Abstract
Most novel active pharmaceutical ingredients have low water solubility; therefore, solubility-enhancing methods are applied. The aim of the present investigation is to study the impact of nine commonly used pharmaceutical excipients (fillers, surfactants, cyclodextrins, polymers) on solubility, permeability and their relationship. This is crucial for ensuring optimal bioavailability. Carbamazepine, naproxen and pimobendan were chosen as model compounds due to their different acid–base properties. Equilibrium solubility was measured by the traditional shake flask method. Effective permeability was determined by the PAMPA model. Measurements of ionizable compounds were carried out at three pH values. The pH-dependent change in the investigated parameters is maintained even in the presence of excipients. Fillers resulted in a slight or no effect, while the impact of other excipients showed a significant concentration dependence. The impact of excipients was influenced by the structure and ionization state of the molecules. The dominance of the ionized form moderates the impact of excipients. The changes in solubility were more pronounced than in the case of permeability. By examining the effect of the ionization state and interactions with excipients, this work supports the development of formulations that enhance solubility with minimal impacts on permeability. Additionally, it can serve as good basis for preformulation studies and design optimization.
1. Introduction
Oral drug administration remains the preferred route for drug delivery due to its non-invasive nature, ease of dosing, cost-effectiveness and high patient compliance [1,2]. However, the efficacy and bioavailability of orally administered drugs are highly influenced by two physicochemical properties: the water solubility and the intestinal permeability. The dissolved form of the active pharmaceutical ingredient (API) is essential for the absorption from the gastrointestinal tract (GI), while adequate permeability ensures transport through the membranes [3,4].
Poor aqueous solubility is a common issue, as it affects approximately 40% of commercial drugs and 70–90% of new drug candidates; thus, they belong to Biopharmaceutical Classification System (BCS) II (low solubility, high permeability) or BCS IV (low solubility, low permeability) [5,6].
Consequently, the development of solubility-enhancing techniques has become a major focus of research over the years. As a result, various formulation strategies, including particle size reduction, amorphous solid dispersions, lipid-based and self-emulsifying systems, have been applied to improve solubility [1,7,8]. In addition to the above-mentioned techniques, formulations contain various types of excipients to enhance drug solubility (complexing agents, surfactants), dissolution rate (disintegrants), and the stability of APIs (polymers) to ensure optimal bioavailability [9,10].
While solubility enhancement has been a primary research focus, its effect on permeability remained mainly overlooked until the pioneering work of Dahan and Miller [11]. Their investigation established an inverse relationship between equilibrium solubility and effective permeability (Pe), both in vitro and in vivo. It highlights the potential for reduced bioavailability, when permeability changes are not considered [12,13]. Such a phenomenon was reported by Beig et al., for example, in the case of carbamazepine in the presence of polyethylene glycol (PEG), as well as etoposide in the presence of sodium lauryl sulphate (SLS) and hydroxypropyl-β-cyclodextrin (HPβCD) [14,15,16]. However, in some cases, the solubility–permeability trade-off is not so obvious. SLS at low concentrations (~10 mM) enhances solubility via micelle formation. At the same time, it decreases the permeability by lowering the free fraction of the drug available for passive transport, which demonstrates the interplay between solubility and permeability [17]. However, at higher concentrations, SLS can solubilize intestinal epithelial cells, leading to the damage of the intestinal barrier, which results in increased permeability [18]. Cyclodextrins (CDs), by contrast, may enhance permeability by facilitating drug transport across aqueous media via complex formation [19].
These findings highlight the crucial role of the solubility–permeability interplay in formulation development, which is confirmed by a recently demonstrated novel formulation development strategy based on this trade-off [20,21,22]. However, in most cases the emphasis is placed on a single drug–excipient interaction instead of a holistic study [11,23,24]. Extended investigations are necessary to systematically evaluate the role of solubility enhancers in modulating both parameters across different concentrations and types of excipients. Furthermore, studies at several pH values are necessary to determine the effect of species distribution on the influencing effect of excipients in the case of ionizable molecules.
The aim of this work is to investigate the impact of nine commonly used excipients on equilibrium solubility, permeability, and their interplay using validated methods. During the selection of excipients, the goal was to identify the most frequently applied categories of excipients by prioritizing those commonly utilized in solubility-enhancing research and present in marketed formulations. To achieve this, two excipients were selected from each category. Thus, the final selection included sugars and sugar alcohols—mannitol and sorbitol—which also exhibit hydrotropic properties, along with lactose, a completely inert filler. Cyclodextrins were represented by hydroxypropyl-β-cyclodextrin (HPβCD) and sulfobutyl-ether-β-cyclodextrin (SBEβCD), known for their ability to form inclusion complexes. Among the surfactants, Tween 80 (Polysorbate 80) and sodium lauryl sulphate (SLS) were selected due to their amphiphilic nature and their frequent application in solubilization. Finally, polymers were represented with polyvinylpyrrolidone K25 (PVP-K25) and polyvinylpyrrolidone/vinyl acetate 64 (PVPVA 64), which are commonly used as solubility enhancers and stabilizers. The occurrence of the excipients was also checked in the FDA database [25]. It contains information (application route, used amount, etc.) about inactive ingredients in drug products approved by the FDA. In formulations, different excipients are applied in various concentrations due to their different functions. While in most cases the concentration of fillers is higher than that of the APIs, the amount of other excipients (e.g., surfactants) is lower. Due to this, 1:0.5, 1:1 and 1:3 mass ratios (APIs:excipients) were investigated during the measurements to model both cases. To ensure a comprehensive analysis, three, BCS II model compounds were chosen: carbamazepine (CAR), naproxen (NAP) and pimobendan (PIMO). Our aim was to examine compounds with different acid–base properties, and to ensure that the pKa value of the ionizable ones would fall within the pH interval of the GI section (pH = 1.0–6.8), so the protonation state would play a decisive role in their absorption [26]. Due to this, three biorelevant pH values were applied, pH = 3.0, pH = 5.0 and pH = 6.5, which simulate the pH of fed stomach, fed intestine and fasted intestine [27,28]. Hence, in addition to excipient effects, the influence of pH was also investigated. Consequently, the main goal of this research is to conduct a comprehensive, multi-factorial investigation to establish a good basis for formulation design.
For the permeability measurements, Parallel Artificial Membrane Permeability Assay (PAMPA) was applied. Due to its high throughput and low material requirements, it is a suitable technique for studies where several measuring layouts are possible [29,30]. Thermodynamic solubility was measured by the saturation shake flask method (SSF) [31,32,33].
2. Materials and Methods
2.1. Materials
CAR (purity >97%) was from TCI Chemical (Tokyo, Japan), NAP (purity 98–102%), and PIMO (purity 98%) was purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA). Table 1 contains some of the most important physicochemical properties and the structures of the APIs. Lactose-monohydrate, sorbitol, mannitol, SLS, PVPVA 64, PVP-K25, and Tween-80 were received from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA), and HPβCD (with the degree of substitution ~4.5) and SBEβCD (with the degree of substitution ~7) were obtained from Cyclolab R&D Ltd. (Budapest, Hungary). The structures of the excipients are presented in Table S1. The buffer components (acetic acid (99–100%), phosphoric acid (>85%), boric acid, sodium hydroxide) were supplied by Molar Chemicals Ltd. (Halásztelek, Hungary). HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid, 238.30 g/mol, purity >99%) was purchased from TCI Chemicals (Tokyo, Japan). GIT lipid was received from Pion Inc. (Billerica, MA, USA).

Table 1.
Structures and properties of model compounds.
2.2. Methods
2.2.1. Thermodynamic Solubility Measurement
Thermodynamic solubility was determined in Britton–Robinson buffer (BRB) at 3 different biorelevant pH values (pH = 3.0, pH = 5.0, and pH = 6.5) at 37 ± 0.5 °C by the SSF method. Excipients were mixed with the APIs at mass ratios of 0.5:1, 1:1, and 3:1 (excipient:API) as a physical mixture. The accurately weighed quantities of the compound were 0.02 g in the case of CAR, 0.002 g in the case of PIMO and 0.02 g for NAP, and the excipients were added in the above-mentioned ratios. The applied volumes were 20 mL, except in the case of NAP at pH = 6.5, where the volume was 5 mL due to its high solubility. In the presence of excess solid, the samples were stirred at 200 rpm for 6 h; after the first hour, the pH was checked and adjusted as necessary, and then it was sedimented for 18 h [35]. At the end of each measurement, the pH was checked again to ensure the accuracy of the results. In cases where the phases did not separate during sedimentation, filtration (Millex GV 0.22 μm, Ø: 3.3 cm, hydrophilic PVDF membrane) was used [36,37]. The μDISS Profiler™ (Pion Inc., Billerica MA, USA) equipped with UV probes was used for in situ concentration measurement. Calibration was performed with the same probes to ensure an appropriate pathlength. The concentration was determined using the 2nd derivative method implemented in AuPRO™ 7.1 software (Pion Inc., Billerica MA, USA).
2.2.2. Determination of Effective Permeability (Pe) with PAMPA
For the determination of Pe, the PAMPA method was applied, which is a plate-based system for the modelling of passive diffusion. Each well of the top (acceptor) compartment of a 96-well STIRWELL™ PAMPA sandwich (Pion Inc., Billerica MA, USA) was coated with 4 µL of GIT lipid (Pion Inc., Billerica MA, USA). The acceptor compartment of the plate was filled with 200 μL of pH = 7.4 HEPES buffer containing 1% of SLS to maintain the sink condition. The bottom (donor) plate was prefilled with 180 μL of the appropriate sample solutions, which were made by adding methanolic stock solution to the adequate buffer already containing the excipient. The concentration of the samples was set based on the saturation concentration of the API in its least soluble form. After assembling the sandwich, it was incubated at 37 ± 0.5 °C. The thickness of unstirred water layer (UWL) in both compartments was set to 60 μm by stirring in Gutbox™ (Pion Inc., Billerica MA, USA) to avoid UWL-limited transport. For CAR, the sandwich was separated after 1 h, for PIMO after 45 min. In the case of NAP at pH = 6.5, it was separated after 45 min, while at pH = 3.0 and pH = 5.0, it was separated after 15 min. The determination of incubation time depended on the permeability of molecules. Subsequently, 150 µL from both the donor and acceptor compartments was transferred to UV plates (Greiner Group AG, Kremsmünster, Austria). UV absorption (λ = 230–400 nm) was measured using a Tecan Infinite M200 UV plate reader (Tecan Group Ltd., Mannedorf, Switzerland). Pe values were calculated using PAMPA Explorer software™ (Pion Inc., Billerica MA, USA).
The Pe can be determined by the following equation [38]:
where VD and VA are the volumes of the donor and acceptor compartment (mL), A is the area of the membrane (0.07 cm2), t is the incubation time (min), τLAG is the steady-state time, CA (t) is the concentration of the acceptor compartment after the incubation, CD (0) is the initial concentration of the donor compartment and CD (t) is the concentration of the donor compartment after the incubation. R is the membrane retention factor:
and is the sink asymmetry ratio (gradient-pH-induced):
2.2.3. Statistical Analysis
Equilibrium solubility measurements were carried out with three parallel experiments. The determination of Pe was performed in duplicate (the parallels within a plate were n = 3). Values are expressed as means ± standard deviation (SD). The permeability and solubility values were compared in each study by analysis of variance using a general linear model and contrast comparison. In the general linear model, the independent variables were the types of excipients at the various mass ratios, while the dependent variables were either permeability or solubility. The analysis was performed separately for each pH condition. For post hoc analysis, general linear hypothesis tests (Dunnett) for multiple comparisons with contrasts were performed. Statistical analysis was performed using the R programming language in R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) [39] and the R Studio Version: 2024.12.1+563 integrated development environment (Posit, Boston, MA, USA). The multiple comparisons were performed using the ‘multcomp’ package Version: 1.4-28 [40].
3. Results
The results of equilibrium solubility were measured in the absence—used as a reference value—and presence of different pharmaceutical excipients at three mass ratios: 1:0.5, 1:1 and 1:3 (API:excipient). Three biorelevant pH values (pH = 3.0, 5.0 and 6.5) were applied for the investigation of the role of the ionization state. To determine Pe, the PAMPA method was used to study the impact of additives on the permeability of our model compounds under the same conditions.
3.1. Equilibrium Solubility
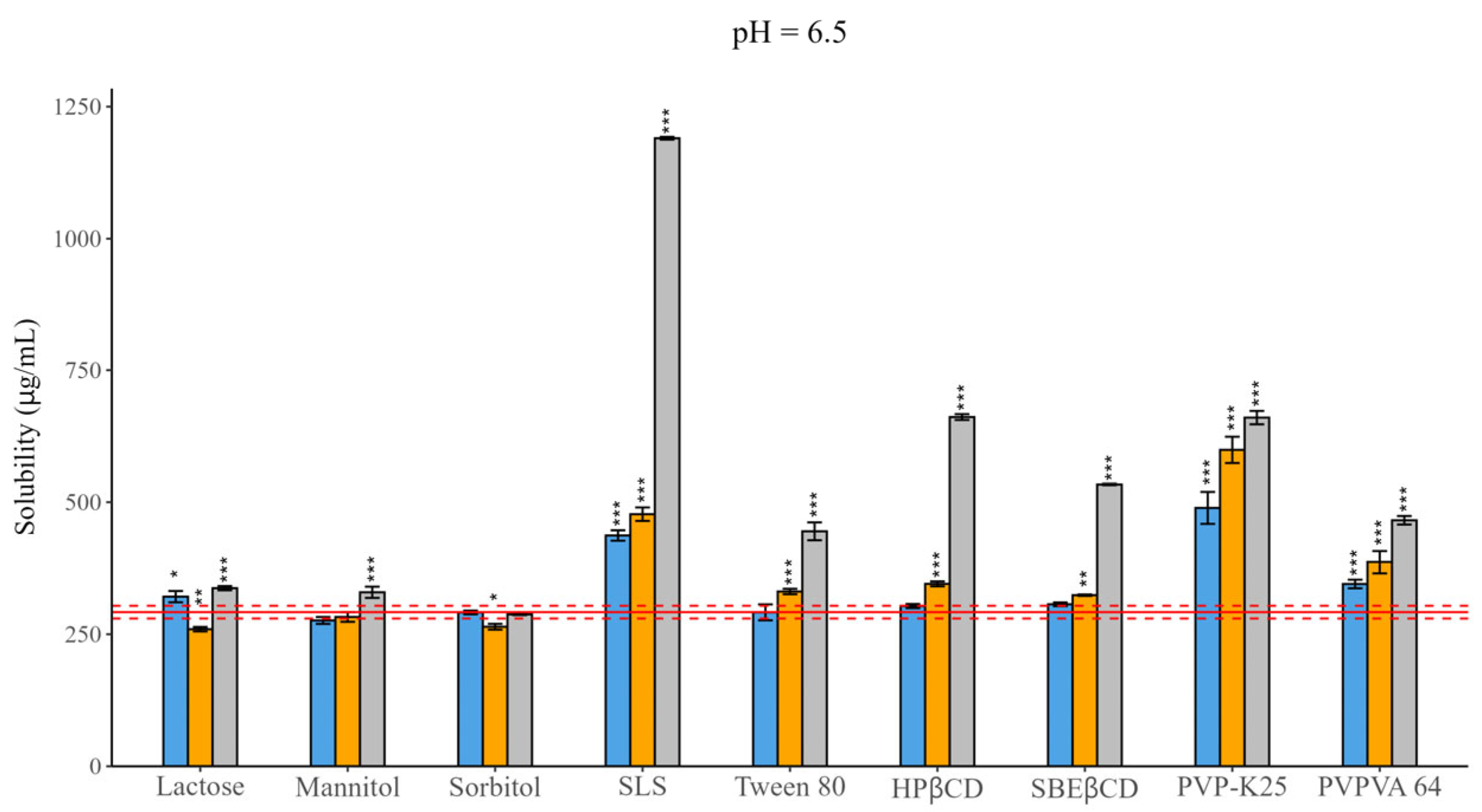
The solubility of CAR, as a neutral substance, is independent from pH. In addition, both ionic excipients, SBEβCD and SLS, are in fully ionized form at all the tested pH levels because of their strongly acidic sulfonic acid group; therefore, the measurements were carried out only at pH = 6.5 [26]. The equilibrium solubility of the molecule was determined to be 292.04 ± 4.88 µg/mL, which shows a good correlation with the published data of El-Massik et al. and Fine-Shamir et al. (~250 µg/mL) [41,42]. The difference can be explained by the different medium and thus the different ionic strength. This value was used as a reference for the study of the impact of the excipients. Based on the statistical analysis, the limit of the significance was p < 0.05. The equilibrium solubility data with different excipients is shown in Figure 1 and the exact values are displayed in Table S2 in the Supplementary Material.
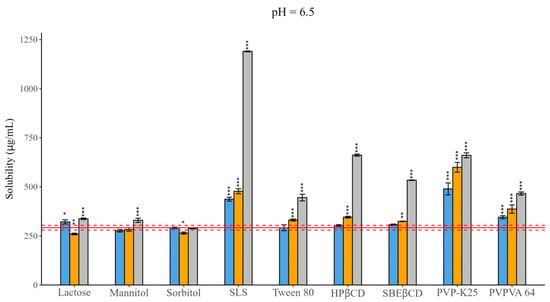
Figure 1.
Effect of excipients on the solubility of CAR at three mass ratios in pH = 6.5 BRB solution (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to sample; p-values are as follows *** <0.001, ** 0.01–0.001, * 0.01–0.05; red line: solubility of CAR without excipients; red dashed line: 95% confidence intervals of the reference). In the case of fillers, mixed effects were observed, for which concentration dependence was not so obvious. Lactose and mannitol resulted in solubility enchantment, while sorbitol did not have a notable impact. The solubilizing effect of SLS was more pronounced under all circumstances compared to that of Tween 80, resulting in a four-fold increase at a 1:3 mass ratio. For CDs, a linear enhancing effect was observed as a function of concentration, although HPβCD complexation was more pronounced (~2-fold) than SBEβCD complexation (~1.5-fold). In the case of polymers, PVP-K25 caused a more significant effect than PVPVA 64.
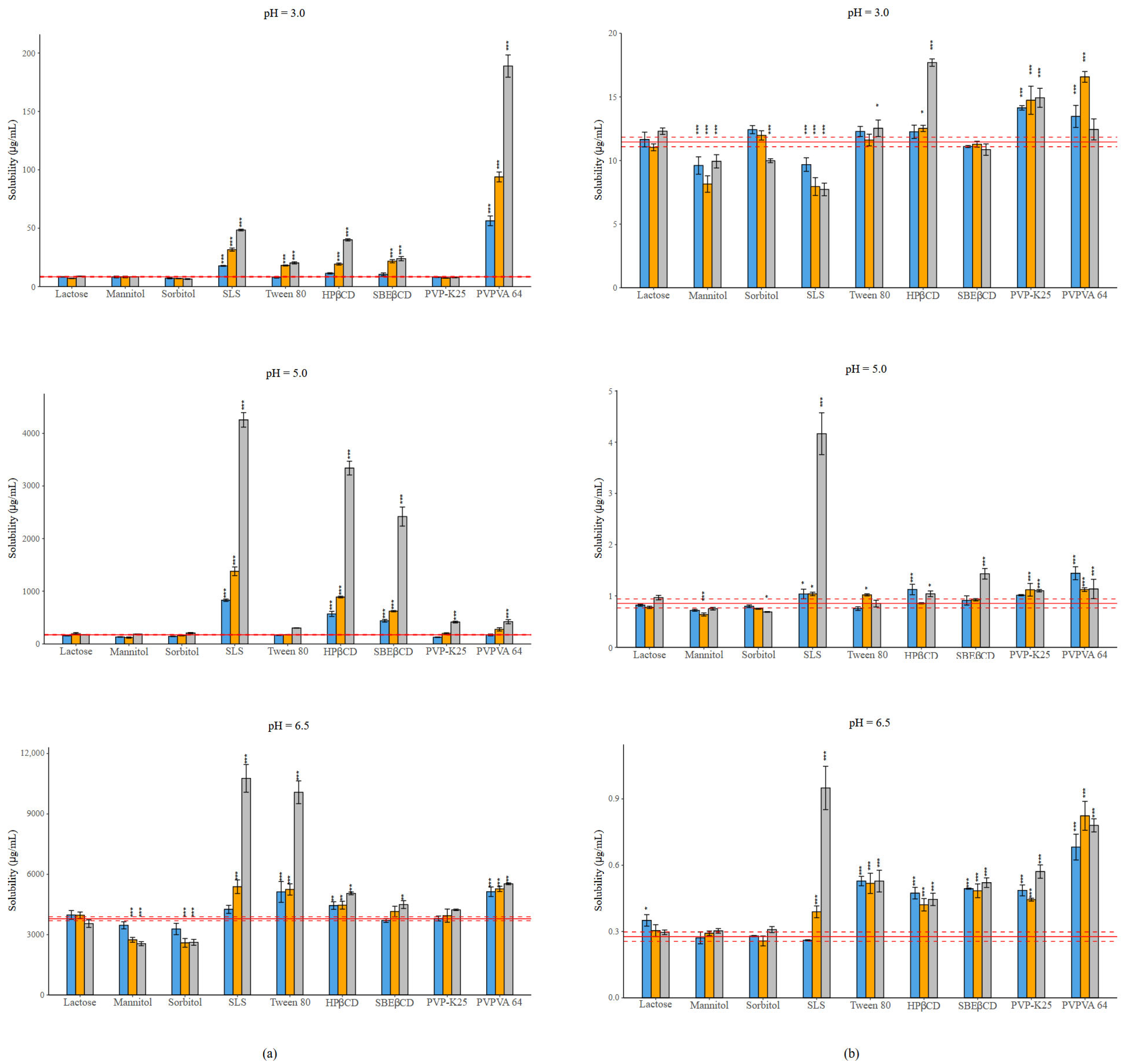
The thermodynamic solubility of NAP and PIMO is pH-dependent [26]. NAP, as an acidic molecule, has nearly 400 times higher solubility at pH = 6.5 compared to its neutral form. The equilibrium solubility values of NAP were as follows: 8.45 ± 0.41 µg/mL (pH = 3.0); 171.97 ± 7.48 µg/mL (pH = 5.0); 3791.55 ± 63.07 µg/mL (pH = 6.5). These values show a good correlation with the literature data [26]. PIMO, as a base, reached its highest solubility at pH = 3.0: 11.46 ± 0.30 µg/mL. As the pH increased, the proportion of the neutral form also increased, leading to decreased solubility, 0.85 ± 0.04 µg/mL at pH = 5.0 and 0.28 ± 0.01 µg/mL at pH = 6.5, where the molecule was fully non-ionized [43]. The effects of the excipients are presented in Figure 2, while Tables S3–S8 contain the measured values. The modifying effect of excipients is more pronounced in the case of NAP compared to PIMO.
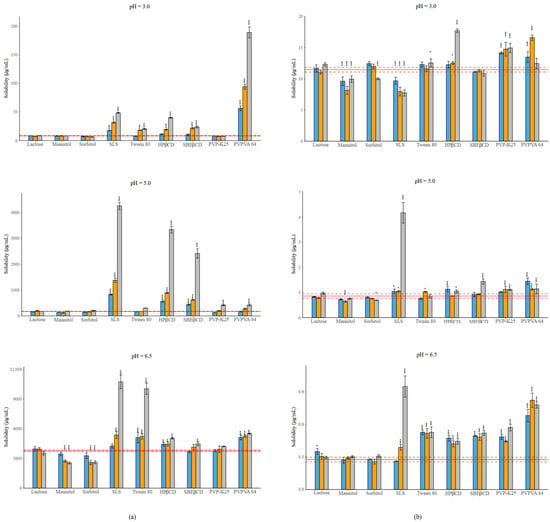
Figure 2.
(a) Effect of excipients on the solubility of NAP at three mass ratios in BRB solutions (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to the sample. p-values are as follows *** <0.001, ** 0.01–0.001; red line: solubility of NAP without excipients; red dashed line: 95% confidence intervals of the reference) at pH = 3.0; at pH = 5.0; at pH = 6.5. (b) Effect of excipients on the solubility of PIMO at three mass ratios in BRB solutions (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to the sample. p values are as follows *** <0.001, * 0.01–0.05; red line: solubility of PIMO without excipients; red dashed line: 95% confidence intervals of the reference) at pH = 3.0; at pH = 5.0; at pH = 6.5. Error bars depict 95% confidence intervals for each bar as opposed to the sample. p values are as follows *** <0.001, ** 0.01–0.001, * 0.01–0.05.
The effects of the different excipients depend on the ionization state of the APIs. In the case of NAP at pH = 3.0, more than 99% of the molecule is non-ionized, while at pH = 5.0, 83% of the molecule is in ionized form, and finally, at pH = 6.5, more than 99% of the molecule exists in ionized form. Due to the basic character of PIMO, the distribution of species will be the opposite at the different pH values.
In the case of fillers, lactose did not cause any change in solubility in either case. Sugar alcohols resulted in a decrease in solubility in the presence of the ionized form of the APIs. In most cases, SLS provided the highest increase in solubility, except for the cationic form of PIMO, where a nearly 30% reduction was noted at a 1:3 mass ratio. The solubilizing effect of Tween 80 is lower than that of SLS under all conditions. CDs showed a mixed effect, although HPβCD showed a more significant effect than SBEβCD. A similar trend, like in the case of CDs, is observed with polymers. The impact of PVP-K25 on NAP was negligible, only causing a significant effect at pH = 5.0 at its highest concentration. On the other hand, the use of PVPVA 64 in most of the cases resulted in a significant solubility increasement, which reached an extremely high value (a nearly 20-fold increase compared to the reference). In the case of PIMO, PVP-K25 and PVPVA 64 caused a similar effect, except at pH = 6.5, where the non-ionized form was dominant, in which case PVPVA 64 reached a higher solubilization.
3.2. Effective Permeability
According to the pH partition hypothesis, transport across the phospholipid bilayer by passive diffusion is favourable for the neutral form of the molecules [26].
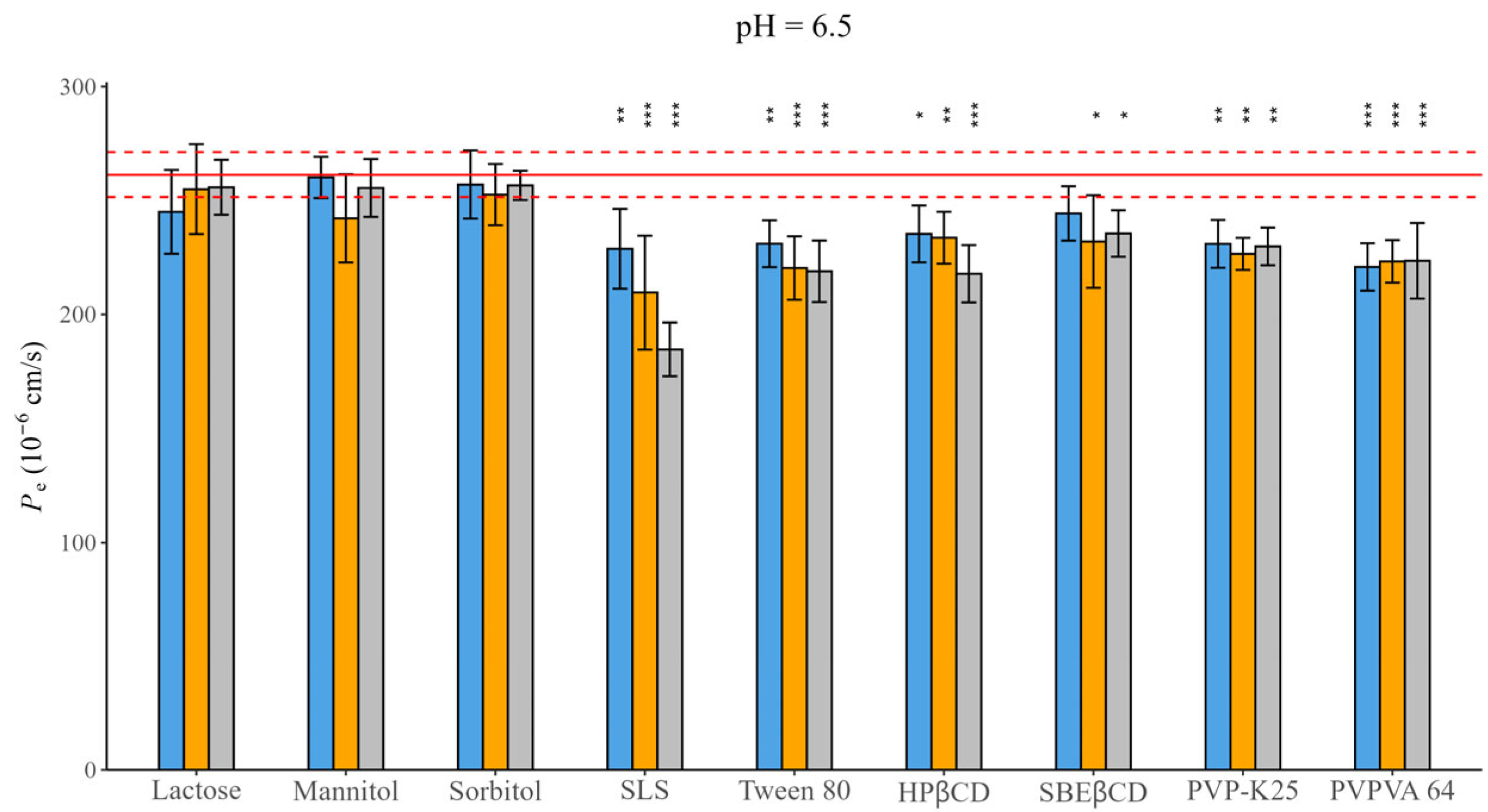
The permeability of CAR was depicted as a single value, 261.38 ± 16.20 10−6 cm/s, measured at pH = 6.5. The excipients moderately affected the permeability, as illustrated in Figure 3; Table S9 contains the exact values. No significant change was observed during the application of fillers, whereas a decrease in permeability was noted with other excipients. SLS caused the most pronounced effect, especially if applied in a high concentration.
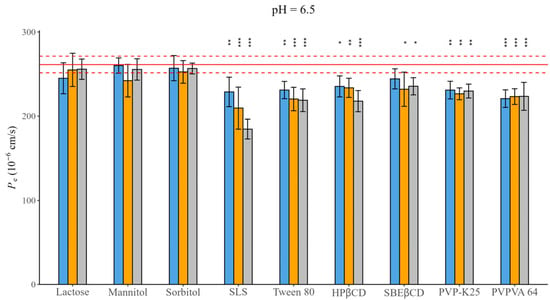
Figure 3.
Effect of excipients on Pe of CAR at three mass ratios in pH = 6.5 BRB solution (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to the sample; p-values are as follows: *** <0.001, ** 0.01–0.001, * 0.01–0.05; red line Pe of CAR without excipients; red dashed line: 95% confidence intervals of the reference).
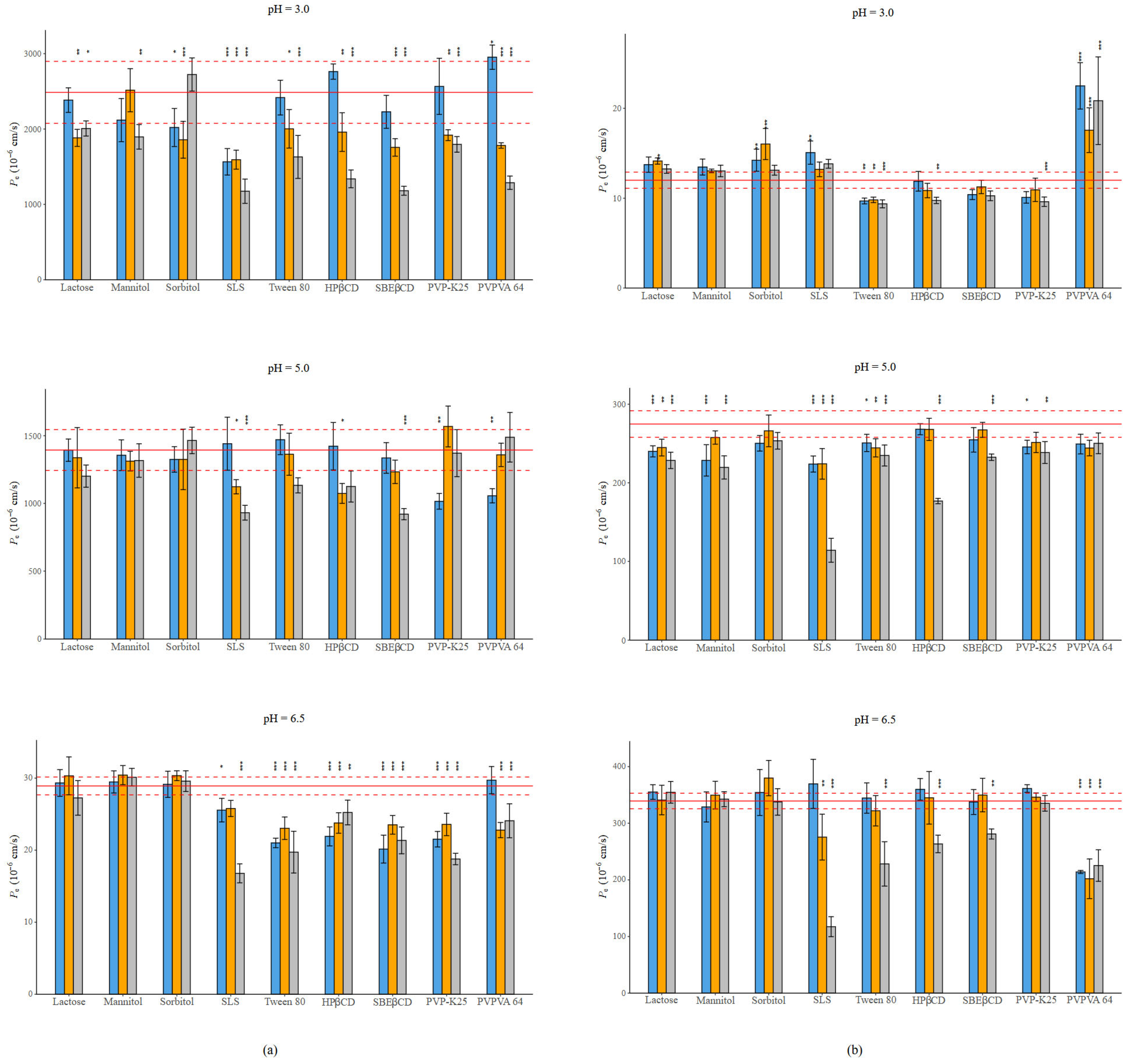
For NAP and PIMO, their Pe is pH-dependent, as is their solubility [26]. NAP exhibited the highest permeability at pH = 3.0: 997.62 ± 46.95 10−6 cm/s, where nearly 99% of the molecule was in its unionized form. As the pH increased, the proportion of the neutral form decreased, leading to decreased permeability as well, with 496.82 ± 32.41 10−6 cm/s at pH = 5.0 and 28.92 ± 1.61 10−6 cm/s at pH = 6.5, where 99% of the molecule was ionized. PIMO, as a basic compound, achieved its maximum permeability at pH = 6.5: 339.67 ± 20.26 10−6 cm/s, followed by 274.82 ± 16.13 10−6 cm/s at pH = 5.0, and the lowest at pH = 3.0: 12.00 ± 1.42 10−6 cm/s. Figure 4 demonstrates the impact of excipients on the permeability of these compounds. Tables S10–S15 contain the exact values.
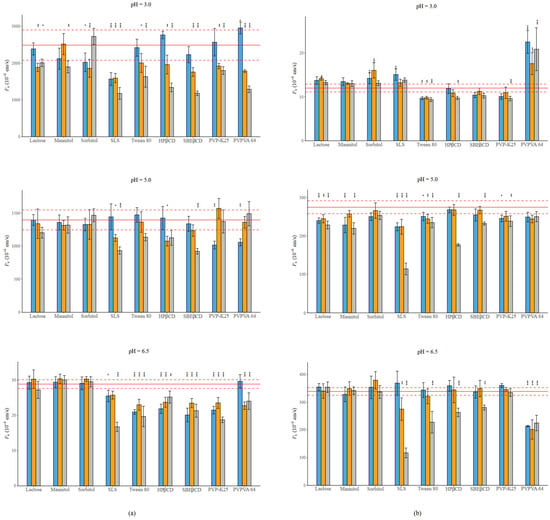
Figure 4.
(a) Effect of excipients on the Pe of NAP at three mass ratios in BRB solutions (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to the sample; p-values are as follows: *** <0.001, ** 0.01–0.001, and * 0.01–0.05; red line: Pe of NAP without excipients; red dashed line: 95% confidence intervals of the reference) at pH = 3.0; at pH = 5.0; at pH = 6.5. (b) Effect of excipients on the Pe of PIMO at three mass ratios in BRB solutions (columns: blue: 1:0.5, orange 1:1, grey 1:3 API: excipient mass ratio; error bars depict 95% confidence intervals for each bar as opposed to the sample; p-values are as follows: *** <0.001, ** 0.01–0.001, and * 0.01–0.05; red line: Pe of PIMO without excipients, red dashed line: 95% confidence intervals of the reference) at pH = 3.0; at pH = 5.0; at pH = 6.5. Error bars depict 95% confidence intervals for each bar as opposed to the sample; p values are as follows: *** <0.001, ** 0.01–0.001, and * 0.01–0.05.
The decrease in permeability is more pronounced in the case of NAP compared to PIMO. The fillers show mixed effect, as well as the polymers. An interesting effect can be noticed in the case of PVPVA 64. When applied with NAP in most cases it causes a decrease in permeability. While next to PIMO, at pH = 3.0 it enhances permeability; however, with the increase in the neutral form, it reduces the Pe. CDs cause a concentration-dependent permeability decrease. In the case of NAP, the effect of SBEβCD is more pronounced than the effect of HPβCD. However, the opposite can be noticed in the case of PIMO. The most significant effect was exerted by SLS, especially at a 1:3 mass ratio under all circumstances in both cases.
4. Discussion
Based on our observations across different pH conditions, in the case of ionizable compounds, the impact of excipients varied based on the ratio of the different species. In general, the most significant changes in the measured parameters were observed in the presence of the neutral form of the APIs, except for sugar alcohols.
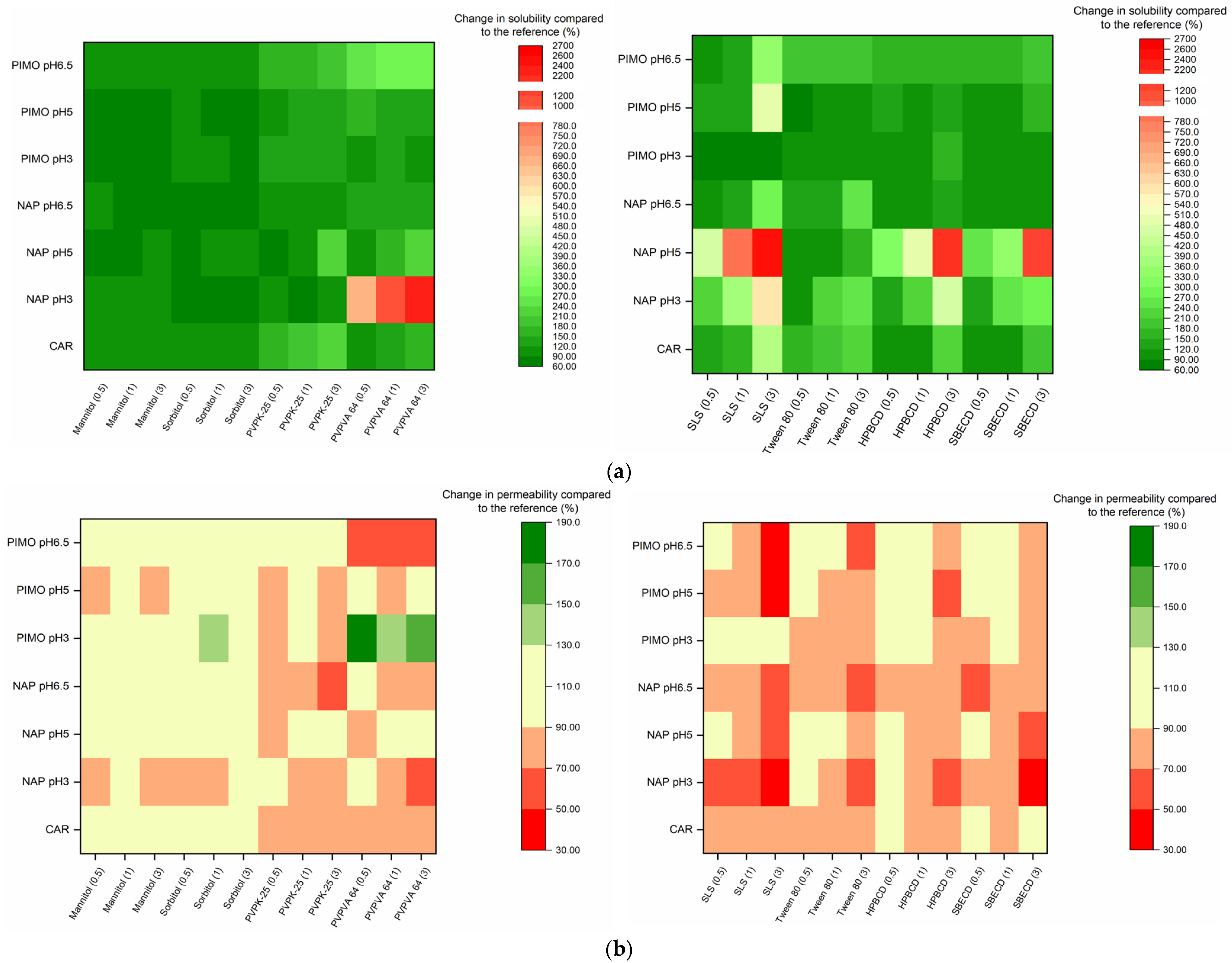
For a better visualization of the excipients’ impact, Figure 5 presents the difference in percentage compared to the reference in the case of both parameters. The measured datasets are demonstrated in Tables S2–S15. Since solubility and permeability affected each other, in the following discussion, the basis of our evaluation is the different groups of excipients and their effect on both parameters.
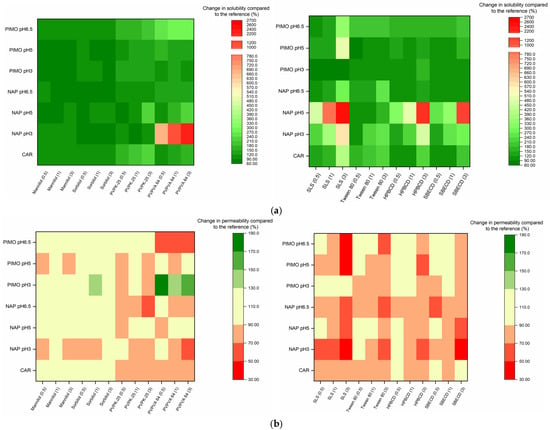
Figure 5.
(a) Change in solubility compared to the reference, expressed as a percentage. (b) Change in permeability compared to the reference, expressed as a percentage.
4.1. Fillers
Based on the results, lactose had no or slight effect on both solubility and permeability. However, sugar alcohols caused broad-ranging effects on both parameters. This may be due to their hydrotropic properties. Hydrotropic agents can reach solubility enhancement via several mechanisms [44,45]. In the case of sugar alcohols, this effect may be attributed to the reduction in water activity [46]. However, to have a solubilizing effect, they need to be applied in high concentrations [47]. In our research, sugar alcohols did not affect the neutral form’s solubility (our highest tested concentration did not reach 10 w/v%), yet they caused a reduction in the solubility of the ionized form. A possible reason is that mannitol and sorbitol increase the strength of hydrogen bonds in water molecules, making the solvent less polar [48]. As a result, the hydrophilic ionic form demonstrates reduced solubility. Their effect on permeability is not so obvious. These findings also show a good correlation with those of previous studies [49,50].
4.2. Surfactants
Surfactants are widely used solubilizing excipients that exert their influence by micelle formation. The critical micelle concentration (CMC) of each surfactant is different. The non-ionic Tween 80 has a lower CMC (0.012–1.05 mM), than the anionic SLS (8.06–8.57 mM, depending on the electrolyte concentration of the used buffer). Despite their respective CMC values, the solubilizing effect of SLS is more pronounced than that of Tween 80. Even though Tween 80 has a wide range of CMC values, its solubilisation capacity can be limited by its large molecular size and ability to form aggregates in the solution [23,51]. These aggregates may limit micelle formation with molecules, diminishing the solubilizing effect. This is demonstrated by the fact that in the presence of SLS, CAR and PIMO leads to a 4-fold increase in solubility at a 1:3 mass ratio, while that of NAP leads to a 20-fold enhancement at pH 5.0. Tween 80 provides an approximate 1.5–2-fold increase under the same conditions. A similar trend can be observed in the decrease in permeability, where SLS was able to cause a nearly 50% reduction. On the other hand, the highest effect of Tween 80 was a 35% reduction in the case of NAP at pH = 3.0. The reason for the decrease in permeability is the reduction in the concentration of the free fraction which is able to penetrate the membranes through micelle formation [45]. These findings show a good correlation with those obtained in the study by Li et al. [23], who investigated the effect of these two surfactants on carbamazepine–nicotinamide co-crystals, and the study by Tőzsér et al., who identified a similar tendency in the case of PIMO and SLS [34]. However, in the case of PIMO at pH = 3.0, SLS significantly reduced the solubility (~33%), likely due to the formation of poorly soluble lauryl sulphate salt. A similar finding was reported by Bhattachar et al. [52]. Due to this, a 10% improvement on permeability can be observed.
4.3. Polymers
Although polymers are not primarily used in formulation development as solubilizing excipients, in certain cases, they can improve the equilibrium solubility by stabilizing solute API molecules in a higher concentration. The effect of PVPVA 64 was, in almost all cases, more significant than that of PVP-K25. In the case of NAP and PVP-K25, our findings show a good correlation with the results of Bolten et al. [53], indicating that PVP polymers do not influence the solubility of NAP. The better effect of PVPVA 64 may be attributed to its copolymer structure, where the hydrophobic vinyl acetate units decrease its overall hydrophilicity compared to those of PVP-K25, giving PVPVA 64 a slightly amphiphilic character. Consequently, PVPVA 64 was observed to be suitable for micelle formation. This may also be one of the reasons for its better solubilizing effect [54,55]. In the case of NAP, the solubility of PVPVA 64 reached an extremely high value, increasing nearly 22-fold in the presence of the neutral form [56]. Nevertheless, the acid–base character of APIs is particularly important with regards to the solubility-improving effect of polymers, as emphasized by Fornells et al. [57]. Their impact on permeability is highly dependent on the ionization state of the APIs. In the case of PIMO at pH = 3.0, where the ionic form of the molecule is dominant, PVP-K25 resulted in around a 15% decrease in permeability, while PVPVA 64 led to a nearly 1.5-fold increase. In the presence of the neutral form, a reversed effect can be observed: PVP-K25 did not significantly affect the permeability, while PVPVA 64 resulted in a decrease of about 35%. However, the concentration dependence of the impact is not so obvious. For NAP, in the case of the neutral form, a significant decrease, around 50%, could be observed. This shows a good correlation with the solubility-enhancing effect. In the presence of the anionic form, PVP-K25’s effect was more pronounced. This is probably due to the various interactions between the polymers and the model compounds, which include both polar and hydrophobic ones [57,58].
4.4. Cyclodextrins
CDs are oligosaccharides containing six, seven or eight monomers and categorized as α-, β- and γ-CDs, respectively. Considering the solubility results, the solubilizing effect of HPβCD was more pronounced compared to those of SBEβCD. The most significant effect was observed with NAP, where solubility increased approximately 20-fold, whereas the enhancement for the other two APIs was around 2-to 3-fold. In case of CAR, these findings show a good correlation with the literature data, where CDs were applied at similarly low concentrations. One of these cases is the work of Volkova et al. In the case of SBEβCD, they also reached around a two-fold increase in solubility [59,60]. On one hand, this difference may be attributed to NAP’s smaller molecular size, which allows for better complexation. On the other hand, it is negatively charged, which can cause electrostatic interactions, resulting in less stable complex formation with SBEβCD [61]. The importance of molecular size is also supported by the fact that although PIMO is positively charged, SBEβCD did not cause a more significant solubility improvement than HPβCD. For permeability measurements, the CDs also reduce the free fraction of the molecules via inclusion complex formation [45,62]. However, most of the CDs at a low concentration do not affect permeability. Based on the literature, in some cases, CDs can improve permeability. One possible reason can be that when a molecule shows UWL-limited transport, CDs can enhance permeability [19]. Furthermore, in some cases, during in vivo measurements, a permeability-enhancing effect can be detected, and one of the possible explanations is complexation with membrane cholesterol [63]. The higher CD concentration resulted in a significant decrease in the permeability of the ionizable molecules. In the case of NAP, the reducing effect of SBEβCD was always more pronounced than that of HPβCD. In most cases, permeability decreased with an increasing concentration of CDs. The exception was at pH = 6.5, where the presence of the ionic form dominated. At this pH, the effect of CDs on the permeability as a function of concentration was not so obvious. For PIMO, the reducing effect of the two CDs was similar to each other and became more pronounced with the increasing concentration of CDs at all pH values [14].
5. Conclusions
In this study, the influence of nine commonly used pharmaceutical excipients (lactose monohydrate, mannitol, sorbitol, HPβCD, SBEβCD, SLS, Tween 80, PVPK-25, PVPVA 64) on the equilibrium solubility and effective permeability of three BCS II drugs (carbamazepine, naproxen, pimobendan) was investigated. The database of experimental results (Tables S2–S15) was obtained through validated methods, compiled from 474 solubility and 1141 permeability measurements. The reliability of the data is indicated by their low standard deviation.
Based on our results, the pH dependence of ionizable API solubility and permeability is confirmed in the presence of excipients.
The main goal of this study was to investigate the effect of different excipients on the relationship between equilibrium solubility and Pe. Based on the results, the solubility–permeability interplay is a phenomenon that must be considered during formulation design, as emphasized by a recently published formulation strategy [20,64]. However, our investigation enabled the evaluation of multiple influencing factors. One key parameter is the structure of APIs. For instance, NAP, which is capable of multiple molecular interactions (hydrophobic, ionic and H-bond), was able to reach even a 20-fold increase in solubility. On the other hand, in the case of CAR, which has a rigid structure and only one polar amide group, these influencing effects are less pronounced; the most significant increase was only four-fold. In the case of PIMO, its flexible structure can limit the interactions with excipients, which can be the reason that the highest effect was around 3.5–4-fold. Furthermore, the ionization state of the APIs plays a crucial role: for NAP and PIMO, the impact of excipients was significantly more pronounced in the neutral form than in the ionized form, particularly in the presence of polymers. Optimal excipient selection results in increased solubility, while minimizing any adverse effects on permeability. During the measurements, certain trends could be observed. Among the fillers, lactose appeared to be the most inert. Surfactants enhanced solubility under most conditions but caused a significant decrease in permeability. Because of this, Tween 80 may be a more suitable alternative to SLS at higher concentrations because its impact on reducing permeability is comparatively lower. CDs improve solubility, but the permeability is not always affected. However, the permeability-reducing effect of SBEβCD is significantly more pronounced compared to that of HPβCD. The effect of PVPVA 64 on both parameters is more favourable than that of PVPK-25.
Based on the results, it can be concluded that the simultaneous evaluation of solubility and permeability under multiple biorelevant pH values, considering the acid–base properties of the APIs, is crucial during the early stages of formulation development. This approach provides a comprehensive understanding of the impact of additives, supporting optimal selection.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/scipharm93020019/s1: Table S1: Structures and solubility values of the examined excipients; Table S2: Solubility of CAR in the presence of the excipients at three mass ratios; Table S3: Solubility of NAP in the presence of the excipients at three mass ratios at pH = 3.0; Table S4: Solubility of NAP in the presence of the excipients at three mass ratios at pH = 5.0; Table S5: Solubility of NAP in the presence of the excipients at three mass ratios at pH = 6.5; Table S6: Solubility of PIMO in the presence of the excipients at three mass ratios at pH = 3.0; Table S7: Solubility of PIMO in the presence of the excipients at three mass ratios at pH = 5.0; Table S8: Solubility of PIMO in the presence of the excipients at three mass ratios at pH = 6.5; Table S9: Permeability of CAR in the presence of the excipients at three mass ratios; Table S10: Permeability of NAP in the presence of the excipients at three mass ratios at pH = 3.0; Table S11: Permeability of NAP in the presence of the excipients at three mass ratios at pH = 5.0; Table S12: Permeability of NAP in the presence of the excipients at three mass ratios at pH = 6.5; Table S13: Permeability of PIMO in the presence of the excipients at three mass ratios at pH = 3.0; Table S14: Permeability of PIMO in the presence of the excipients at three mass ratios at pH = 5.0; Table S15: Permeability of PIMO in the presence of the excipients at three mass ratios at pH = 6.5.
Author Contributions
Conceptualization: K.T.-N. and B.S.; methodology: R.A.; validation: R.S.; formal analysis: V.B., R.S. and R.A.; investigation: V.B. and P.T.; writing—original draft preparation V.B., R.A. and K.T.-N.; writing—review and editing: K.T.-N., B.S. and P.T.; statistical analysis: A.M. visualization: P.T. and R.S.; supervision: K.T.-N. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data can be provided by the authors upon request. No publicly accessible archive storage is available.
Acknowledgments
V.B. would like to thank to Gergely Völgyi for giving us the opportunity to begin this research under his guidance. V.B. also expresses her appreciation to György Tibor Balogh for valuable advices.
Conflicts of Interest
Bálint Sinkó is the employee of Pion Inc., Billerica, MA, USA. The authors declare no conflicts of interest.
Abbreviations
| API | Active Pharmaceutical Ingredients |
| Pe | Effective Permeability |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| SSF | Saturated Shake Flask Method |
| CAR | Carbamazepine |
| NAP | Naproxen |
| PIMO | Pimobendan |
| BCS | Biopharmaceutical Classification System |
| CDs | Cyclodextrins |
| HPβCD | Hydroxypropyl-β-cyclodextrin |
| SBEβCD | Sulfobutylether-β-cyclodextrin |
| PVP-K25 | Polyvinylpyrrolidone K25 |
| PVPVA 64 | Polyvinylpyrrolidone/vinyl acetate 64 |
| SLS | Sodium Lauryl Sulphate |
| Tween 80 | Polysorbat 80 |
| CMC | Critical Micelle Concentration |
References
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of Nanoparticulate Strategies for Solubility Enhancement of Poorly Soluble Drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G.; Marra, F.; Titomanlio, G.; d’Amore, M.; Barba, A.A. Gastrointestinal Behavior and ADME Phenomena: I. In Vitro Simulation. J. Drug Deliv. Sci. Technol. 2016, 35, 272–283. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation Design for Poorly Water-Soluble Drugs Based on Biopharmaceutics Classification System: Basic Approaches and Practical Applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 46, 413–420. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical Approaches for Enhancing Solubility and Oral Bioavailability of Poorly Soluble Drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Xie, B.; Liu, Y.; Li, X.; Yang, P.; He, W. Solubilization Techniques Used for Poorly Water-Soluble Drugs. Acta Pharm. Sin. B 2024, 14, 4683–4716. [Google Scholar] [CrossRef]
- Jagtap, S.; Magdum, C.; Jadge, D.; Jagtap, R. Solubility Enhancement Technique: A Review. J. Pharm. Sci. Res. 2018, 10, 2205–2211. [Google Scholar]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The Effect of the Particle Size Reduction on the Biorelevant Solubility and Dissolution of Poorly Soluble Drugs with Different Acid-Base Character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M.; Hoffman, A.; Amidon, G.E.; Amidon, G.L. The Solubility-Permeability Interplay in Using Cyclodextrins as Pharmaceutical Solubilizers: Mechanistic Modeling and Application to Progesterone. J. Pharm. Sci. 2010, 99, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The Solubility–Permeability Interplay and Oral Drug Formulation Design: Two Heads Are Better than One. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Miller, J.M.; Beig, A.; Krieg, B.J.; Carr, R.A.; Borchardt, T.B.; Amidon, G.E.; Amidon, G.L.; Dahan, A. The Solubility-Permeability Interplay: Mechanistic Modeling and Predictive Application of the Impact of Micellar Solubilization on Intestinal Permeation. Mol. Pharm. 2011, 8, 1848–1856. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. Oral Delivery of Lipophilic Drugs: The Tradeoff between Solubility Increase and Permeability Decrease When Using Cyclodextrin-Based Formulations. PLoS ONE 2013, 8, e68237. [Google Scholar] [CrossRef]
- Beig, A.; Miller, J.M.; Dahan, A. The Interaction of Nifedipine with Selected Cyclodextrins and the Subsequent Solubility-Permeability Trade-Off. Eur. J. Pharm. Biopharm. 2013, 85, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Beig, A.; Miller, J.M.; Lindley, D.; Dahan, A. Striking the Optimal Solubility-Permeability Balance in Oral Formulation Development for Lipophilic Drugs: Maximizing Carbamazepine Blood Levels. Mol. Pharm. 2017, 14, 319–327. [Google Scholar] [CrossRef]
- Beig, A.; Miller, J.M.; Lindley, D.; Carr, R.A.; Zocharski, P.; Agbaria, R.; Dahan, A. Head-To-Head Comparison of Different Solubility-Enabling Formulations of Etoposide and Their Consequent Solubility-Permeability Interplay. J. Pharm. Sci. 2015, 104, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of Surfactant Excipients in Oral Drug Formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Maw, P.D.; Loftsson, T.; Jansook, P. A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery. Pharmaceuticals 2022, 15, 1447. [Google Scholar] [CrossRef]
- Kádár, S.; Tőzsér, P.; Nagy, B.; Farkas, A.; Nagy, Z.K.; Tsinman, O.; Tsinman, K.; Csicsák, D.; Völgyi, G.; Takács-Novák, K.; et al. Flux-Based Formulation Development—A Proof of Concept Study. AAPS J. 2022, 24, 22. [Google Scholar] [CrossRef]
- Pawar, B.M.; Rahman, S.N.R.; Pawde, D.M.; Goswami, A.; Shunmugaperumal, T. Orally Administered Drug Solubility-Enhancing Formulations: Lesson Learnt from Optimum Solubility-Permeability Balance. AAPS PharmSciTech 2021, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Fine-Shamir, N.; Dahan, A. Ethanol-Based Solubility-Enabling Oral Drug Formulation Development: Accounting for the Solubility-Permeability Interplay. Int. J. Pharm. 2024, 653, 123893. [Google Scholar] [CrossRef]
- Li, M.; Qiao, N.; Wang, K. Influence of Sodium Lauryl Sulfate and Tween 80 on Carbamazepine-Nicotinamide Cocrystal Solubility and Dissolution Behaviour. Pharmaceutics 2013, 5, 508–524. [Google Scholar] [CrossRef]
- Nayak, A.K.; Panigrahi, P.P. Solubility Enhancement of Etoricoxib by Cosolvency Approach. ISRN Phys. Chem. 2012, 2012, 820653. [Google Scholar] [CrossRef]
- FDA—Inactive Ingredients Database. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredients-database-download (accessed on 25 February 2025).
- Avdeef, A. Absorption and Drug Development: Solubility, Permeability and Charge State, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bergström, C.A.S.; Holm, R.; Jørgensen, S.A.; Andersson, S.B.E.; Artursson, P.; Beato, S.; Borde, A.; Box, K.; Brewster, M.; Dressman, J.; et al. Early Pharmaceutical Profiling to Predict Oral Drug Absorption: Current Status and Unmet Needs. Eur. J. Pharm. Sci. 2014, 57, 173–199. [Google Scholar] [CrossRef]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Avdeef, A. The Rise of PAMPA. Expert Opin. Drug Metab. Toxicol. 2005, 1, 325–342. [Google Scholar] [CrossRef]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of Equilibrium Solubility Measurement by Saturation Shake-Flask Method Using Hydrochlorothiazide as Model Compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Veseli, A.; Žakelj, S.; Kristl, A. A Review of Methods for Solubility Determination in Biopharmaceutical Drug Characterization. Drug Dev. Ind. Pharm. 2019, 45, 1717–1724. [Google Scholar] [CrossRef]
- Tong, W. Practical Aspects of Solubility Determination in Pharmaceutical Preformulation. In Solvent Systems and Their Selection in Pharmaceutics and Biopharmaceutics. Biotechnology: Pharmaceutical Aspects; Augustijns, P., Brewster, M.E., Eds.; Springer: New York, NY, USA, 2007; Volume VI. [Google Scholar]
- Tőzsér, P.; Kovács, L.L.; Kádár, S.; Csicsák, D.; Sóti, P.; Völgyi, G.; Sinkó, B.; Nagy, Z.K.; Borbás, E. The Effect of Surfactants and PH Modifying Agents on the Dissolution and Permeation of Pimobendan. Period. Polytech. Chem. Eng. 2023, 67, 1–10. [Google Scholar] [CrossRef]
- Avdeef, A.; Fuguet, E.; Llinàs, A.; Ràfols, C.; Bosch, E.; Völgyi, G.; Verbic, T.; Boldyreva, E.; Takács-Novák, K. Equilibrium Solubility Measurement of Ionizable Drugs—Consensus Recommendations for Improving Data Quality. Admet Dmpk 2016, 4, 117–178. [Google Scholar] [CrossRef]
- Völgyi, G.; Csicsák, D.; Takács-Novák, K. Right Filter-Selection for Phase Separation in Equilibrium Solubility Measurement. Eur. J. Pharm. Sci. 2018, 123, 98–105. [Google Scholar] [CrossRef]
- Marín, M.T.; Margarit, M.V.; Salcedo, G.E. Characterization and Solubility Study of Solid Dispersions of Flunarizine and Polyvinylpyrrolidone. Il Farm. 2002, 57, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Sinkó, B.; Kökösi, J.; Avdeef, A.; Takács-Novák, K. A PAMPA Study of the Permeability-Enhancing Effect of New Ceramide Analogues. Chem. Biodivers. 2009, 6, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- El-Massik, M.A.; Abdallah, O.Y.; Galal, S.; Daabis, N.A. Towards a Universal Dissolution Medium for Carbamazepine. Drug Dev. Ind. Pharm. 2006, 32, 893–905. [Google Scholar] [CrossRef]
- Fine-Shamir, N.; Beig, A.; Miller, J.M.; Dahan, A. The Solubility, Permeability and the Dose as Key Factors in Formulation Development for Oral Lipophilic Drugs: Maximizing the Bioavailability of Carbamazepine with a Cosolvent-Based Formulation. Int. J. Pharm. 2020, 582, 119307. [Google Scholar] [CrossRef]
- Völgyi, G.; Baka, E.; Box, K.J.; Comer, J.E.A.; Takács-Novák, K. Study of PH-Dependent Solubility of Organic Bases. Revisit of Henderson-Hasselbalch Relationship. Anal. Chim. Acta 2010, 673, 40–46. [Google Scholar] [CrossRef]
- Jain, P.; Goel, A.; Sharma, S.; Parmar, M. Solubility enhancement techniques with special emphasis on hydrotrophy. Int. J. Pharma Prof. Res. 2010, 1, 34–45. [Google Scholar]
- Fine-Shamir, N.; Dahan, A. Solubility-Enabling Formulations for Oral Delivery of Lipophilic Drugs: Considering the Solubility-Permeability Interplay for Accelerated Formulation Development. Expert Opin. Drug Deliv. 2024, 21, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Molinier, V.; Aubry, J.M. Sugar-Based Hydrotropes: Preparation, Properties and Applications. Carbohydr. Chem. 2014, 40, 51–72. [Google Scholar]
- Abdullah Ali, H.; Kamal Omer, H. Solubility Enhancement of a Poorly Water-Soluble Drug Using Hydrotropy and Mixed Hydrotropy-Based Solid Dispersion Techniques. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 7161660. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.W.; Albers, E. Effect of Hydrotropic Substances on the Complexation of Sparingly Soluble Drugs with Cyclodextrin Derivatives and the Influence of Cyclodextrin Complexation on the Pharmacokinetics of the Drugs. J. Pharm. Sci. 1991, 80, 599–604. [Google Scholar] [CrossRef]
- Dash, R.P.; Srinivas, N.R.; Babu, R.J. Use of Sorbitol as Pharmaceutical Excipient in the Present Day Formulations–Issues and Challenges for Drug Absorption and Bioavailability. Drug Dev. Ind. Pharm. 2019, 45, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Straughn, A.B.; Sadrieh, N.; Meyer, M.; Faustino, P.J.; Ciavarella, A.B.; Meibohm, B.; Yates, C.R.; Hussain, A.S. A Modern View of Excipient Effects on Bioequivalence: Case Study of Sorbitol. Pharm. Res. 2007, 24, 73–80. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Rosés, M.; Bosch, E. Critical Micelle Concentration of Surfactants in Aqueous Buffered and Unbuffered Systems. Anal. Chim. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Bhattachar, S.N.; Risley, D.S.; Werawatganone, P.; Aburub, A. Weak Bases and Formation of a Less Soluble Lauryl Sulfate Salt/Complex in Sodium Lauryl Sulfate (SLS) Containing Media. Int. J. Pharm. 2011, 412, 95–98. [Google Scholar] [CrossRef]
- Bolten, D.; Lietzow, R.; Türk, M. Solubility of Ibuprofen, Phytosterol, Salicylic Acid, and Naproxen in Aqueous Solutions. Chem. Eng. Technol. 2013, 36, 426–434. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Shi, X.B.; Chang, J.H.; Wang, R.X.; Zhou, J.Y.; Liu, P. Amorphous Solid Dispersions of Glycyrrhetinic Acid: Using Soluplus, PVP, and PVPVA as the Polymer Matrix to Enhance Solubility, Bioavailability, and Stability. AAPS PharmSciTech 2025, 26, 18. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Qiao, N.; Yardley, V.; Li, M. Investigating Permeation Behavior of Flufenamic Acid Cocrystals Using a Dissolution and Permeation System. Mol. Pharm. 2018, 15, 4257–4272. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.M.; Olesen, N.E.; Holm, P.; Langguth, P.; Holm, R.; Rades, T. Influence of Polymer Molecular Weight on Drug–Polymer Solubility: A Comparison between Experimentally Determined Solubility in PVP and Prediction Derived from Solubility in Monomer. J. Pharm. Sci. 2015, 104, 2905–2912. [Google Scholar] [CrossRef]
- Fornells, E.; Fuguet, E.; Mañé, M.; Ruiz, R.; Box, K.; Bosch, E.; Ràfols, C. Effect of Vinylpyrrolidone Polymers on the Solubility and Supersaturation of Drugs; a Study Using the Cheqsol Method. Eur. J. Pharm. Sci. 2018, 117, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.E.; Tao, J.; Zhang, G.G.Z.; Yu, L. Solubilities of Crystalline Drugs in Polymers: An Improved Analytical Method and Comparison of Solubilities of Indomethacin and Nifedipine in PVP, PVP/VA, and PVAc. J. Pharm. Sci. 2010, 99, 4023–4031. [Google Scholar] [CrossRef]
- Volkova, T.; Simonova, O.; Perlovich, G. Mechanistic Insight in Permeability through Different Membranes in the Presence of Pharmaceutical Excipients: A Case of Model Hydrophobic Carbamazepine. Pharmaceutics 2024, 16, 184. [Google Scholar] [CrossRef]
- Medarević, D.; Kachrimanis, K.; Djurić, Z.; Ibrić, S. Influence of Hydrophilic Polymers on the Complexation of Carbamazepine with Hydroxypropyl-β-Cyclodextrin. Eur. J. Pharm. Sci. 2015, 78, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Roy, S.; Kumar, A.; Mahmood, S.; Khodapanah, N.; Thomas, S.; Agatemor, C.; Ghosal, K. Physicochemical Characterization, Molecular Docking, and in Vitro Dissolution of Glimepiride-Captisol Inclusion Complexes. ACS Omega 2020, 5, 19968–19977. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. The Use of Captisol (SBE7-β-CD) in Oral Solubility-Enabling Formulations: Comparison to HPβCD and the Solubility-Permeability Interplay. Eur. J. Pharm. Sci. 2015, 77, 73–78. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Effects on Drug Permeation through Biological Membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef]
- Borbás, E.; Nagy, Z.K.; Nagy, B.; Balogh, A.; Farkas, B.; Tsinman, O.; Tsinman, K.; Sinkó, B. The Effect of Formulation Additives on in Vitro Dissolution-Absorption Profile and in Vivo Bioavailability of Telmisartan from Brand and Generic Formulations. Eur. J. Pharm. Sci. 2018, 114, 310–317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).