Activity of β-Caryophyllene Oxide and Benznidazole Mixture Against Trypanosoma cruzi and In Silico Prediction of Anti-Trypanocidal Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Benznidazole and β-Caryophyllene Oxide
2.2. Cell Culture
2.3. Cell Viability Assay in Murine Macrophages
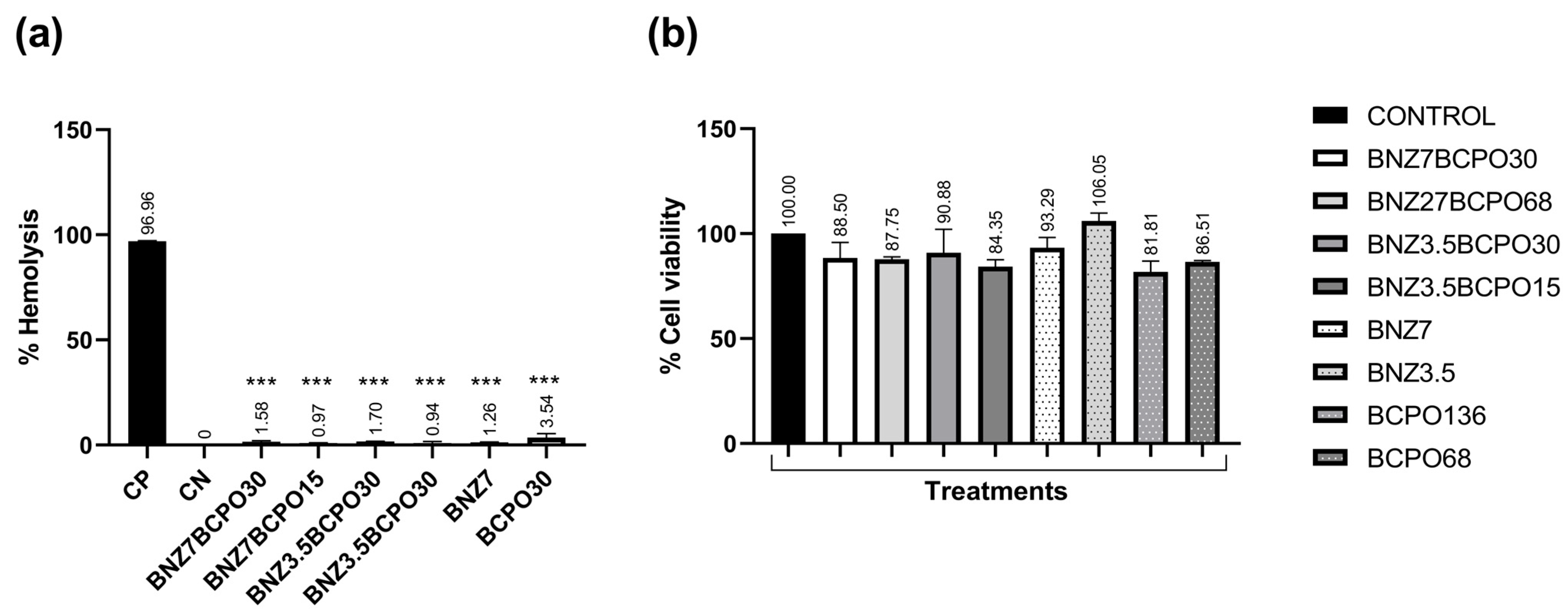
2.4. Hemolysis Assay
2.5. Trypanocidal Assay
2.6. Drug Combination Analysis
2.7. Bioinformatics Tools
2.8. Ligands
2.9. Molecular Modeling of TcABCG1
2.10. Docking Analysis
2.11. Statistical Analyses
3. Results
3.1. Cytotoxic Activity
3.2. Trypanocidal Activity
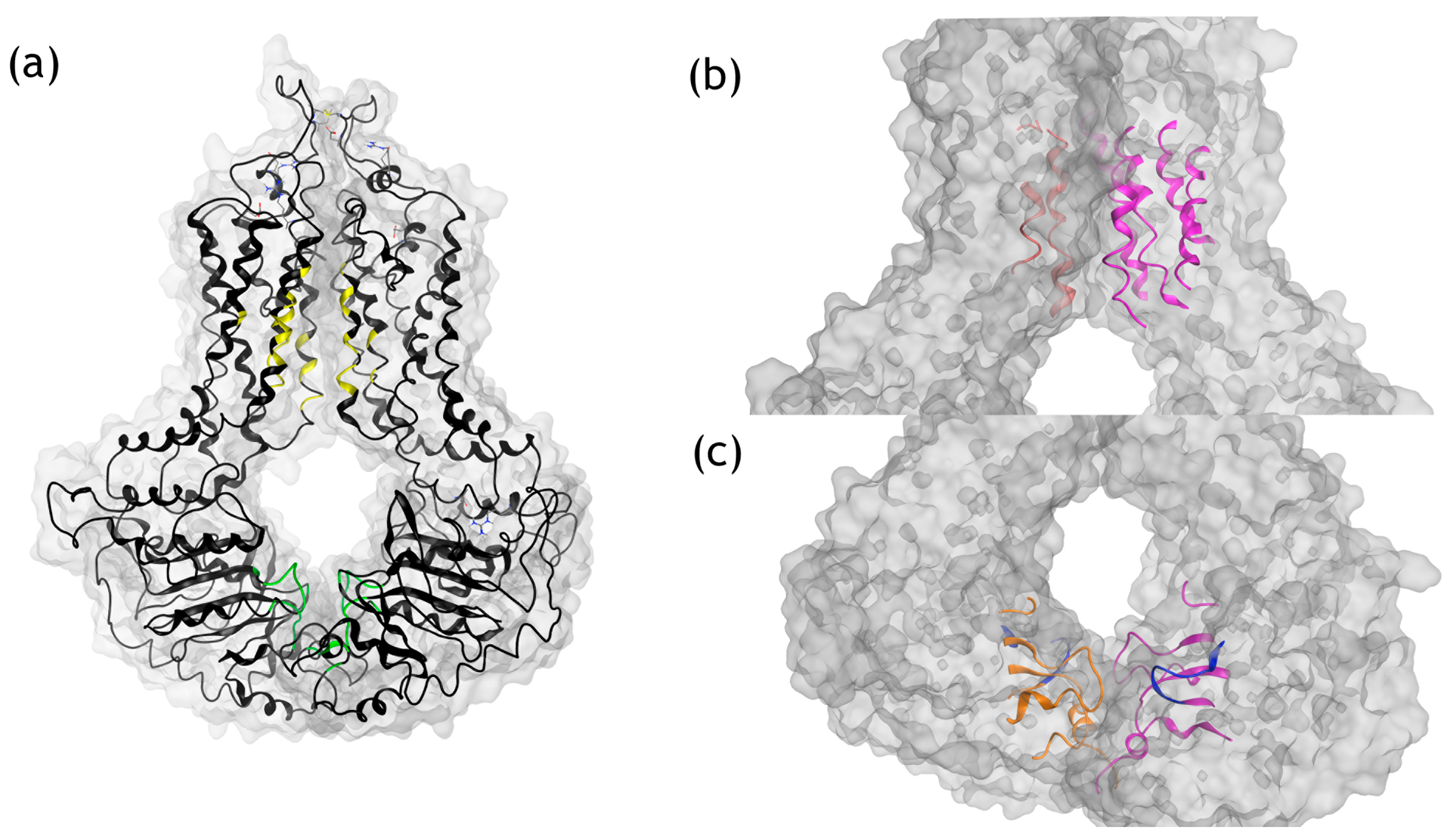
3.3. Molecular Modeling Analysis of TcABCG1
3.4. Docking of β-Caryophyllene Oxide in the TcABCG1 Pockets
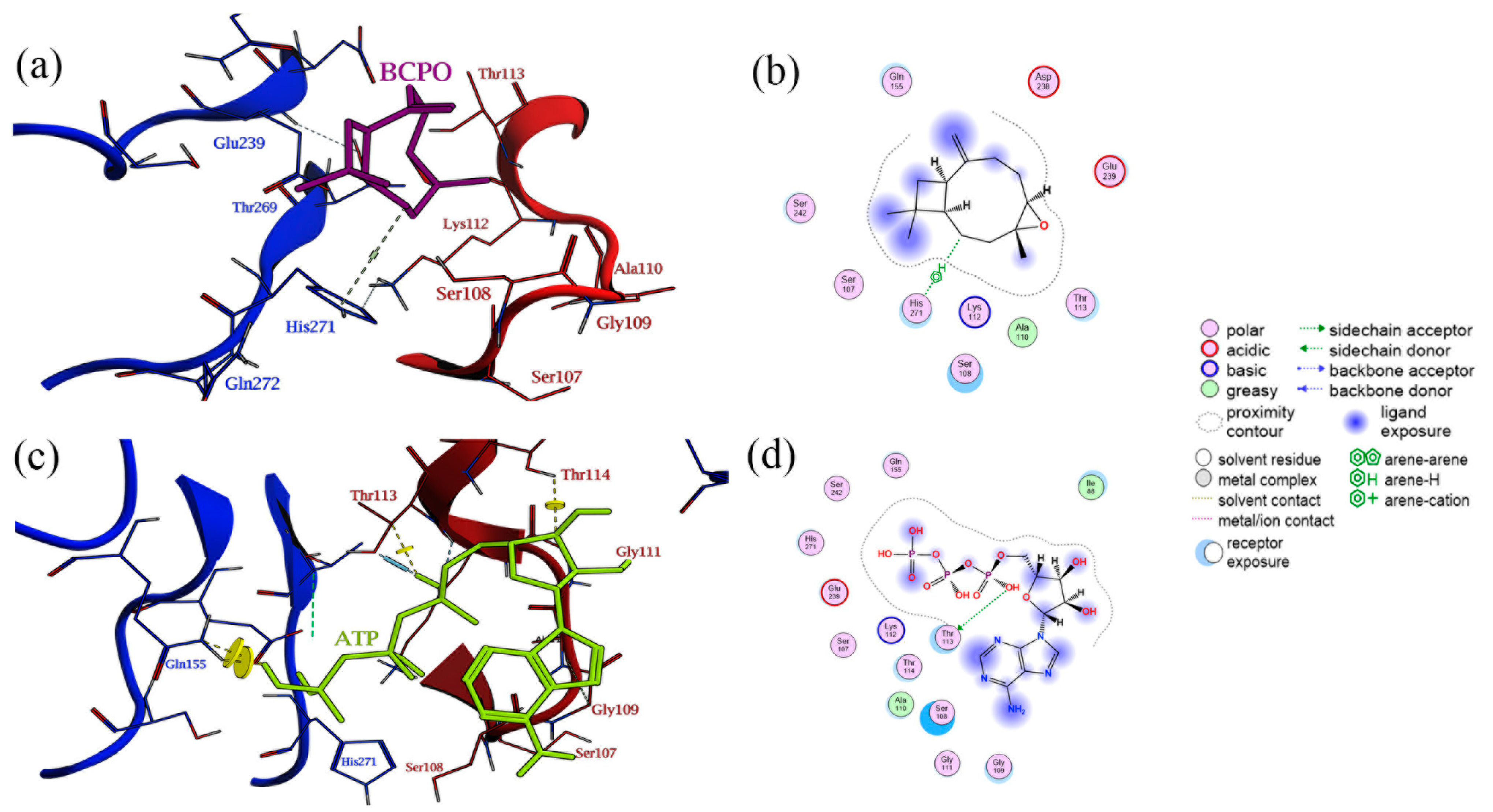
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ATP | Adenosine triphosphate |
| BCPO | β-Caryophyllene oxide |
| BNZ | Benznidazole |
| CD | Chagas disease |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribonucleotide Acid |
| FBS | Fetal bovine serum |
| FIC | Fractional inhibitory concentration |
| LIT | Liver-infusion tryptose |
| PBS | Phosphate-buffer saline |
| RMSD | Root Mean Square Deviation |
| TcPgP | T. cruzi P-Glycoprotein |
| T. cruzi | Trypanosoma cruzi |
References
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N.; Luquetti, A.O. Chagas Disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a Bug and a Hard Place: Trypanosoma Cruzi Genetic Diversity and the Clinical Outcomes of Chagas Disease. Expert Rev. Anti. Infect. Ther. 2015, 13, 995–1029. [Google Scholar] [CrossRef]
- Jaramillo, L.; Mejía, C.; Martínez, L.; Vera, S. Enfermedad de Chagas: Una Mirada Alternativa Al Tratamiento. Rev. Cub. Med. Trop. 2017, 69, 1–13. [Google Scholar]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies Against Trypanosoma Cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef]
- Vela, A.; Coral-Almeida Id, M.; Sereno Id, D.; Costales Id, J.A.; Id, C.B.; Fré Dé Rique Brenièreid, S. In Vitro Susceptibility of Trypanosoma Cruzi Discrete Typing Units (DTUs) to Benznidazole: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009269. [Google Scholar] [CrossRef]
- Ashley, E.A.; Phyo, A.P. Drugs in Development for Malaria. Drugs 2018, 78, 861–879. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug Combinations: A Strategy to Extend the Life of Antibiotics in the 21st Century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B. Combination Therapy Strategies for the Treatment of Malaria. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef] [PubMed]
- Polanco-Hernández, G.; Escalante-Erosa, F.; García-Sosa, K.; Rosado, M.E.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Giménez-Turba, A.; Salamanca, E.; Peña-Rodríguez, L.M. Synergistic Effect of Lupenone and Caryophyllene Oxide against Trypanosoma Cruzi. Evid.-Based Complement. Altern. Med. 2013, 2013, 435398. [Google Scholar] [CrossRef] [PubMed]
- Moreno, É.M.; Leal, S.M.; Stashenko, E.E.; García, L.T. Induction of Programmed Cell Death in Trypanosoma Cruzi by Lippia Alba Essential Oils and Their Major and Synergistic Terpenes (Citral, Limonene and Caryophyllene Oxide). BMC Complement. Altern. Med. 2018, 18, 225. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Gullì, M.; Facchinetti, R.; Minacori, M.; Mancinelli, R.; Percaccio, E.; Scuderi, C.; Eufemi, M.; Di Sotto, A. Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis. Pharmaceutics 2022, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, R.; Saleh, E.; Hashim, A.; Soliman, N.; Dallah, A.; Elrasheed, A.; Elakraa, G. The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy. Front. Pharmacol. 2017, 7, 237140. [Google Scholar] [CrossRef] [PubMed]
- da Costa, K.M.; Valente, R.C.; Salustiano, E.J.; Gentile, L.B.; De-Lima, L.F.; Mendonça-Previato, L.; Previato, J.O. Functional Characterization of ABCC Proteins from Trypanosoma Cruzi and Their Involvement with Thiol Transport. Front. Microbiol. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Araujo, R.G.A.; Moreno, M.; Franco, J.; Aguiar, P.H.N.; Nunes, S.L.; Silva, M.N.; Ienne, S.; Machado, C.R.; Brandão, A. A Novel ABCG-like Transporter of Trypanosoma Cruzi Is Involved in Natural Resistance to Benznidazole. Mem. Inst. Oswaldo Cruz 2015, 110, 433–444. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Perdomo, V.G.; Luquita, M.G.; Villanueva, S.S.M.; Arias, A.; Theile, D.; Weiss, J.; Mottino, A.D.; Ruiz, M.L.; Catania, V.A. Regulation of Biotransformation Systems and ABC Transporters by Benznidazole in HepG2 Cells: Involvement of Pregnane X-Receptor. PLoS Negl. Trop. Dis. 2012, 6, e1951. [Google Scholar] [CrossRef]
- Exeltis USA, Inc. Benznidazole Tablets: Highlights of Prescribing Information. benznidazoletablets.com. Available online: https://www.benznidazoletablets.com/assets/pdf/draft-pi.pdf (accessed on 21 August 2025).
- Bayne, K. Revised Guide for the Care and Use of Laboratory Animals Available, 8th ed.; The National Academies Press: Washington, DC, USA, 1996; Volume 39. [Google Scholar]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Diario Oficial de la Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=762506&fecha=22/08/2001#gsc.tab=0 (accessed on 21 August 2025).
- Tamayo, J.; Chan, I.; Arana, V.; Villa, F.; Torres, J.; Araujo, J.; Aguilar, F.; Rejón, M.; Castro, N.; Vargas, R.; et al. In Vitro and in Vivo Anti-Inflammatory Properties of Mayan Propolis. Eur. J. Inflamm. 2020, 6, 951–952. [Google Scholar] [CrossRef]
- del Guzmán Marín, E.; Zavala Castro, J.; Acosta Viana, K.; Rosado Barrera, M. Importancia de La Caracterización de Cepas de Trypanosoma Cruzi. Rev. Biomédica 1999, 10, 177–184. [Google Scholar]
- Martínez-Pavetti, A.M.; Infanzón, B.; Rojas, A.; Aria, L.; López, L.; Meza, T.; de Guillen, I.; Acosta de Hetter, M.E. Antigenic Profile Characterization of Soluble Protein Extracts from Two Trypanosoma Cruzi Strains. Mem. Del Inst. Investig. En Cienc. La Salud 2017, 15, 27–34. [Google Scholar] [CrossRef]
- Fajardo, E.F.; Cabrine-Santos, M.; Ferreira, K.A.M.; Lages-Silva, E.; Ramírez, L.E.; Pedrosa, A.L. Semisolid Liver Infusion Tryptose Supplemented with Human Urine Allows Growth and Isolation of Trypanosoma Cruzi and Trypanosoma Rangeli Clonal Lineages. Rev. Soc. Bras. Med. Trop. 2016, 49, 369–372. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Krichen, F.; Karoud, W.; Sila, A.; Abdelmalek, B.E.; Ghorbel, R.; Ellouz-Chaabouni, S.; Bougatef, A. Extraction, Characterization and Antimicrobial Activity of Sulfated Polysaccharides from Fish Skins. Int. J. Biol. Macromol. 2015, 75, 283–289. [Google Scholar] [CrossRef]
- Barbosa, J.M.C.; Pedra-Rezende, Y.; Pereira, L.D.; de Melo, T.G.; Barbosa, H.S.; Lannes-Vieira, J.; de Castro, S.L.; Daliry, A.; Salomão, K. Benznidazole and Amiodarone Combined Treatment Attenuates Cytoskeletal Damage in Trypanosoma Cruzi-Infected Cardiac Cells. Front. Cell. Infect. Microbiol. 2022, 12, 975931. [Google Scholar] [CrossRef]
- Mary, O.G.; Zaituni, M.S.; Faith, M.P.; Lughano, K.J.M.; Robinson, M.H.; John, O.E.; Box, P.O.; Box, P.O.; Frederiksberg, C. Antibacterial effects of single and combined crude extracts of synadenium glaucescens and commiphora swynnertonii. Afr. J. Infect. Dis. 2022, 16, 9–16. [Google Scholar] [CrossRef]
- Willis, J.A.; Cheburkanov, V.; Chen, S.; Soares, J.M.; Kassab, G.; Blanco, K.C.; Bagnato, V.S.; de Figueiredo, P.; Yakovlev, V.V. Breaking down Antibiotic Resistance in Methicillin-Resistant Staphylococcus Aureus: Combining Antimicrobial Photodynamic and Antibiotic Treatments. Proc. Natl. Acad. Sci. USA 2022, 119, e2208378119. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Farrell, C.M.; Feldgarden, M.; Fine, A.M.; Funk, K.; et al. Database Resources of the National Center for Biotechnology Information in 2023. Nucleic Acids Res. 2023, 51, D29–D38. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Copley, R.R.; Doerks, T.; Ponting, C.P.; Bork, P. SMART: A Web-Based Tool for the Study of Genetically Mobile Domains. Nucleic Acids Res. 2000, 28, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; De Castro, E.; Langendijk-Genevaux, P.S.; Pagni, M.; Sigrist, C.J.A. The PROSITE Database. Nucleic Acids Res. 2006, 34, 227–230. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE); 2022.02; Chemical Computing Group ULC: Montreal, QC, Canada, 2023.
- Dionisio, K.L.; Phillips, K.; Price, P.S.; Grulke, C.M.; Williams, A.; Biryol, D.; Hong, T.; Isaacs, K.K. Data Descriptor: The Chemical and Products Database, a Resource for Exposure-Relevant Data on Chemicals in Consumer Products. Sci. Data 2018, 5, 180125. [Google Scholar] [CrossRef]
- Mahgoub, R.E.; Atatreh, N.; Ghattas, M.A. Using Filters in Virtual Screening: A Comprehensive Guide to Minimize Errors and Maximize Efficiency. In Annual Reports in Medicinal Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 59, pp. 99–136. [Google Scholar] [CrossRef]
- Liang, J.; Edelsbrunner, H.; Woodward, C. Anatomy of Protein Pockets and Cavities: Measurement of Binding Site Geometry and Implications for Ligand Design. Protein Sci. 1998, 7, 1884–1897. [Google Scholar] [CrossRef]
- Luna-Vázquez-gómez, R.; Arellano-García, M.E.; García-Ramos, J.C.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Casillas-Figueroa, F.; Ruiz-Ruiz, B.; Bogdanchikova, N.; Pestryakov, A. Hemolysis of Human Erythrocytes by ArgovitTM Agnps from Healthy and Diabetic Donors: An in Vitro Study. Materials 2021, 14, 2792. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Device—Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Pagano, M.; Faggio, C. The Use of Erythrocyte Fragility to Assess Xenobiotic Cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Diniz, L.d.F.; Gonçalves, K.R.; WonDollinger, R.S.; Assíria, T.; Ribeiro, I.; Bahia, M.T. Synergic Effect of Allopurinol in Combination with Nitroheterocyclic Compounds against Trypanosoma Cruzi. Antimicrob. Agents Chemother. 2019, 63, e02264-18. [Google Scholar] [CrossRef]
- Kessler, R.L.; Contreras, V.T.; Marliére, N.P.; Aparecida Guarneri, A.; Villamizar Silva, L.H.; Mazzarotto, G.A.C.A.; Batista, M.; Soccol, V.T.; Krieger, M.A.; Probst, C.M. Recently Differentiated Epimastigotes from Trypanosoma Cruzi Are Infective to the Mammalian Host. Mol. Microbiol. 2017, 104, 712–736. [Google Scholar] [CrossRef]
- De Souza, W.; Barrias, E.S. May the Epimastigote Form of Trypanosoma Cruzi Be Infective? Acta Trop. 2020, 212, 105688. [Google Scholar] [CrossRef]
- Jäger, A.V.; Muiá, R.P.; Campetella, O. Stage-Specific Expression of Trypanosoma Cruzi Trans-Sialidase Involves Highly Conserved 3′ Untranslated Regions. FEMS Microbiol. Lett. 2008, 283, 182–188. [Google Scholar] [CrossRef]
- Chiurillo, M.A.; Ahmed, M.; González, C.; Raja, A.; Lander, N. Gene Editing of Putative CAMP and Ca2+-Regulated Proteins Using an Efficient Cloning-Free CRISPR/Cas9 System in Trypanosoma Cruzi. J. Eukaryot. Microbiol. 2023, 70, e12999. [Google Scholar] [CrossRef]
- Rivera, H.N.; McAuliffe, I.; Aderohunmu, T.L.; Wiegand, R.E.; Montgomery, S.P.; Bradbury, R.S.; Handali, S. Evaluation of the Performance of Ortho T. Cruzi ELISA Test System for the Detection of Antibodies to Trypanosoma Cruzi. J. Clin. Microbiol. 2022, 60, e00134-22. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Ferreira, R.C.; Ienne, S.; Zingales, B. ABCG-like Transporter of Trypanosoma Cruzi Involved in Benznidazole Resistance: Gene Polymorphisms Disclose Inter-Strain Intragenic Recombination in Hybrid Isolates. Infect. Genet. Evol. 2015, 31, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Quebrada Palacio, L.P.; González, M.N.; Hernandez-Vasquez, Y.; Perrone, A.E.; Parodi-Talice, A.; Bua, J.; Postan, M. Phenotypic Diversity and Drug Susceptibility of Trypanosoma Cruzi TcV Clinical Isolates. PLoS ONE 2018, 13, e0203462. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; D’ávila, D.A.; Silva, M.N.; Galvão, L.M.; Macedo, A.M.; Chiari, E.; Gontijo, E.D.; Zingales, B. Trypanosoma Cruzi Benznidazole Susceptibility In Vitro Does Not Predict the Therapeutic Outcome of Human Chagas Disease. Mem. Inst. Oswaldo Cruz 2010, 105, 918–924. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Fan, C.; Chen, J.; Xu, S.; Liu, Z. Rationally Screened and Designed ABCG2-Binding Aptamers for Targeting Cancer Stem Cells and Reversing Multidrug Resistance. Anal. Chem. 2022, 94, 7375–7382. [Google Scholar] [CrossRef]
- Yu, Q.; Ni, D.; Kowal, J.; Manolaridis, I.; Jackson, S.M.; Stahlberg, H.; Locher, K.P. Structures of ABCG2 under Turnover Conditions Reveal a Key Step in the Drug Transport Mechanism. Nat. Commun. 2021, 12, 4376. [Google Scholar] [CrossRef] [PubMed]
- Bulusu, G.; Desiraju, G.R. Strong and Weak Hydrogen Bonds in Protein–Ligand Recognition. J. Indian Inst. Sci. 2020, 100, 31–41. [Google Scholar] [CrossRef]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting p-Glycoprotein through Caryophyllane Sesquiterpenes in Hepg2 Cells: An in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhong, L.S.; Huang, C.; Guo, Y.Y.; Jin, F.J.; Hu, Y.Z.; Zhao, Z.B.; Ren, Z.; Wang, Y.F. β-Caryophyllene Acts as a Ferroptosis Inhibitor to Ameliorate Experimental Colitis. Int. J. Mol. Sci. 2022, 23, 16055. [Google Scholar] [CrossRef]
- Simões-Silva, M.R.; De Araújo, J.S.; Peres, R.B.; Da Silva, P.B.; Batista, M.M.; De Azevedo, L.D.; Bastos, M.M.; Bahia, M.T.; Boechat, N.; Soeiro, M.N.C. Repurposing Strategies for Chagas Disease Therapy: The Effect of Imatinib and Derivatives against Trypanosoma Cruzi. Parasitology 2019, 146, 1006–1012. [Google Scholar] [CrossRef]




| T. cruzi Epi/Cell (μg/mL) | |||
|---|---|---|---|
| Y (IC50) | H4 (IC50) | Macrophages (CC50) | |
| BNZ Mixture | 0.90 ± 0.5 | 4.67 ± 0.21 | 41.53 ± 0.20 |
| BCPO Mixture | 4.82 ± 0.17 | 8.25 ± 0.32 | 173.5 ± 0.35 |
| ΣFIC | 0.23 | 0.16 | 1 |
| Pharmacological interaction | Synergy | Synergy | No interaction or addition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, L.P.; Hernández-Cuevas, N.A.; Acosta-Viana, K.Y.; Arana-Argáez, V.E.; Torres-Romero, J.C.; Polanco-Hernández, G.M. Activity of β-Caryophyllene Oxide and Benznidazole Mixture Against Trypanosoma cruzi and In Silico Prediction of Anti-Trypanocidal Interaction. Sci. Pharm. 2025, 93, 40. https://doi.org/10.3390/scipharm93030040
López-López LP, Hernández-Cuevas NA, Acosta-Viana KY, Arana-Argáez VE, Torres-Romero JC, Polanco-Hernández GM. Activity of β-Caryophyllene Oxide and Benznidazole Mixture Against Trypanosoma cruzi and In Silico Prediction of Anti-Trypanocidal Interaction. Scientia Pharmaceutica. 2025; 93(3):40. https://doi.org/10.3390/scipharm93030040
Chicago/Turabian StyleLópez-López, Luis P., Nora A. Hernández-Cuevas, Karla Y. Acosta-Viana, Víctor E. Arana-Argáez, Julio C. Torres-Romero, and Glendy M. Polanco-Hernández. 2025. "Activity of β-Caryophyllene Oxide and Benznidazole Mixture Against Trypanosoma cruzi and In Silico Prediction of Anti-Trypanocidal Interaction" Scientia Pharmaceutica 93, no. 3: 40. https://doi.org/10.3390/scipharm93030040
APA StyleLópez-López, L. P., Hernández-Cuevas, N. A., Acosta-Viana, K. Y., Arana-Argáez, V. E., Torres-Romero, J. C., & Polanco-Hernández, G. M. (2025). Activity of β-Caryophyllene Oxide and Benznidazole Mixture Against Trypanosoma cruzi and In Silico Prediction of Anti-Trypanocidal Interaction. Scientia Pharmaceutica, 93(3), 40. https://doi.org/10.3390/scipharm93030040










