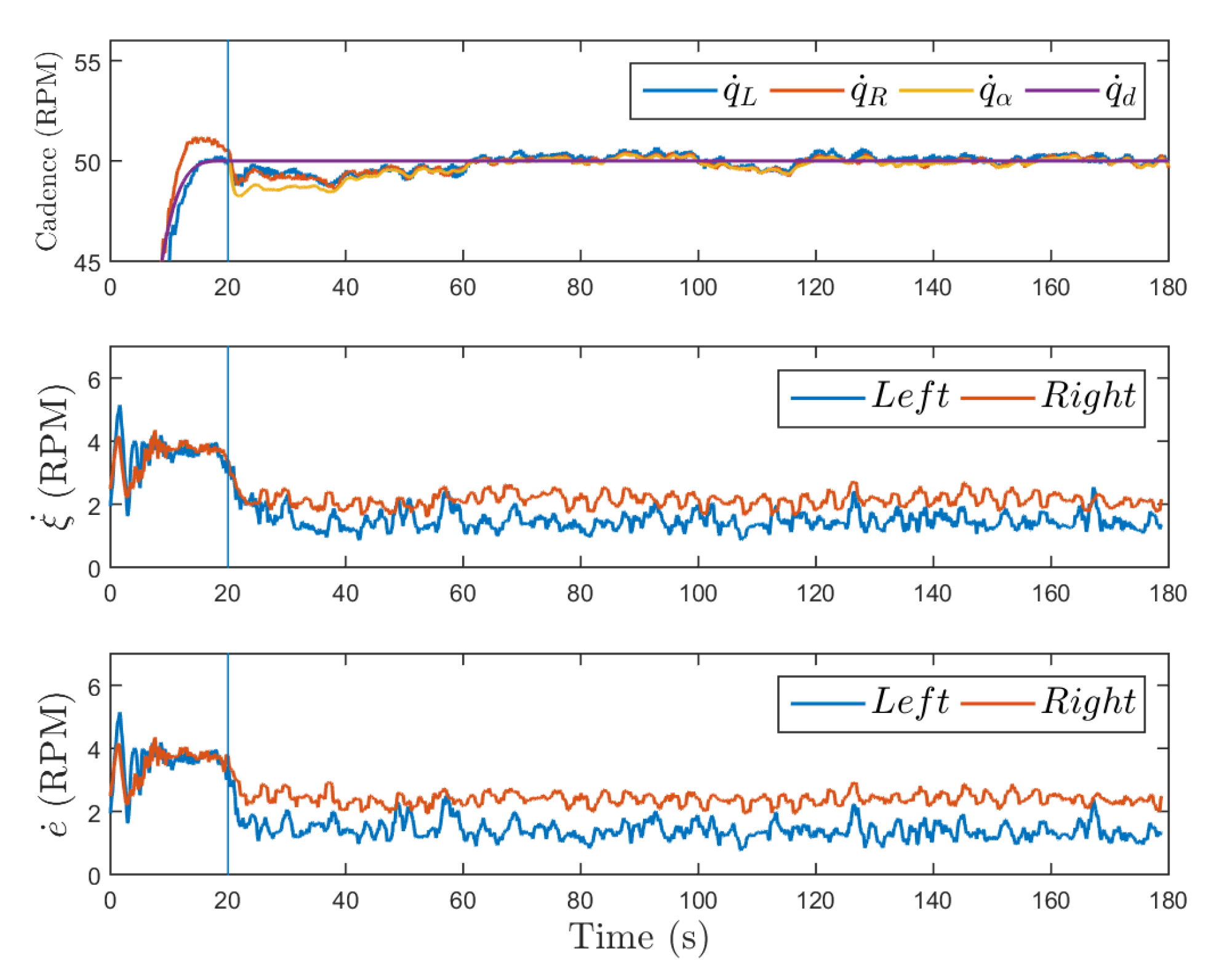
3.1.1. Robust Cadence Control
3.1.2. Adaptive Cadence Control
3.2. Direct Torque Control
3.2.1. Robust Direct Torque Control
3.2.2. Adaptive Direct Torque Control
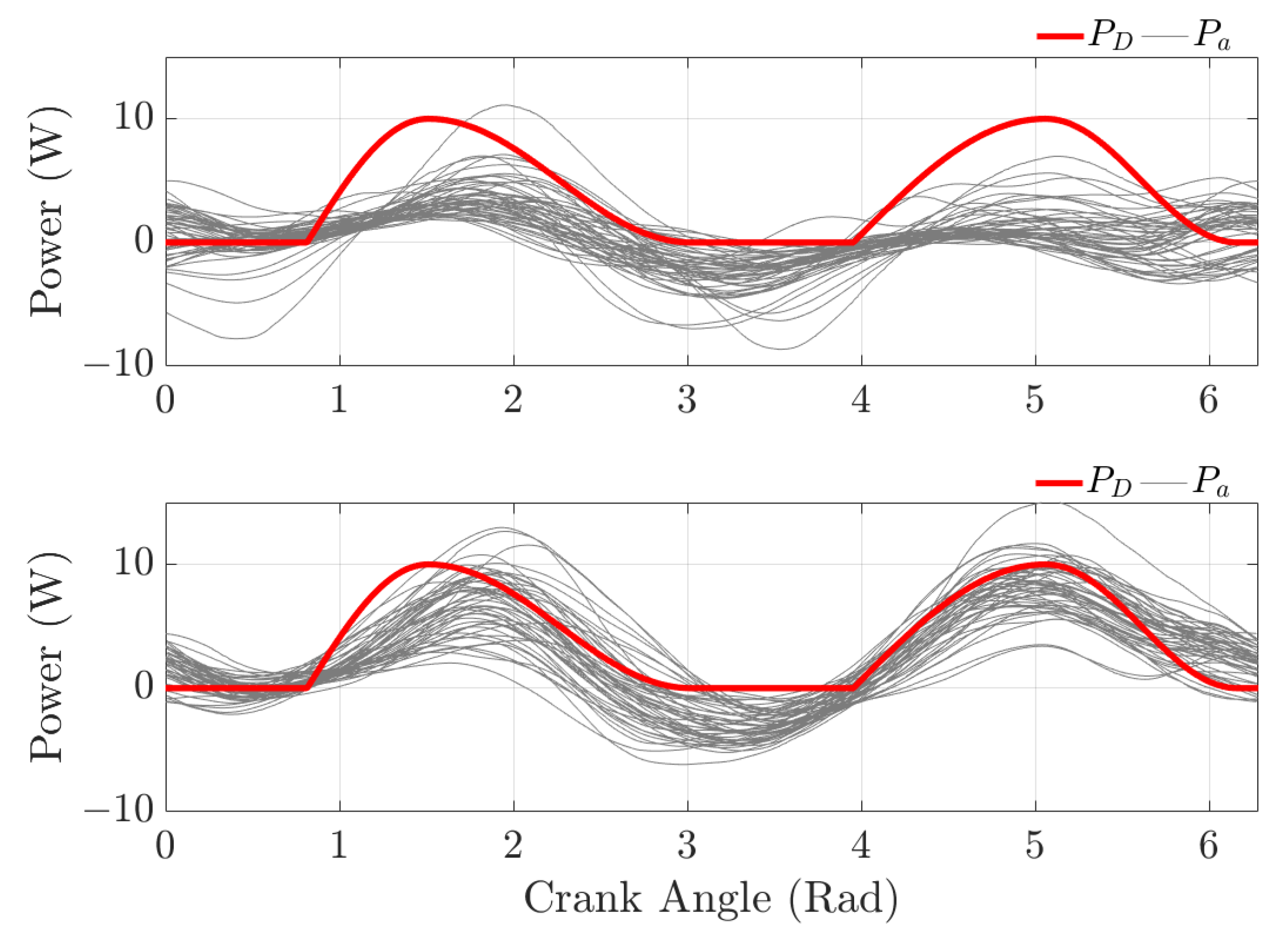
3.3. Indirect Torque Control
3.3.1. Robust Indirect Torque Control
3.3.2. Adaptive Indirect Torque Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallace, S.E.; Kimbarow, M.L. Cognitive Communication Disorders; Plural Publishing: San Diego, CA, USA, 2016; pp. 253–277. [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gilleespie, C.; et al. Heart disease and stroke statistics—2017 update. Circulation 2017, 135, 146–603. [Google Scholar] [CrossRef]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. J. Spinal Cord Med. 2012, 35, 197–198. [Google Scholar] [CrossRef]
- Ho, C.H.; Triolo, R.J.; Elias, A.L.; Kilgore, K.L.; DiMarco, A.F.; Bogie, K.; Vette, A.H.; Audu, M.L.; Kobetic, R.; Chang, S.R.; et al. Functional Electrical Stimulation and Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 631–654. [Google Scholar] [CrossRef]
- Lynch, C.; Popovic, M. Functional Electrical Stimulation. IEEE Control Syst. Mag. 2008, 28, 40–50. [Google Scholar]
- Peckham, P.H.; Knutson, J.S. Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 2005, 7, 327–360. [Google Scholar] [CrossRef]
- Edgerton, V.R.; de Leon, R.D.; Harkema, S.J.; Hodgson, J.A.; London, N.; Reinkensmeyer, D.J.; Roy, R.R.; Talmadge, R.J.; Tillakaratne, N.J.; Timoszyk, W.; et al. Retraining the injured spinal cord. J. Physiol. 2001, 533, 15–22. [Google Scholar] [CrossRef]
- Edgerton, V.; Roy, R.R. Paralysis recovery in humans and model systems. Curr. Opin. Neurobiol. 2002, 12, 658–667. [Google Scholar] [CrossRef]
- Jones, M.L.; Evans, N.; Tefertiller, C.; Backus, D.; Sweatman, M.; Tansey, K.; Morrison, S. Activity-Based Therapy for Recovery of Walking in Individuals With Chronic Spinal Cord Injury: Results From a Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2014, 95, 2239–2246. [Google Scholar] [CrossRef]
- Peng, C.W.; Chen, S.C.; Lai, C.H.; Chen, C.J.; Chen, C.C.; Mizrahi, J.; Handa, Y. Review: Clinical Benefits of Functional Electrical Stimulation Cycling Exercise for Subjects with Central Neurological Impairments. J. Med. Biol. Eng. 2011, 31, 1–11. [Google Scholar] [CrossRef]
- Harrington, A.T.; McRae, C.G.A.; Lee, S.C.K. Evaluation of functional electrical stimulation to assist cycling in four adolescents with spastic cerebral palsy. J. Pediatr. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Griffin, L.; Decker, M.; Hwang, J.; Wang, B.; Kitchen, K.; Ding, Z.; Ivy, J. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2009, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Berry, H.R.; Perret, C.; Saunders, B.A.; Kakebeeke, T.H.; Donaldson, N.D.N.; Allan, D.B.; Hunt, K.J. Cardiorespiratory and Power Adaptation to Stimulated Cycle Training in Paraplegia. Med. Sci. Sports Exerc. 2008, 40, 1573–1580. [Google Scholar] [CrossRef]
- Hunt, K.J.; Stone, B.; Negård, N.O.; Schauer, T.; Fraser, M.H.; Cathcart, A.J.; Ferrario, C.; Ward, S.A.; Grant, S. Control Strategies for Integration of Electric Motor Assist and Functional Electrical Stimulation in Paraplegic Cycling: Utility for Exercise Testing and Mobile Cycling. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 89–101. [Google Scholar] [CrossRef]
- Bellman, M.J.; Downey, R.J.; Parikh, A.; Dixon, W.E. Automatic Control of Cycling Induced by Functional Electrical Stimulation with Electric Motor Assistance. IEEE Trans. Autom. Sci. Eng. 2017, 14, 1225–1234. [Google Scholar] [CrossRef]
- Duenas, V.H.; Cousin, C.A.; Parikh, A.; Freeborn, P.; Fox, E.J.; Dixon, W.E. Motorized and Functional Electrical Stimulation Induced Cycling via Switched Repetitive Learning Control. IEEE Trans. Control Syst. Technol. 2019, 27, 1468–1479. [Google Scholar] [CrossRef]
- Cousin, C.A.; Duenas, V.; Rouse, C.; Bellman, M.; Freeborn, P.; Fox, E.; Dixon, W.E. Closed-Loop Cadence and Instantaneous Power Control on a Motorized Functional Electrical Stimulation Cycle. IEEE Trans. Control Syst. Technol. 2019, 28, 2276–2291. [Google Scholar] [CrossRef]
- Duenas, V.; Cousin, C.A.; Ghanbari, V.; Fox, E.J.; Dixon, W.E. Torque and Cadence Tracking in Functional Electrical Stimulation Induced Cycling using Passivity-Based Spatial Repetitive Learning Control. Automatica 2020, 115, 108852. [Google Scholar] [CrossRef]
- Cousin, C.A.; Rouse, C.A.; Duenas, V.H.; Dixon, W.E. Controlling the Cadence and Admittance of a Functional Electrical Stimulation Cycle. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1181–1192. [Google Scholar] [CrossRef]
- Cousin, C.A.; Rouse, C.A.; Dixon, W.E. Split-Crank Functional Electrical Stimulation Cycling: An Adapting Admitting Rehabilitation Robot. IEEE Trans. Control Syst. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Hogan, N. Impedance Control: An Approach to Manipulation: Part I-Theory, Part II-Implementation, Part III-Applications. J. Dyn. Syst. Meas. Control 1985, 107, 1–24. [Google Scholar] [CrossRef]
- Zhang, J.; Cheah, C.C. Passivity and stability of human–robot interaction control for upper-limb rehabilitation robots. IEEE Trans. Robot. 2015, 31, 233–245. [Google Scholar] [CrossRef]
- Li, Y.; Sam Ge, S.; Yang, C. Learning impedance control for physical robot–environment interaction. Int. J. Control 2012, 85, 182–193. [Google Scholar] [CrossRef]
- Li, Y.; Ge, S.S. Impedance learning for robots interacting with unknown environments. IEEE Trans. Control Syst. Technol. 2014, 22, 1422–1432. [Google Scholar] [CrossRef]
- Ranatunga, I.; Lewis, F.L.; Popa, D.O.; Tousif, S.M. Adaptive Admittance Control for Human–Robot Interaction Using Model Reference Design and Adaptive Inverse Filtering. IEEE Trans. Control Syst. Technol. 2017, 25, 278–285. [Google Scholar] [CrossRef]
- Anaya, F.; Thangavel, P.; Yu, H. Hybrid FES—Robotic gait rehabilitation technologies: A review on mechanical design, actuation, and control strategies. Int. J. Intell. Robot. Appl. 2018, 2, 1–28. [Google Scholar] [CrossRef]
- Del Ama, A.J.; Gil-Agudo, Á.; Pons, J.L.; Moreno, J.C. Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J. Neuroeng. Rehab. 2014, 11, 27. [Google Scholar] [CrossRef]
- Khalil, H.K. Nonlinear Systems, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Slotine, J.J.E.; Li, W. On the adaptive control of robot manipulators. Int. J. Robot. Res. 1987, 6, 49–59. [Google Scholar] [CrossRef]
- Narendra, K.; Annaswamy, A. Stable Adaptive Systems; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- Arimoto, S.; Kawamura, S.; Miyazaki, F. Bettering Operation of Dynamic Systems by Learning: A New Control Theory for Servomechanism or Mechatronics Systems. In Proceedings of the 23rd IEEE Conference on Decision and Control, Las Vegas, NV, USA, 12–14 December 1984; pp. 1064–1069. [Google Scholar]
- Messner, W.; Horowitz, R.; Kao, W.W.; Boals, M. A New Adaptive Learning Rule. IEEE Trans. Autom. Control 1991, 36, 188–197. [Google Scholar] [CrossRef]
- Reed, B. The physiology of neuromuscular electrical stimulation. Pediatr. Phys. Ther. 1997, 9, 96–102. [Google Scholar] [CrossRef]
- Mushahwar, V.K.; Jacobs, P.L.; Normann, R.A.; Triolo, R.J.; Kleitman, N. New Funtional Electrical Stimulation Approaches to Standing and Walking. J. Neural Eng. 2007, 4, S181–S197. [Google Scholar] [CrossRef]
- Kobetic, R.; To, C.S.; Schnellenberger, J.R.; Audu, M.L.; Bulea, T.C.; Gaudio, R.; Pinault, G.; Tashman, S.; Triolo, R.J. Development of hybrid orthosis for standing, walking, and stair climbing after spinal cord injury. J. Rehabil. Res. Dev. 2009, 46, 447–462. [Google Scholar] [CrossRef]
- Alibeji, N.A.; Molazadeh, V.; Dicianno, B.E.; Sharma, N. A Control Scheme That Uses Dynamic Postural Synergies to Coordinate a Hybrid Walking Neuroprosthesis: Theory and Experiments. Front. Neurosci. 2018, 12, 159. [Google Scholar] [CrossRef]
- Bo, A.; de Fonseca, L.; de Sousa, A. FES-induced co-activation of antagonist muscles for upper limb control and disturbance rejection. Med. Eng. Phys. 2016, 38, 1176–1184. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular Electrical Stimulation for Skeletal Muscle Function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Binder-Macleod, S.A.; Halden, E.E.; Jungles, K.A. Effects of stimulation intensity on the physiological responses of human motor units. Med. Sci. Sports Exerc. 1995, 27, 556–565. [Google Scholar] [CrossRef]
- Doucet, B.M.; Griffin, L. Maximal versus submaximal intensity stimulation with variable patterns. Muscle Nerve 2008, 37, 770–777. [Google Scholar] [CrossRef]
- Kebaetse, M.B.; Binder-Macleod, S.A. Strategies that improve human skeletal muscle performance during repetitive, non-isometric contractions. Pflügers Arch. 2004, 448, 525–532. [Google Scholar] [CrossRef]
- Karu, Z.Z.; Durfee, W.K.; Barzilai, A.M. Reducing muscle fatigue in FES applications by stimulating with N-let pulse trains. IEEE Trans. Biomed. Eng. 1995, 42, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Popović, L.Z.; Malesevic, N.M. Muscle fatigue of quadriceps in paraplegics: Comparison between single vs. multi-pad electrode surface stimulation. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 2–6 September 2009; pp. 6785–6788. [Google Scholar] [CrossRef]
- Merletti, R.; Knaflitz, M.; De Luca, C.J. Electrically evoked myoelectric signals. Crit. Rev. Biomed. Eng. 1992, 19, 293–340. [Google Scholar] [PubMed]
- Downey, R.; Merad, M.; Gonzalez, E.; Dixon, W. The Time-Varying Nature of Electromechanical Delay and Muscle Control Effectiveness in Response to Stimulation-Induced Fatigue. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Binder-Macleod, S.A.; McLaughlin, W.A. Effects of asynchronous stimulation on the human quadriceps femoris muscle. Arch. Phys. Med. Rehabil. 1997, 78, 294–297. [Google Scholar] [CrossRef]
- Figoni, S.F.; Rodgers, M.M.; Glaser, R.M.; Hooker, S.P.; Feghri, P.D.; Ezenwa, B.N.; Mathews, T.; Suryaprasad, A.G.; Gupta, S.C. Physiologic responses of paraplegics and quadriplegics to passive and active leg cycle ergometry. J. Am. Paraplegia Soc. 1990, 13, 33–39. [Google Scholar] [CrossRef]
- Gregory, C.M.; Bickel, C.S. Recruitment Patterns in Human Skeletal Muscle During Electrical Stimulation. Phys. Ther. 2005, 85, 358–364. [Google Scholar] [CrossRef]
- Bickel, C.S.; Gregory, C.M.; Dean, J.C. Motor unit recruitment during neuromuscular electrical stimulation: A critical appraisal. Eur. J. Appl. Physiol. 2011, 111, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Schauer, T.; Negård, N.O.; Previdi, F.; Hunt, K.J.; Fraser, M.H.; Ferchland, E.; Raisch, J. Online identification and nonlinear control of the electrically stimulated quadriceps muscle. Control Eng. Pract. 2005, 13, 1207–1219. [Google Scholar] [CrossRef]
- Dolbow, D.; Gorgey, A.; Ketchum, J.; Gater, D. Home-based functional electrical stimulation cycling enhances quality of life in individuals with spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 324–329. [Google Scholar] [CrossRef]
- Ambrosini, E.; Ferrante, S.; Ferrigno, G.; Molteni, F.; Pedrocchi, A. Cycling Induced by Electrical Stimulation Improves Muscle Activation and Symmetry During Pedaling in Hemiparetic Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; Ferrante, S.; Schauer, T.; Ferrigno, G.; Molteni, F.; Pedrocchi, A. Design of a symmetry controller for cycling induced by electrical stimulation: Preliminary results on post-acute stroke patients. Artif. Organs 2010, 34, 663–667. [Google Scholar] [CrossRef]
- Van der Loos, M.; Worthen-Chaudhari, L.; Schwandt, D. A Split-Crank Bicycle Ergometer Uses Servomotors to Provide Programmable Pedal Forces for Studies in Human Biomechanics. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 445–452. [Google Scholar] [CrossRef]
- Ting, L.; Kautz, S.; Brown, D.; Zajac, F. Contralateral movement and extensor force generation alter flexion phase muscle coordination in pedaling. J. Neurophysiol. 2000, 83, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
- Elmer, S.J.; McDaniel, J.; Martin, J.C. Biomechanics of counterweighted one-legged cycling. J. Appl. Biomech. 2016, 32, 78–85. [Google Scholar] [CrossRef]
- Bellman, M. Control of Cycling Induced by Functional Electrical Stimulation: A Switched Systems Theory Approach. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2015. [Google Scholar]
- Idsø, E.S. Development of a Mathematical Model of a Rider-Tricycle System; Technical Report; Department of Engineering Cybernetics, Norwegian University of Science and Technology: Trondheim, Norway, 2002. [Google Scholar]
- Ferrarin, M.; Pedotti, A. The Relationship Between Electrical Stimulus and Joint Torque: A Dynamic Model. IEEE Trans. Rehabil. Eng. 2000, 8, 342–352. [Google Scholar] [CrossRef]
- Ferrarin, M.; Palazzo, F.; Riener, R.; Quintern, J. Model-Based Control of FES-Induced Single Joint Movements. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 245–256. [Google Scholar] [CrossRef]
- Riener, R.; Ferrarin, F.; Pavan, E.E.; Frigo, C.A. Patient-Driven Control of FES-Supported Standing Up and Sitting Down: Experimental Results. IEEE Trans. Rehabil. Eng. 2000, 8, 523–529. [Google Scholar] [CrossRef]
- Hunt, K.J.; Fang, J.; Saengsuwan, J.; Grob, M.; Laubacher, M. On the efficiency of FES cycling: A framework and systematic review. Technol. Health Care 2012, 20, 395–422. [Google Scholar] [CrossRef]
- Grohler, M.; Angeli, T.; Eberharter, T.; Lugner, P.; Mayr, W.; Hofer, C. Test bed with force-measuring crank for static and dynamic investigations on cycling by means of functional electrical stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 169–180. [Google Scholar] [CrossRef]
- Newham, D.; Donaldson, N.D.N. FES cycling. Oper. Neuromod. 2007, 395–402. [Google Scholar] [CrossRef]
- Duffell, L.D.; Donaldson, N.D.N.; Perkins, T.A.; Rushton, D.N.; Hunt, K.J.; Kakebeeke, T.H.; Newham, D.J. Long-term intensive electrically stimulated cycling by spinal cord–injured people: Effect on muscle properties and their relation to power output. Muscle Nerve 2008, 38, 1304–1311. [Google Scholar] [CrossRef]
- Berkelmans, R. FES cycling. J. Autom. Control 2008, 18, 73–76. [Google Scholar] [CrossRef]
- Szecsi, J.; Straube, A.; Fornusek, C. Comparison of the pedalling performance induced by magnetic and electrical stimulation cycle ergometry in able-bodied subjects. Med. Eng. Phys. 2014, 36, 484–489. [Google Scholar] [CrossRef]
- Van der Schaft, A. L2-Gain and Passivity Techniques in Nonlinear Control; Springer-Verlag London Ltd.: London, UK, 1996. [Google Scholar]
- Krstic, M.; Kanellakopoulos, I.; Kokotovic, P.V. Nonlinear and Adaptive Control Design; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Liberzon, D. Switching in Systems and Control; Birkhauser: Boston, MA, USA, 2003. [Google Scholar]
- Stegman, P.; Crawford, C.S.; Andujar, M.; Nijholt, A.; Gilbert, J.E. Brain-Computer Interface Software: A Review and Discussion. IEEE Trans. Hum. Mach. Syst. 2020, 50, 101–115. [Google Scholar] [CrossRef]






| Ref. | Motor Assignment | Muscle Assignment | Setpoint * | Tracking Errors (Able-Bodied) | Tracking Errors (NI) | Participants | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Tracking Objective | Type | Objective | Torque | Cadence | Torque | Cadence | Torque | Able | NI | |
| (Nm) | (RPM) | (Nm) | (RPM) | (Nm) | |||||||
| [15] | Robust | Cadence | Robust | Cadence | N/A | 0.00 ± 2.91 | N/A | N/A | N/A | 5 | 0 |
| [16] | Adaptive | Cadence | Adaptive | Cadence | N/A | 0.03 ± 3.70 | N/A | 0.04 ± 3.31 | N/A | 5 | 3 |
| [17] | Robust | Cadence | Robust | Direct Torque | 1.91 | 0.01 ± 1.03 | 0.00 ± 0.17 | 0.02 ± 1.87 | 0.00 ± 0.47 | 7 | 6 |
| [18] | Robust | Cadence | Adaptive | Direct Torque | 1.91 | 0.02 ± 1.17 | 0.42 ± 0.30 | 0.01 ± 1.23 | 0.21 ± 0.11 | 5 | 3 |
| [19] | Robust | Indirect Torque | Robust | Cadence | 0.50, 0.00 | 1.75 ± 2.06 | −0.30 ± 0.55 | 4.76 ± 1.36 | −0.53 ± 0.48 | 3 | 4 |
| [20] | Adaptive | Indirect Torque | Robust | Cadence | 0.50, 0.23 | 1.03 ± 1.47 | −0.33 ± 3.60 | 0.77 ± 1.66 | −0.20 ± 2.51 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cousin, C.; Duenas, V.; Dixon, W. FES Cycling and Closed-Loop Feedback Control for Rehabilitative Human–Robot Interaction. Robotics 2021, 10, 61. https://doi.org/10.3390/robotics10020061
Cousin C, Duenas V, Dixon W. FES Cycling and Closed-Loop Feedback Control for Rehabilitative Human–Robot Interaction. Robotics. 2021; 10(2):61. https://doi.org/10.3390/robotics10020061
Chicago/Turabian StyleCousin, Christian, Victor Duenas, and Warren Dixon. 2021. "FES Cycling and Closed-Loop Feedback Control for Rehabilitative Human–Robot Interaction" Robotics 10, no. 2: 61. https://doi.org/10.3390/robotics10020061
APA StyleCousin, C., Duenas, V., & Dixon, W. (2021). FES Cycling and Closed-Loop Feedback Control for Rehabilitative Human–Robot Interaction. Robotics, 10(2), 61. https://doi.org/10.3390/robotics10020061







