Radioprotective Efficacy of Phosphorus-Containing Polymer Complexes of Amifostine WR-2721
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Phosphorus-Containing Polymer Complexes of Amifostine (WR-2721)
2.1.1. Obtaining Poly(oxyethylene H-phosphonate)s
2.1.2. Obtaining the Poly(hydroxyoxyethylene phosphate)s
2.1.3. Obtaining Amifostine (WR-2721)
2.1.4. Immobilization of Amifostine (WR2721) on Poly(hydroxyoxyethylene phosphate)s
2.2. Cells, Cell Culture Conditions and Irradiation
2.3. Clonogenic Survival Assay
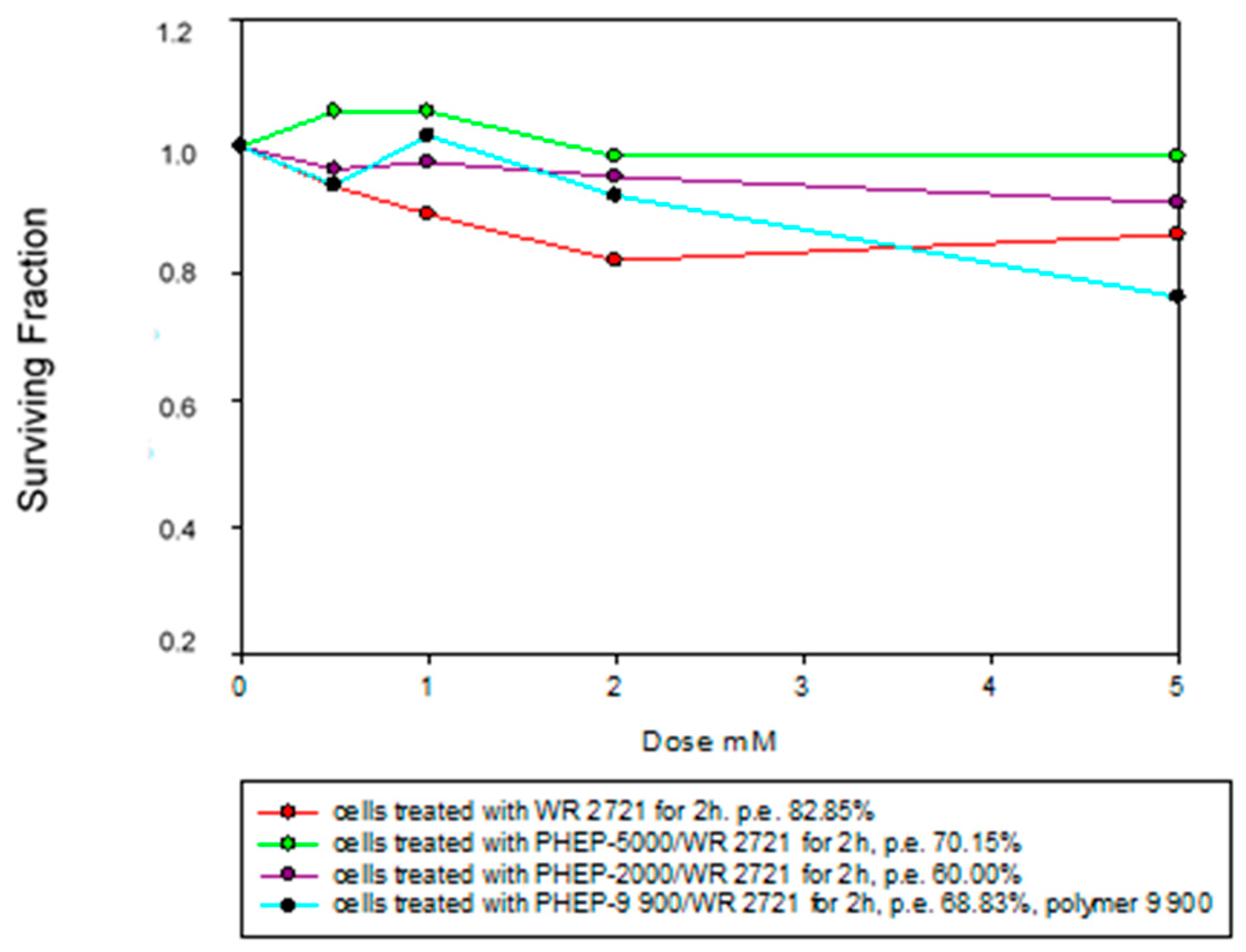
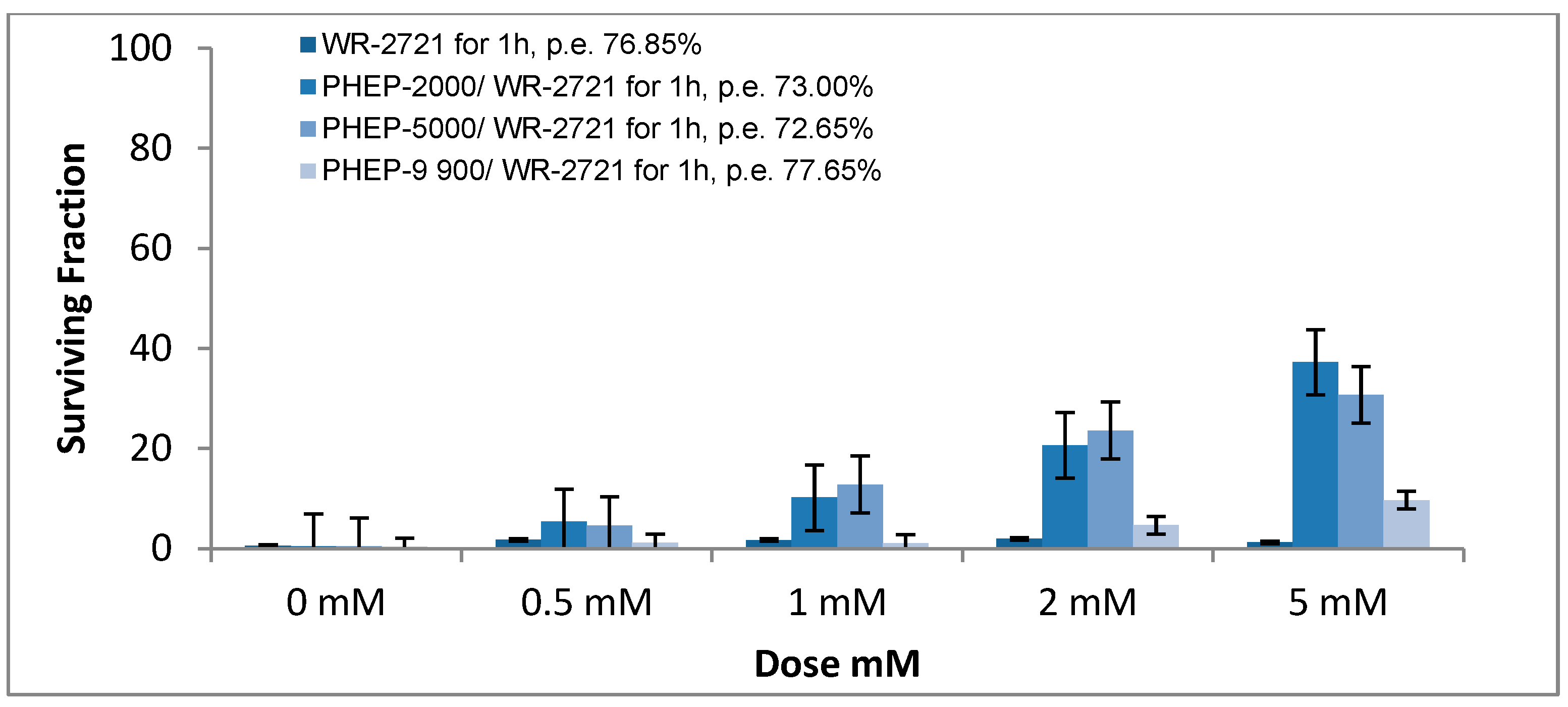
3. Results
Radioprotective Efficiency
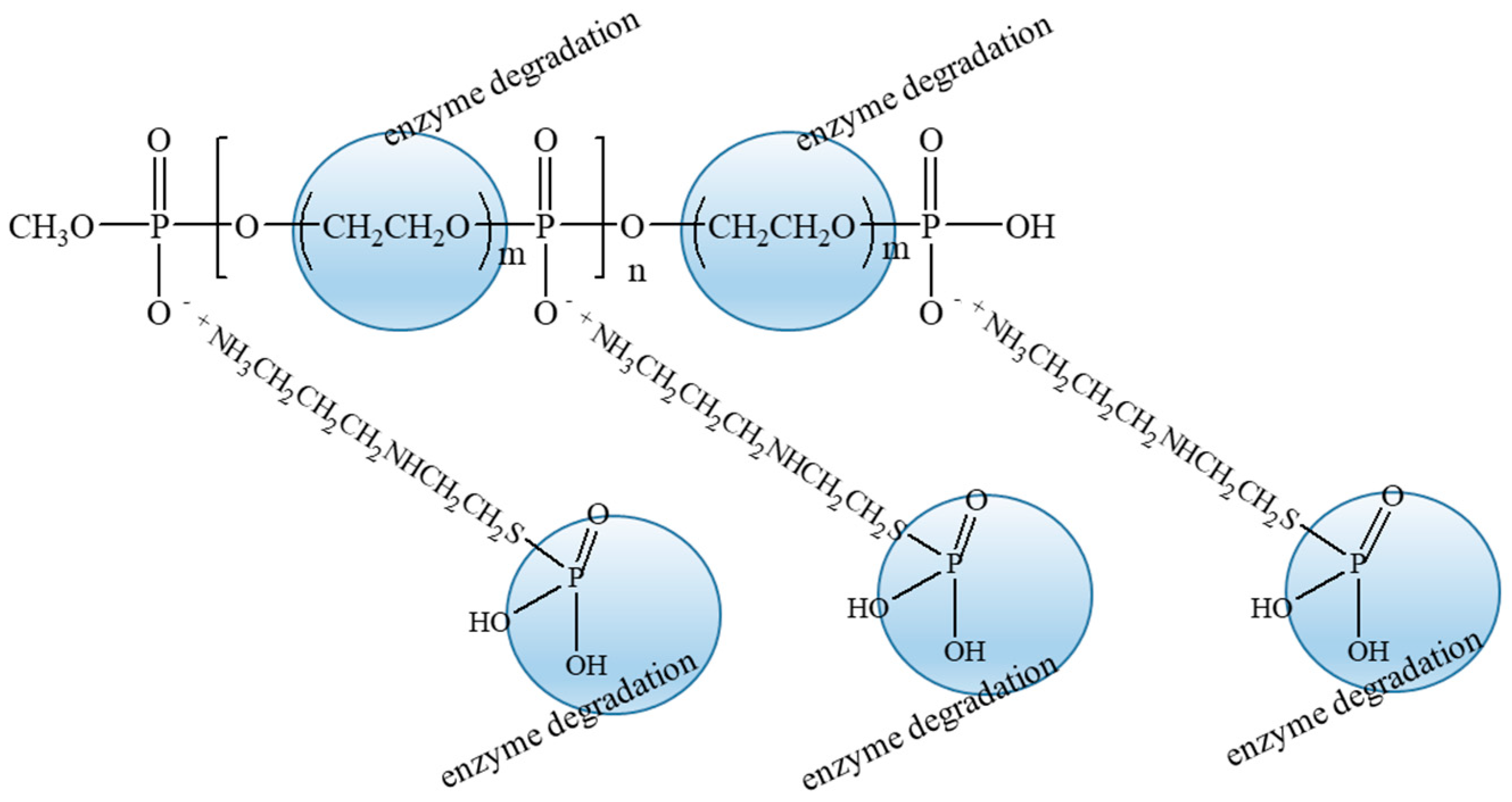
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cm | Centimeter |
| Da | Dalton |
| Gy | Gray, units used to measure the amount of radiation absorbed |
| h | Hour |
| kV | Kilovoltage |
| mA | Milliamperage |
| MEF | Mouse embryonic fibroblasts |
| min | Minute |
| mM | Millionaire |
| mm | Millimeter |
References
- Guo, J.; Zhao, Z.; Shang, Z.-F.; Tang, Z.; Zhu, H.; Zhang, K. Nanodrugs with intrinsic radioprotective exertion: Turning the double-edged sword into a single-edged knife. Exploration 2023, 3, 20220119. [Google Scholar] [CrossRef]
- Zheng, K.; Zhu, X.; Guo, S.; Zhang, X. Gamma-ray-responsive drug delivery systems for radiation protection. Chem. Eng. J. 2023, 463, 142522. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Gościniak, A.; Formanowicz, D.; Cielecka-Piontek, J. Natural Guardians: Natural Compounds as Radioprotectors in Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 6937. [Google Scholar] [CrossRef]
- Dest, V.M. Radioprotectants: Adding quality of life to survivorship? Semin. Oncol. Nurs. 2006, 22, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, J.; Zhu, J.; Chen, X.; Zhou, L.; Ma, W.; Lin, Y. A DNA tetrahedron-based nanosuit for efficient delivery of amifostine and multi-organ radioprotection. Bioact. Mater. 2024, 39, 191–205. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin. Drug Saf. 2019, 18, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Burkon, P.; Petyrek, P.; Spurny, V. Cytoprotective effects of amifostine in the treatment of tumors. Vnitr. Lek. 2003, 49, 673–678. [Google Scholar]
- Koukourakis, M.I. Amifostine:is there evidence of tumor protection. Semin. Oncol. 2003, 30, 18–30. [Google Scholar] [CrossRef]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 2023, 20, 5383–5395. [Google Scholar] [CrossRef]
- Molkentine, J.M.; Fujimoto, T.N.; Horvath, T.D.; Grossberg, A.J.; Garcia, C.J.G.; Deorukhkar, A.; Bonilla, M.d.l.C.; Lin, D.; Samuel, E.L.G.; Chan, W.K.; et al. Enteral Activation of WR-2721 Mediates Radioprotection and Improved Survival from Lethal Fractionated Radiation. Sci. Rep. 2019, 9, 1949. [Google Scholar] [CrossRef]
- Davidson, D.E.; Grenan, M.M.; Sweeney, T.R. Radiation Sensitizers. Their Use in the Clinical Management of Cancer; Brady, L.W., Ed.; Mason Publ.: New York, NY, USA, 1980; p. 309. [Google Scholar]
- Kessler, L.; Koo, C.; Richter, C.-P.; Tan, X. Hearing loss during chemotherapy: Prevalence, mechanisms, and protection. Am. J. Cancer Res. 2024, 14, 4597. [Google Scholar] [CrossRef]
- Dunst, J.; Semlin, S.; Pigorsch, S.; Müller, A.C.; Reese, T. Itermittent use of amifostine during postoperative radiochemotherapy and acute toxicity in rectal cancer patients. Strahlenther. Onkol. 2000, 176, 416–421. [Google Scholar] [CrossRef]
- Fatome, M.; Courteille, F.; Laval, J.D.; Roman, V. Radioprotective activity of ethylcellulose microspheres containing WR2721, after oral administration. Int. J. Radiat. Biol. 1987, 52, 21–29. [Google Scholar] [CrossRef]
- Pamujula, S.; Graves, R.A.; Kishore, V.; Mandal, T.K. Preparation and in vivo characterization of amifostine biodegradable microcapsules. Eur. J. Pharm. Biopharm. 2004, 57, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Pamujula, S.; Graves, R.A.; Freeman, T.; Srinivasan, V.; Bostanian, L.A.; Kishore, V.; Mandal, T.K. Oral delivery of spray dried PLGA/aminphostine nanoparticles. J. Pharm. Pharmacol. 2004, 56, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Pamujula, S.; Kishore, V.; Rider, B.; Fermin, C.D.; Graves, R.A.; Agrawal, K.C.; Mandal, T.K. Radioprotection in mice following oral delivery of amifostine nanoparticles. Int. J. Radiat. Biol. 2005, 81, 251–257. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and Properties of Pharmacologically Active Polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Pires, C.P.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Joana Lopes, J.; Macário-Soares, A.; Diana Peixoto, D.; Giram, S.P.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Y.; Mukerabigwi, J.F.; Wang, H.; Cao, Y. Polymer Conjugate as the New Promising Drug Delivery System for Combination Therapy against Cancer. Curr. Top. Med. Chem. 2024, 24, 1101–1119. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Tietilu, A.D.; Yücel, O.; Erol, T.; Akgüner, Z.P.; Darıcı, H.; Alarcin, E.; Emik, S. Hyperbranched polymer-based nanoparticle drug delivery platform for the nucleus-targeting in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 81, 104195. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Buqué, A.; Zitvogel, L.; Kroemer, G.; Lorenzo Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 2021, 39, 310–345. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.S.; Pandey, A.; Jha, A.K.; Badwaik, H.K.R.; Alexander, A. Polyethylene Glycol–Based Polymer-Drug Conjugates: Novel Design and Synthesis Strategies for Enhanced Therapeutic Efficacy and Targeted Drug Delivery. Appl. Biochem. Biotechnol. 2024, 196, 7325–7361. [Google Scholar] [CrossRef]
- Huang, S.; Xu, M.; Da, Q.; Jing, L.; Wang, H. Mitochondria-Targeted Nitronyl Nitroxide Radical Nanoparticles for Protection against Radiation-Induced Damage with Antioxidant Effects. Cancers 2024, 16, 351. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Wang, H.; Zhang, M.; He, J.; Peihong, N. Advanced Biomaterials Derived from Functional Polyphosphoesters: Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2024, 16, 51876–51898. [Google Scholar] [CrossRef] [PubMed]
- Kraicheva, I.; Bogomilova, A.; Tsacheva, I.; Momekov, G.; Momekova, D.; Troev, K. Synthesis, NMR characterization and in vitro cytotoxicity evaluation of new poly(oxyethylene aminophosphonate)s. Eur. J. Med. Chem. 2010, 45, 6039–6044. [Google Scholar] [CrossRef]
- CYan, C.V.; Pham, C.-D.; Arthur, K.; Yang, L.K.; Muller, L.F. Aliphatic amines are viable pro-drug moieties in phosphonoamidate drugs. Bioorganic Med. Chem. Lett. 2020, 30, 127656. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Mao, H.-Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Del. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Pelosi, C.; Tinè, R.M.; Wurm, R.F. Main-chain water-soluble polyphosphoesters: Multi-functional polymers as degradable PEG-alternatives for biomedical applications. Eur. Polym. J. 2020, 141, 110079. [Google Scholar] [CrossRef]
- Troev, K. Chemistry and Application of H-Phosphonates; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V. Design, Synthesis and Actual Applications of the Polymers Containing Acidic P–OH Fragments: Part 1. Polyphosphodiesters. Int. J. Mol. Sci. 2022, 23, 14857. [Google Scholar] [CrossRef]
- Troev, K.; Tsatcheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of aminothiols on poly(oxyethylene H-phosphonate)s and poly(oxyethylene phosphate)s-An approach to polymeric protective agents for radiotherapy of cancer. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 7, 1349–1363. [Google Scholar] [CrossRef]
- Mochizuki, K.; Mitova, V.; Makino, K.; Terada, H.; Takeuchi, I.; Troev, K. pH-Sensitive Amphiphilic Diblock Polyphosphoesters with Lactate Units: Synthesis and Application as Drug Carriers. Int. J. Mol. Sci. 2024, 25, 4518. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Makino, K.; Terada, H.; Takeuchi, I.; Mitova, V.; Troev, K. Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications. Molecules 2023, 28, 5243. [Google Scholar] [CrossRef] [PubMed]

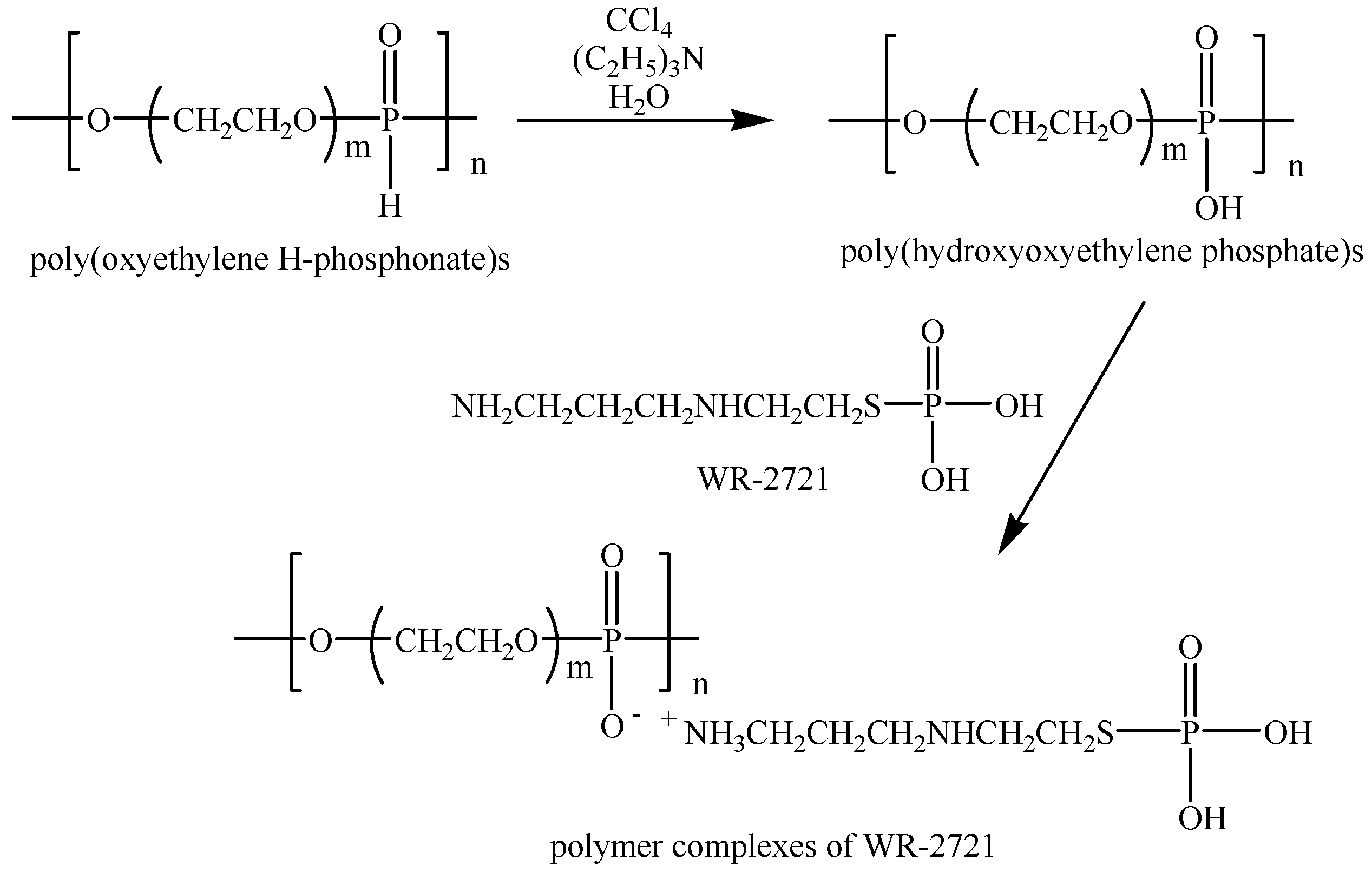




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsacheva, I.; Uzun, D. Radioprotective Efficacy of Phosphorus-Containing Polymer Complexes of Amifostine WR-2721. Sci. Pharm. 2025, 93, 21. https://doi.org/10.3390/scipharm93020021
Tsacheva I, Uzun D. Radioprotective Efficacy of Phosphorus-Containing Polymer Complexes of Amifostine WR-2721. Scientia Pharmaceutica. 2025; 93(2):21. https://doi.org/10.3390/scipharm93020021
Chicago/Turabian StyleTsacheva, Ivelina, and Dzhamal Uzun. 2025. "Radioprotective Efficacy of Phosphorus-Containing Polymer Complexes of Amifostine WR-2721" Scientia Pharmaceutica 93, no. 2: 21. https://doi.org/10.3390/scipharm93020021
APA StyleTsacheva, I., & Uzun, D. (2025). Radioprotective Efficacy of Phosphorus-Containing Polymer Complexes of Amifostine WR-2721. Scientia Pharmaceutica, 93(2), 21. https://doi.org/10.3390/scipharm93020021






