Antidiabetic Effect of Dihydrobetulonic Acid Derivatives as Pparα/γ Agonists
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Compounds Activity and Specificity In Vitro
2.2. Dual-Luciferase Reporter Assay to Study the Compounds Activity and Specificity
2.3. Testing Substances for PPARα/γ Activation
2.4. Animals
2.5. The OGTT
2.6. The ITT
2.7. The AY Mice Experiment Design
2.8. Biochemical Assays
2.9. Histological Examination
2.10. Statistical Analysis
3. Results
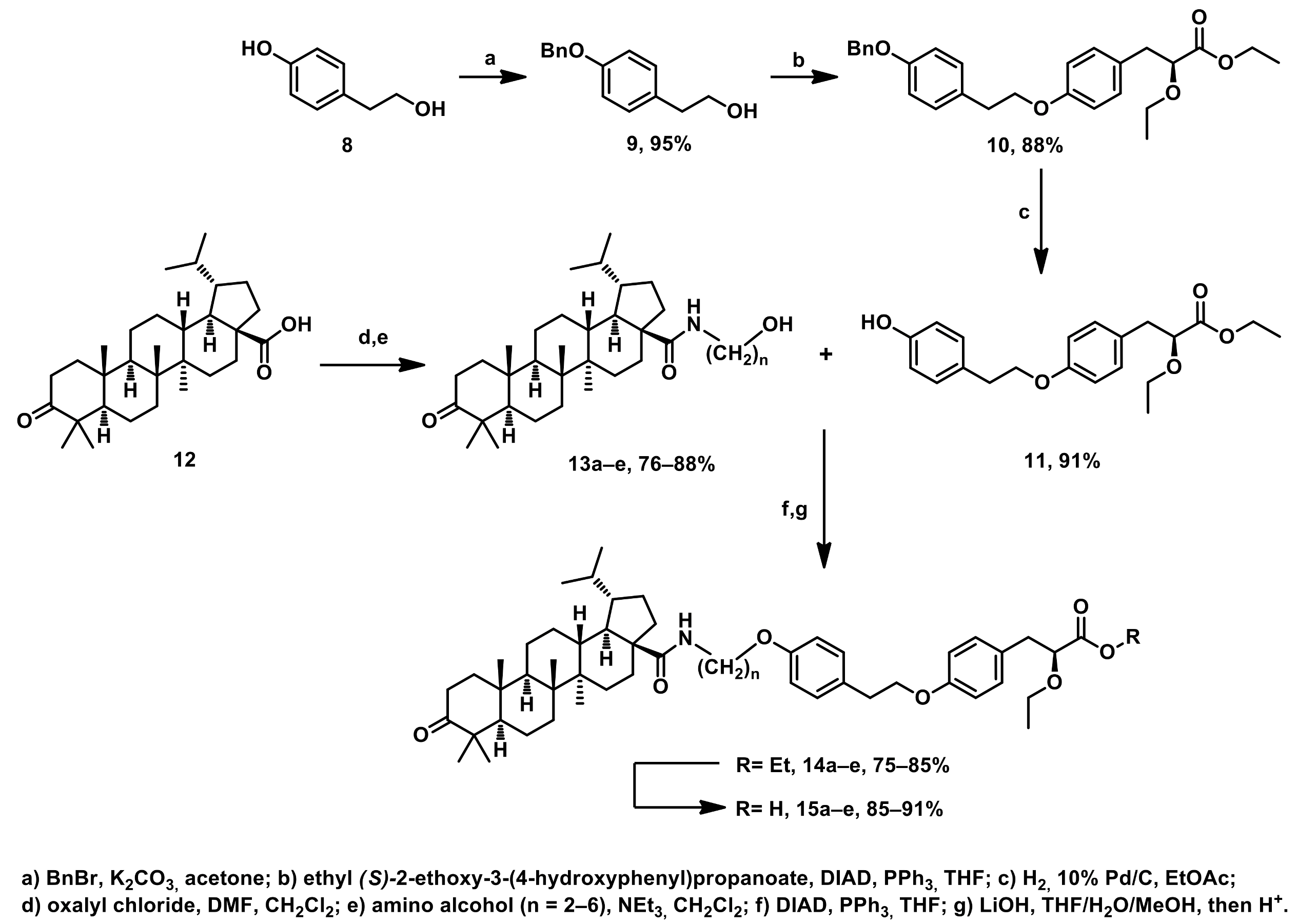
3.1. Chemistry
3.2. Biological Testing
In Vitro PPARα and PPARγ Receptors Agonist Activity Evaluation
3.3. In Vivo Experiment
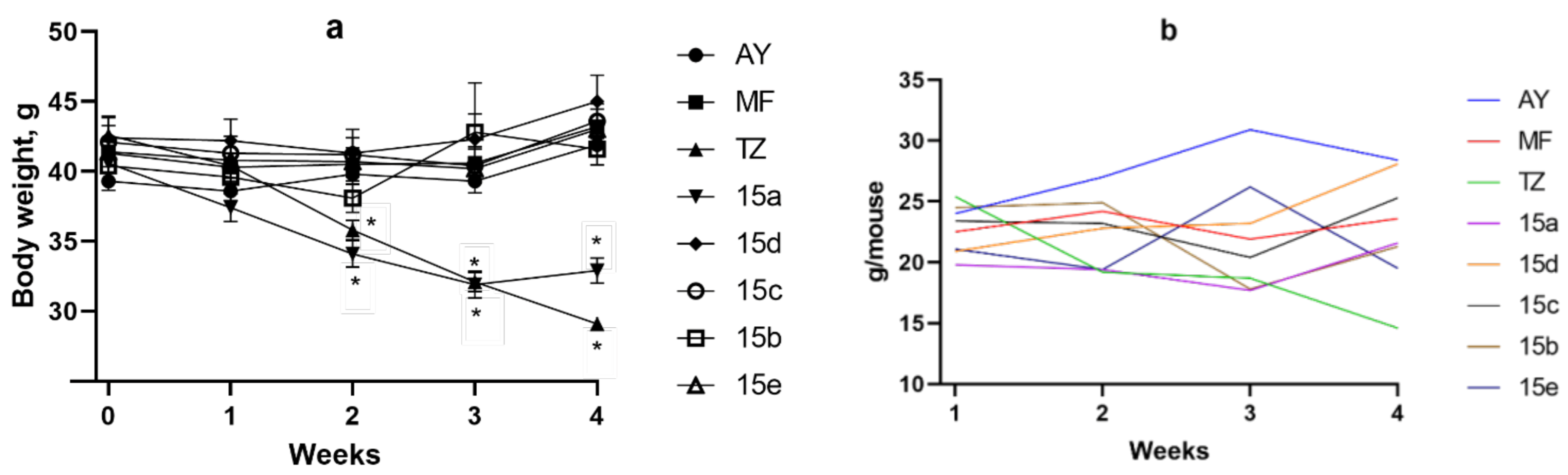
Body Weight and Food Consumption Dynamics
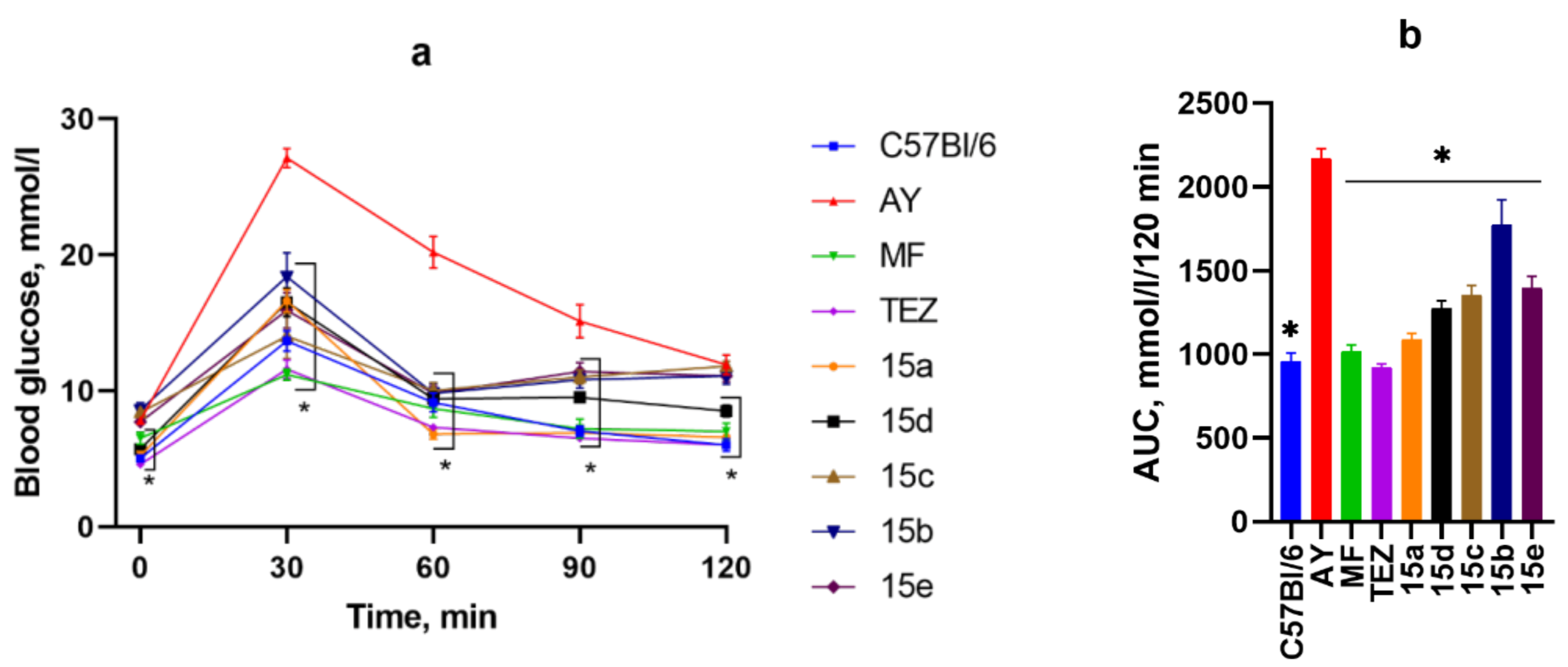
3.4. OGTT After 2 Weeks of Substance Administration
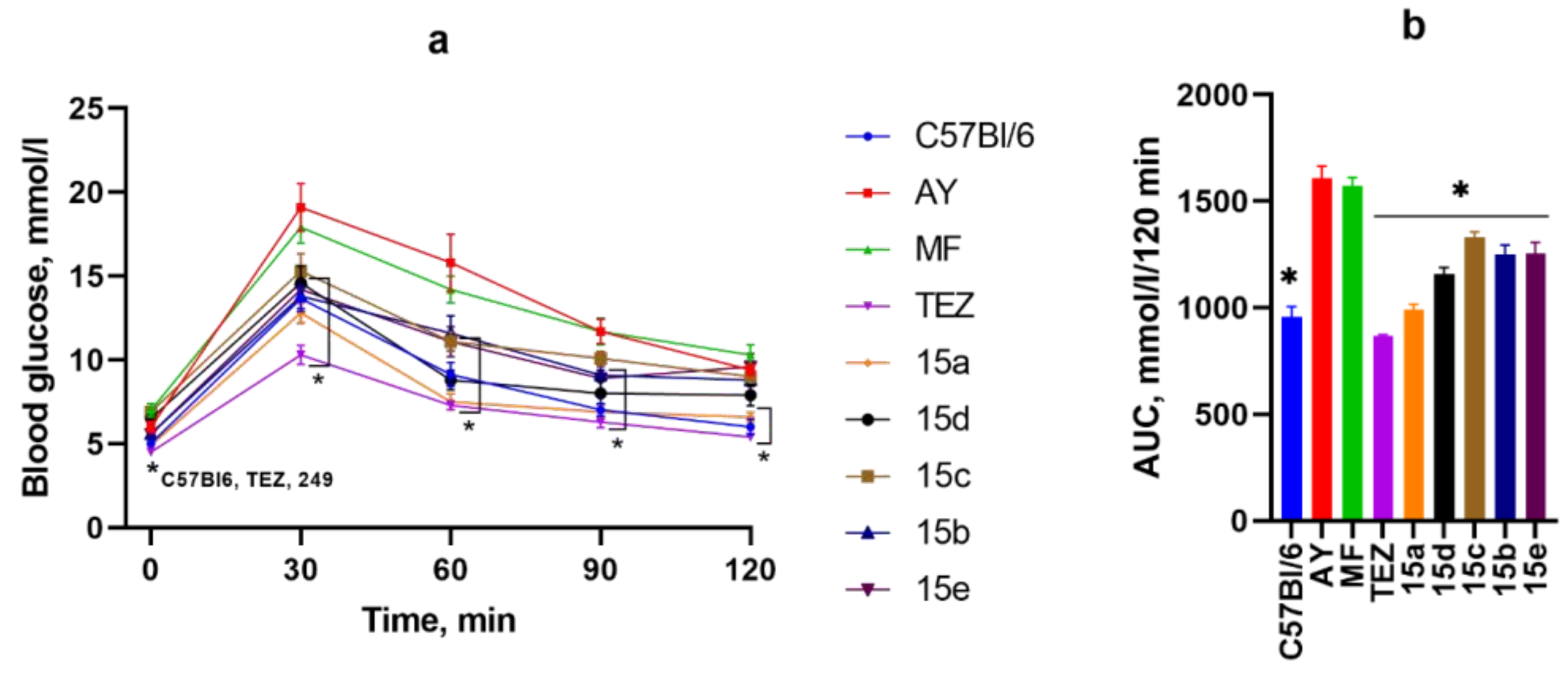
3.5. OGTT After 4 Weeks of Substance Administration
3.6. ITT
3.7. Biochemical Blood Analysis
3.8. Mass of Animal Organs and Tissues
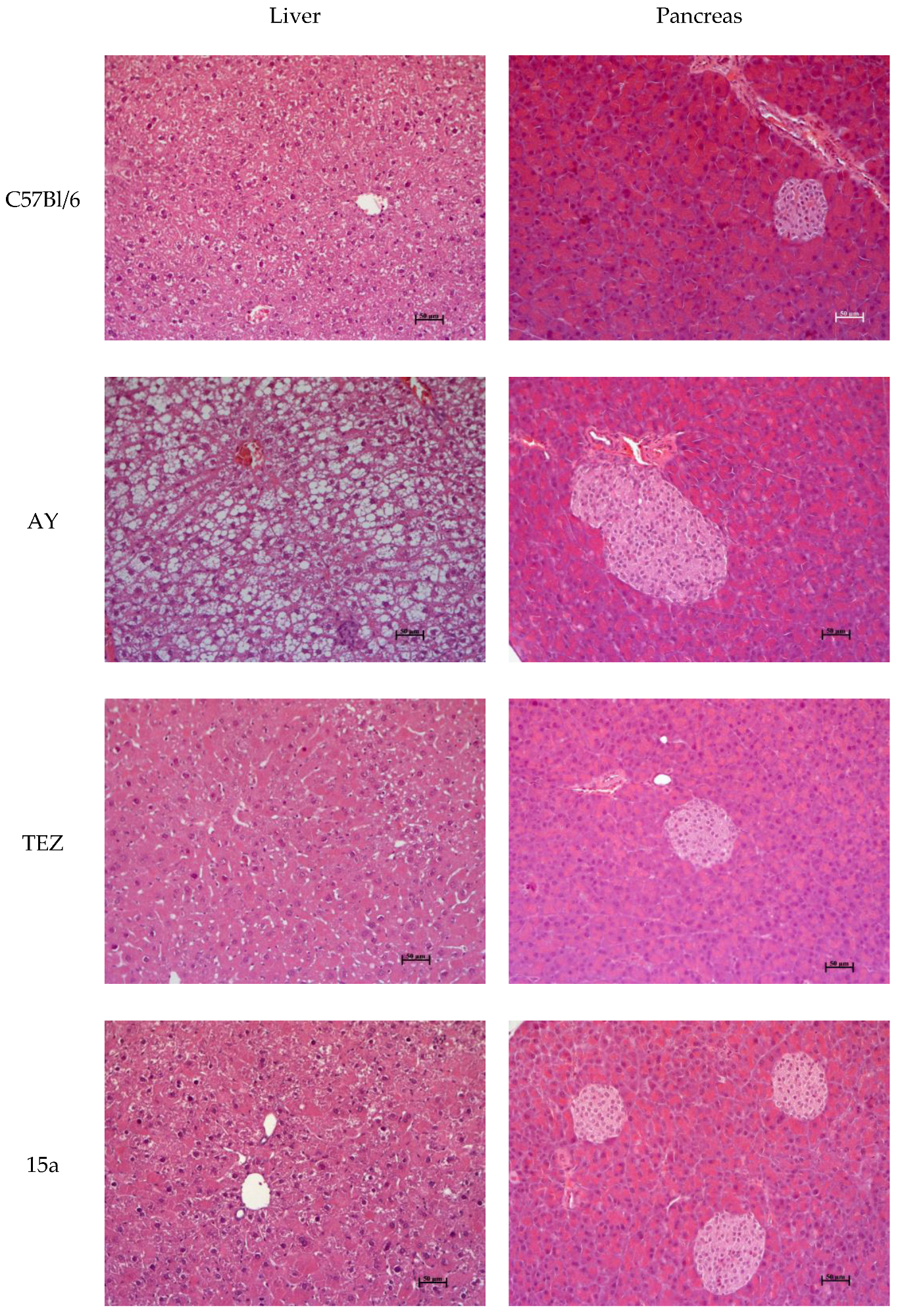
3.9. Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Kalliora, C.; Drosatos, K. The Glitazars Paradox: Cardiotoxicity of the Metabolically Beneficial Dual PPARα and PPARγ Activation. J. Cardiovasc. Pharmacol. 2020, 76, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef] [PubMed]
- Doumas, M.; Imprialos, K.; Stavropoulos, K.; Athyros, V.G. Pharmacological Management of Type 2 Diabetes Complications. Curr. Vasc. Pharmacol. 2020, 18, 101–103. [Google Scholar] [CrossRef]
- Takada, I.; Makishima, M. Peroxisome proliferator-activated receptor agonists and antagonists: A patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Alnuaimi, S.; Reljic, T.; Abdulla, F.S.; Memon, H.; Al-Ali, S.; Smith, T.; Serdarevic, F.; Velija Asimi, Z.; Kumar, A.; Semiz, S. PPAR agonists as add-on treatment with metformin in management of type 2 diabetes: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 8809. [Google Scholar] [CrossRef]
- Balachandran, K. Dual PPAR α/γ Agonists: Continuing Cardiac Concerns. Indian J. Endocrinol. Metab. 2019, 23, 586–587. [Google Scholar]
- Bhosle, D.; Bhosle, V.; Bobde, J.; Bhagat, A.; Shaikh, H.; Kadam, R. Study of Saroglitazar in Treatment Of Pre-diabetes with Dyslipidemia: STOP-D. J. Assoc. Physicians India 2018, 66, 14–17. [Google Scholar]
- Dutta, D.; Bhattacharya, S.; Surana, V.; Aggarwal, S.; Singla, R.; Khandelwal, D.; Sharma, M. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1759–1768. [Google Scholar] [CrossRef]
- Jain, N.; Bhansali, S.; Kurpad, A.; Hawkins, M.; Sharma, A.; Kaur, S.; Rastogi, A.; Bhansali, A. Effect of a Dual PPAR α/γ agonist on Insulin Sensitivity in Patients of Type 2 Diabetes with Hypertriglyceridemia- Randomized double-blind placebo-controlled trial. Sci. Rep. 2019, 9, 19017. [Google Scholar] [CrossRef]
- Ji, L.; Song, W.; Fang, H.; Li, W.; Geng, J.; Wang, Y.; Guo, L.; Cai, H.; Yang, T.; Li, H.; et al. Efficacy and safety of chiglitazar, a novel peroxisome proliferator-activated receptor pan-agonist, in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, phase 3 trial (CMAP). Sci. Bull. 2021, 66, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Ai, G.; Chen, B.; Yao, H.; Cao, H.; Pan, D.; Lu, X. Impact of chiglitazar on glycemic control in type 2 diabetic patients with metabolic syndrome and insulin resistance: A pooled data analysis from two phase III trials. J. Diabetes 2024, 16, e13484. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Al-Assaf, H. Hepatoprotective and antioxidant effect of corosolic acid on carbon tetrachloride induced hepatotoxicity. Afr. J. Pharm. Pharmacol. 2013, 7, 673–678. [Google Scholar] [CrossRef]
- Semenov, D.E.; Zhukova, N.A.; Ivanova, E.P.; Sorokina, I.V.; Baiev, D.S.; Nepomnyashchikh, G.I.; Tolstikova, T.G.; Biryukova, M.S. Hepatoprotective Properties of Betulonic Acid Amide and Heptral in Toxic Liver Injury Induced by Carbon Tetrachloride in Combination with Ethanol. Bull. Exp. Biol. Med. 2015, 158, 336–341. [Google Scholar] [CrossRef]
- Afrose, S.; Hossain, M.S.; Maki, T.; Tsujii, H. Karaya root saponin exerts a hypocholesterolemic response in rats fed a high-cholesterol diet. Nutr. Res. 2009, 29, 350–354. [Google Scholar] [CrossRef]
- Tai, M.M. A Mathematical Model for the Determination of Total Area Under Glucose Tolerance and Other Metabolic Curves. Diabetes Care 1994, 17, 152–154. [Google Scholar] [CrossRef]
- Wu, M.; Ye, W.; Zheng, Y.; Zhang, S. Oxamate Enhances the Anti-Inflammatory and Insulin-Sensitizing Effects of Metformin in Diabetic Mice. Pharmacology 2017, 100, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, V.; Blokhin, M.; Kuranov, S.; Khvostov, M.; Baev, D.; Borisova, M.S.; Luzina, O.; Tolstikova, T.G.; Salakhutdinov, N.F. Triterpenic Acid Amides as a Promising Agent for Treatment of Metabolic Syndrome. Sci. Pharm. 2021, 89, 4. [Google Scholar] [CrossRef]
- Kuranov, S.O.; Luzina, O.A.; Onopchenko, O.; Pishel, I.; Zozulya, S.; Gureev, M.; Salakhutdinov, N.F.; Krasavin, M. Exploring bulky natural and natural-like periphery in the design of p-(benzyloxy)phenylpropionic acid agonists of free fatty acid receptor 1 (GPR40). Bioorg. Chem. 2020, 99, 103830. [Google Scholar] [CrossRef]
- Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules 2021, 26, 3435. [Google Scholar] [CrossRef] [PubMed]
- Astrazeneca. Process for The Preparation of 2-Ethoxy-3-[4-(2-(Methanesulphonyloxyphenyl)-Ethoxy) Phenil] Propanoic Acid. WO2003082812A2, 9 October 2023.
- Tan, C.K.; Zhuang, Y.; Wahli, W. Synthetic and natural Peroxisome Proliferator-Activated Receptor (PPAR) agonists as candidates for the therapy of the metabolic syndrome. Expert Opin. Ther. Targets 2017, 21, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Blokhin, M.E.; Kuranov, S.O.; Khvostov, M.V.; Fomenko, V.V.; Luzina, O.A.; Zhukova, N.A.; Elhajjar, C.; Tolstikova, T.G.; Salakhutdinov, N.F. Terpene-Containing Analogues of Glitazars as Potential Therapeutic Agents for Metabolic Syndrome. Curr. Issues Mol. Biol. 2023, 45, 2230–2247. [Google Scholar] [CrossRef] [PubMed]
- Chira, E.C.; McMillen, T.S.; Wang, S.; Haw, A.; O’Brien, K.D.; Wight, T.N.; Chait, A. Tesaglitazar, a dual peroxisome proliferator-activated receptor alpha/gamma agonist, reduces atherosclerosis in female low-density lipoprotein receptor deficient mice. Atherosclerosis 2007, 195, 100–109. [Google Scholar] [CrossRef][Green Version]
- Chaput, E.; Saladin, R.; Silvestre, M.; Edgar, A.D. Fenofibrate and Rosiglitazone Lower Serum Triglycerides with Opposing Effects on Body Weight. Biochem. Biophys. Res. Commun. 2000, 271, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Kashireddy, P.; Borensztajn, J. The peroxisome-proliferator-activated receptor alpha agonist ciprofibrate severely aggravates hypercholesterolaemia and accelerates the development of atherosclerosis in mice lacking apolipoprotein E. Biochem. J. 2003, 373(Pt 3), 941–947. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef]
- Meyer, K.; Völkl, A.; Endele, R.; Kühnle, H.F.; Pill, J. Species differences in induction of hepatic enzymes by BM 17.0744, an activator of peroxisome proliferator-activated receptor alpha (PPARα). Arch. Toxicol. 1999, 73, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, Y.; Zhang, X.; Li, P.; Shang, L.; Chen, X.; Zeng, J. Targeting peroxisomal fatty acid oxidation improves hepatic steatosis and insulin resistance in obese mice. J. Biol. Chem. 2023, 299, 102845. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Laybutt, D.R.; Kaneto, H.; Bonner-Weir, S.; Sharma, A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 2001, 50, S154–S159. [Google Scholar] [CrossRef] [PubMed]



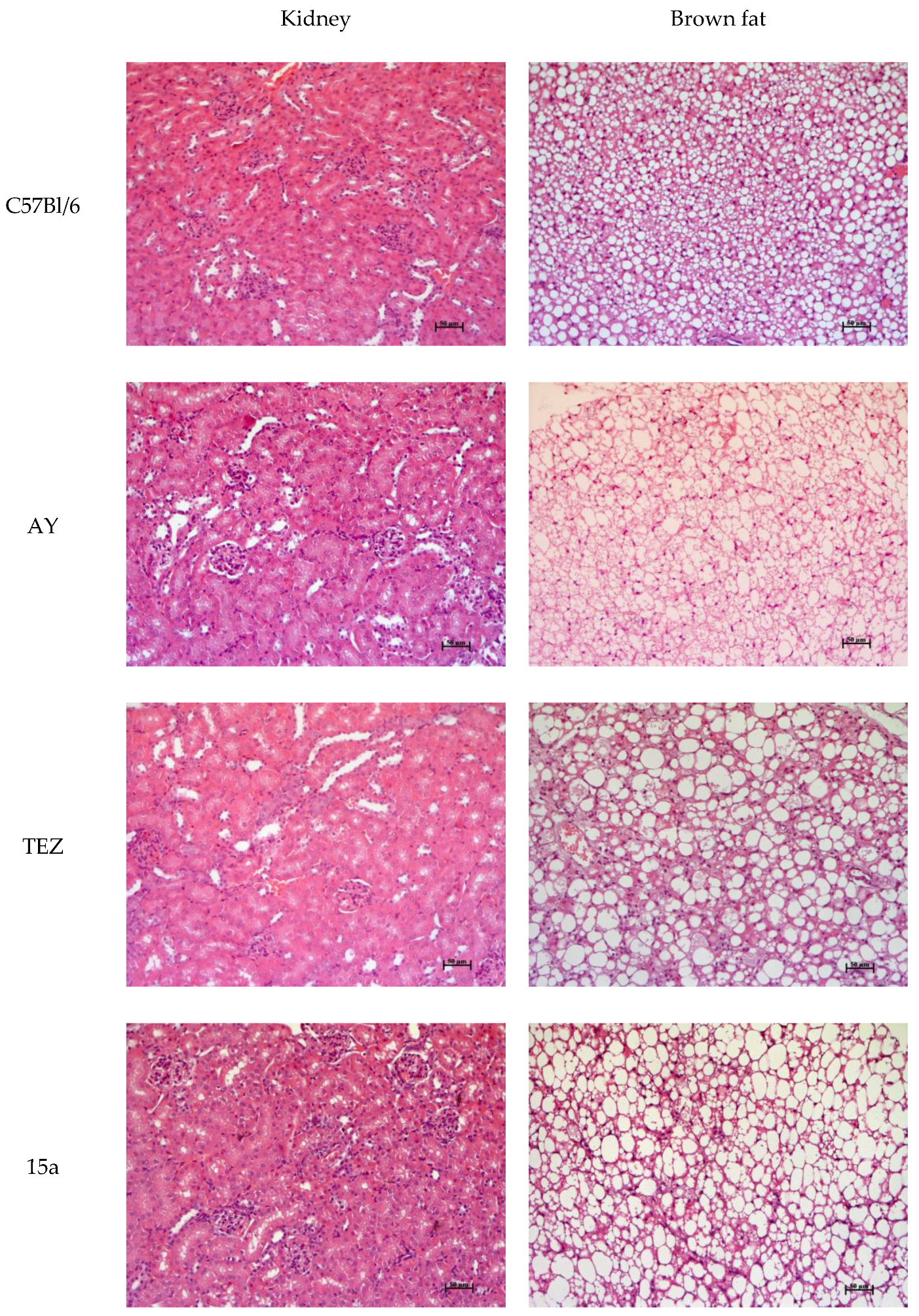
| Group | TC, mmol/L | TG, mmol/L | ALT, U/L | AST, U/L | ALP, U/L |
|---|---|---|---|---|---|
| C57Bl/6 | 3.88 ± 0.03 | 1.32 ± 0.05 | 13.10 ± 1.55 | 26.19 ± 3.66 | 68.37 ± 10.03 |
| AY | 3.87 ± 0.03 | 1.39 ± 0.06 | 14.55 ± 2.94 | 27.36 ± 5.75 | 79.50 ± 13.55 |
| MF | 3.95 ± 0.04 | 1.43 ± 0.06 | 5.24 ± 1.10 * | 41.32 ± 6.68 | 121.70 ± 17.61 |
| TEZ | 3.85 ± 0.02 | 1.12 ± 0.03 * | 20.52 ± 3.14 | 58.06 ± 9.00 * | 383.92 ± 74.95 * |
| 15a | 3.88 ± 0.03 | 1.19 ± 0.04 * | 16.59 ± 2.77 | 50.63 ± 9.14 | 264.28 ± 29.62 * |
| 15d | 3.83 ± 0.02 | 1.32 ± 0.04 | 20.20 ± 3.07 | 50.63 ± 8.46 | 165.81 ± 16.56 * |
| 15c | 3.80 ± 0.01 | 1.27 ± 0.02 | 24.44 ± 3.47 | 47.64 ± 7.25 | 130.37 ± 20.19 |
| 15b | 3.81 ± 0.02 | 1.20 ± 0.02 * | 21.24 ± 2.49 | 49.47 ± 5.27 * | 135.55 ± 13.65 * |
| 15e | 3.89 ± 0.04 | 1.41 ± 0.05 | 19.79 ± 2.80 | 36.67 ± 7.42 | 143.36 ± 19.56 * |
| Body Mass, g. | Liver Mass, g. | Gonadal Fat Mass, g. | Interscapular Fat Mass, g. | Interscapular Brown Fat Mass, g. | |
|---|---|---|---|---|---|
| C57Bl/6 | 26.2 ± 0.36 | 1.31 ± 0.04 | 0.3 ± 0.03 | - | 0.17 ± 0.011 |
| AY | 41.1 ± 0.40 | 1.76 ± 0.10 | 2.1 ± 0.20 | 0.94 ± 0.08 | 0.31 ± 0.03 |
| MF | 40.3 ± 1.45 | 1.59 ± 0.04 | 2.4 ± 0.14 | 1.15 ± 0.07 | 0.26 ± 0.029 |
| TEZ | 26.0 ± 0.77 * | 2.58 ± 0.22 * | 0.4 ± 0.07 * | 0.44 ± 0.08 * | 0.22 ± 0.006 * |
| 15a | 31.5 ± 0.84 * | 2.14 ± 0.11 * | 1.2 ± 0.09 * | 0.64 ± 0.03 * | 0.20 ± 0.026 * |
| 15d | 40.3 ±1.07 | 2.08 ± 0.13 | 2.2 ± 0.12 | 1.04 ± 0.07 | 0.36 ± 0.074 |
| 15c | 42.2 ± 1.36 | 2.11 ± 0.22 | 2.4 ± 0.15 | 1.11 ± 0.08 | 0.32 ± 0.034 |
| 15b | 43.6 ± 1.77 | 2.19 ± 0.21 | 2.3 ± 0.17 | 1.16 ± 0.08 | 0.30 ± 0.051 |
| 15e | 41.7 ± 0.50 | 2.00 ±0.09 | 2.3 ± 0.18 | 1.05 ± 0.15 | 0.30 ± 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khvostov, M.V.; Blokhin, M.E.; Borisov, S.A.; Fomenko, V.V.; Meshkova, Y.V.; Zhukova, N.A.; Nikonova, S.V.; Pavlova, S.V.; Pogosova, M.A.; Medvedev, S.P.; et al. Antidiabetic Effect of Dihydrobetulonic Acid Derivatives as Pparα/γ Agonists. Sci. Pharm. 2024, 92, 65. https://doi.org/10.3390/scipharm92040065
Khvostov MV, Blokhin ME, Borisov SA, Fomenko VV, Meshkova YV, Zhukova NA, Nikonova SV, Pavlova SV, Pogosova MA, Medvedev SP, et al. Antidiabetic Effect of Dihydrobetulonic Acid Derivatives as Pparα/γ Agonists. Scientia Pharmaceutica. 2024; 92(4):65. https://doi.org/10.3390/scipharm92040065
Chicago/Turabian StyleKhvostov, Mikhail V., Mikhail E. Blokhin, Sergey A. Borisov, Vladislav V. Fomenko, Yulia V. Meshkova, Natalia A. Zhukova, Sophia V. Nikonova, Sophia V. Pavlova, Maria A. Pogosova, Sergey P. Medvedev, and et al. 2024. "Antidiabetic Effect of Dihydrobetulonic Acid Derivatives as Pparα/γ Agonists" Scientia Pharmaceutica 92, no. 4: 65. https://doi.org/10.3390/scipharm92040065
APA StyleKhvostov, M. V., Blokhin, M. E., Borisov, S. A., Fomenko, V. V., Meshkova, Y. V., Zhukova, N. A., Nikonova, S. V., Pavlova, S. V., Pogosova, M. A., Medvedev, S. P., Luzina, O. A., & Salakhutdinov, N. F. (2024). Antidiabetic Effect of Dihydrobetulonic Acid Derivatives as Pparα/γ Agonists. Scientia Pharmaceutica, 92(4), 65. https://doi.org/10.3390/scipharm92040065








