Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Gelatin Hydrolysate
2.2. Optimization and Modeling of Enzymatic Hydrolysis Process by Response Surface Methodology
2.3. Antioxidant Activity
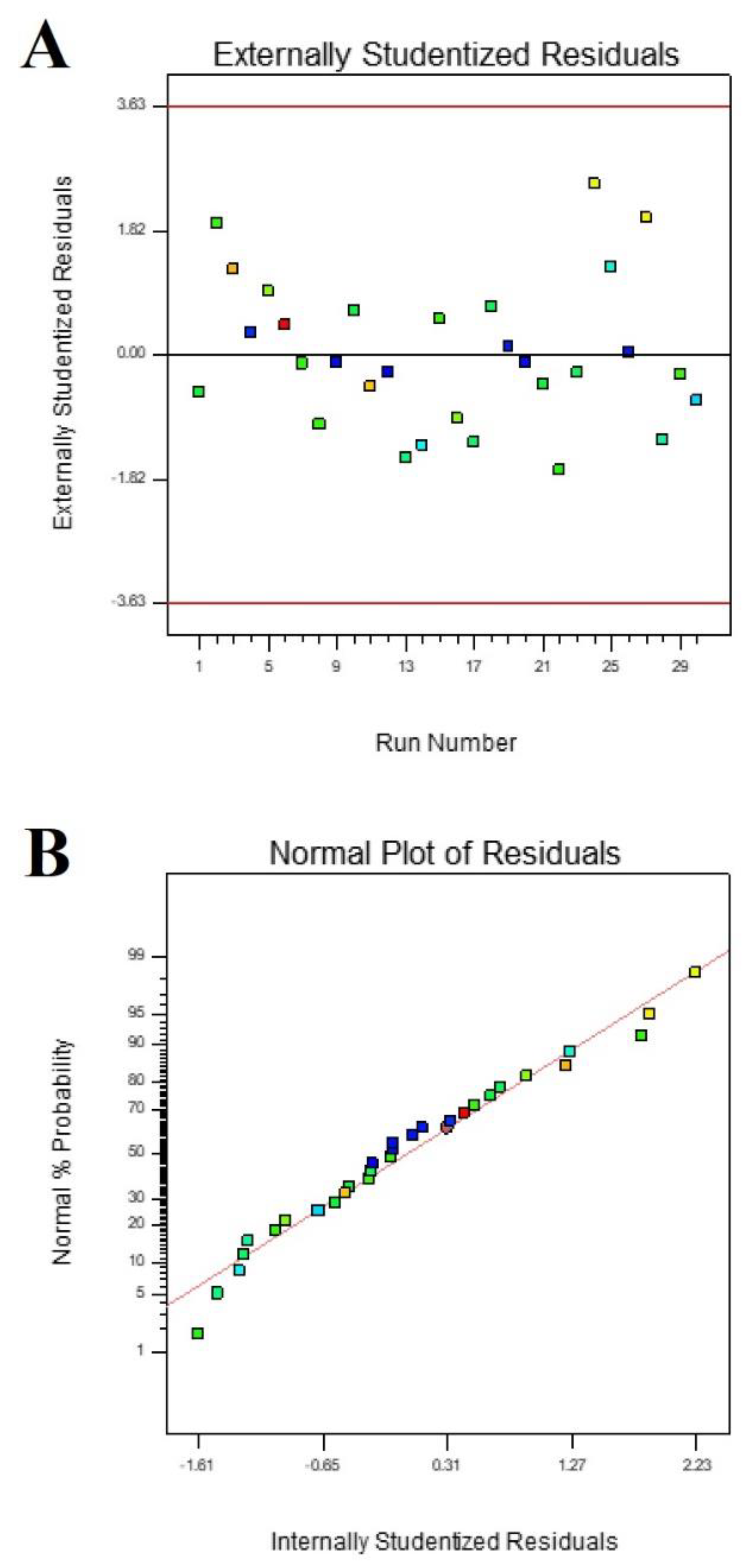
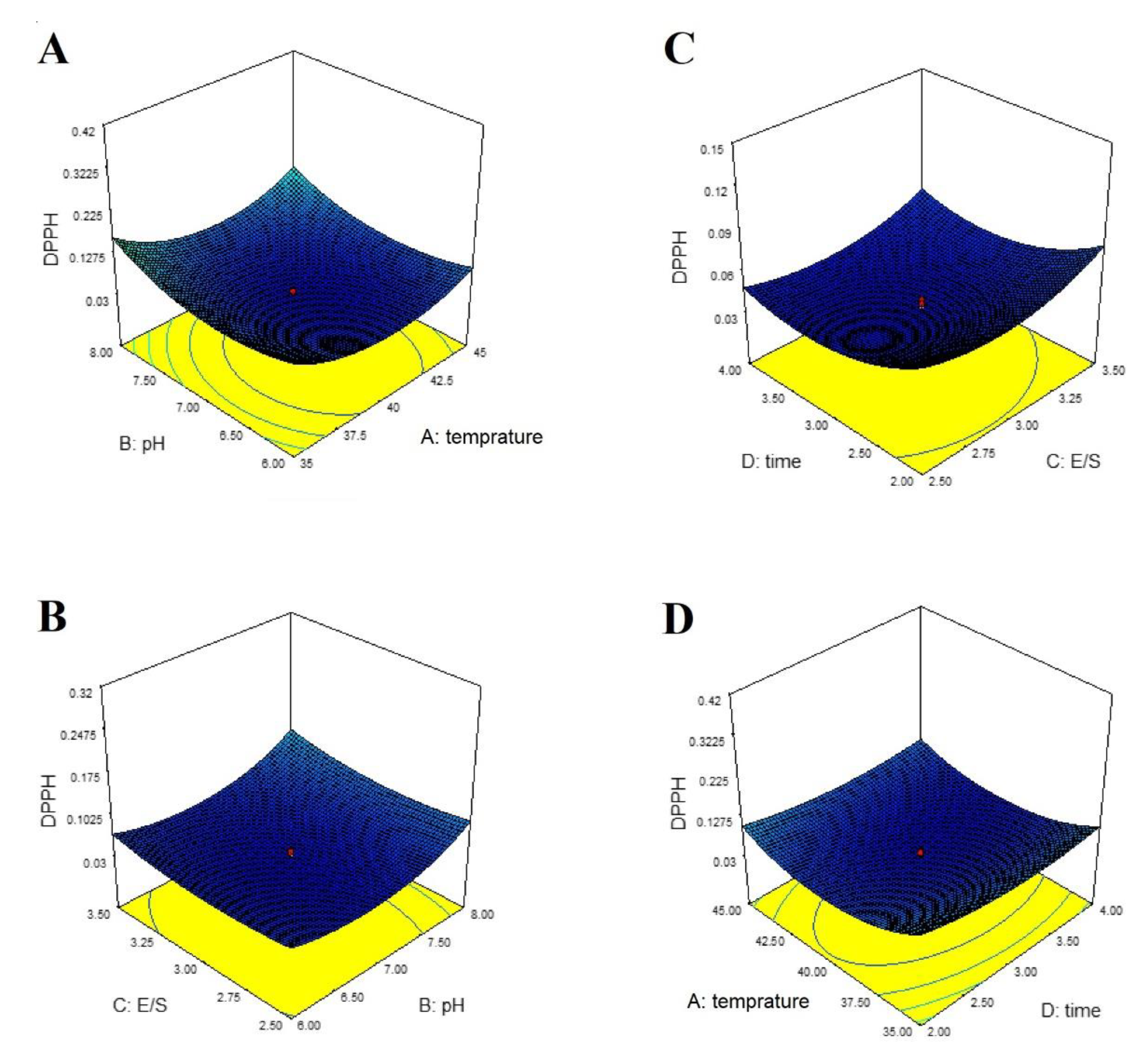
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annual review of food science and technology 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.; Dayah, A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Nasri, M. Bioactive peptides from fish collagen byproducts: A review. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 309–333. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Saleh, N.I.M.; Ghani, W.A.W.A.K.; Harun, M.R.; Kamal, S.M.M. Optimization of enzymatic hydrolysis for the production of antioxidative peptide from Nannochloropsis gaditana using response surface methodology. Adapt. Chall. 2019, 27, 41–55. [Google Scholar]
- Intiquilla, A.; Jiménez-Aliaga, K.; Zavaleta, A.I.; Hernández-Ledesma, B. Production of Antioxidant Hydrolyzates from a Lupinus mutabilis (Tarwi) Protein Concentrate with Alcalase: Optimization by Response Surface Methodology. Nat. Prod. Commun. 2018, 13, 1934578X1801300626. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Cantalapiedra, J.; Zapata, C.; Franco, J.M.; Franco, D. Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds. Adv. Food Nutr. Res. 2019, 92, 127–185. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Qiu, Y.-T.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Naik, L.; Mann, B.; Bajaj, R.; Sangwan, R.B.; Sharma, R. Process Optimization for the Production of Bio-functional Whey Protein Hydrolysates: Adopting Response Surface Methodology. Int. J. Pept. Res. Ther. 2013, 19, 231–237. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Sarbon, N.M. A response surface approach on hydrolysis condition of eel (Monopterus Sp.) protein hydrolysate with antioxidant activity. Int. Food Res. J. 2017, 24, 1081–1093. [Google Scholar]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, identification and activity evaluation. Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Naqash, S.Y.; Nazeer, R.A. Optimization of enzymatic hydrolysis conditions for the production of antioxidant peptides from muscles of Nemipterus japonicus and Exocoetus volitans using response surface methodology. Amino Acids 2011, 43, 337–345. [Google Scholar] [CrossRef] [PubMed]
| Run No. | Temperature (°C) | pH | E/Sa | Time (h) | Antioxidant Activity (%) |
|---|---|---|---|---|---|
| 1 | 45 | 8 | 2.5 | 4 | 15.1 |
| 2 | 45 | 6 | 3.5 | 4 | 18.5 |
| 3 | 40 | 9 | 3 | 3 | 31.3 |
| 4 | 40 | 7 | 3 | 3 | 3.9 |
| 5 | 35 | 8 | 2.5 | 4 | 22.6 |
| 6 | 30 | 7 | 3 | 3 | 41.5 |
| 7 | 45 | 8 | 3.5 | 4 | 18.3 |
| 8 | 35 | 8 | 3.5 | 4 | 18.6 |
| 9 | 40 | 7 | 3 | 3 | 3.3 |
| 10 | 40 | 7 | 3 | 1 | 14.7 |
| 11 | 50 | 7 | 3 | 3 | 30.4 |
| 12 | 40 | 7 | 3 | 3 | 3.1 |
| 13 | 45 | 6 | 3.5 | 2 | 12.4 |
| 14 | 40 | 7 | 4 | 3 | 8.6 |
| 15 | 35 | 6 | 2.5 | 2 | 18.7 |
| 16 | 35 | 8 | 3.5 | 2 | 22.2 |
| 17 | 40 | 5 | 3 | 3 | 13.3 |
| 18 | 45 | 6 | 2.5 | 4 | 13.5 |
| 19 | 40 | 7 | 3 | 3 | 3.6 |
| 20 | 40 | 7 | 3 | 3 | 3.3 |
| 21 | 35 | 6 | 3.5 | 4 | 14.9 |
| 22 | 35 | 8 | 2.5 | 2 | 17.9 |
| 23 | 35 | 6 | 2.5 | 4 | 13.5 |
| 24 | 35 | 6 | 3.5 | 2 | 26.7 |
| 25 | 40 | 7 | 2 | 3 | 9.6 |
| 26 | 40 | 7 | 3 | 3 | 3.5 |
| 27 | 45 | 8 | 3.5 | 2 | 28.6 |
| 28 | 45 | 6 | 2.5 | 2 | 11.3 |
| 29 | 45 | 8 | 2.5 | 2 | 18.9 |
| 30 | 40 | 7 | 3 | 5 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Brück, W.M.; Vahidi, R.; Mahmoudi, R.; Khanjari, A. Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology. Sci. Pharm. 2022, 90, 62. https://doi.org/10.3390/scipharm90040062
Pakbin B, Allahyari S, Dibazar SP, Brück WM, Vahidi R, Mahmoudi R, Khanjari A. Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology. Scientia Pharmaceutica. 2022; 90(4):62. https://doi.org/10.3390/scipharm90040062
Chicago/Turabian StylePakbin, Babak, Samaneh Allahyari, Shaghayegh Pishkhan Dibazar, Wolfram Manuel Brück, Roghayeh Vahidi, Razzagh Mahmoudi, and Ali Khanjari. 2022. "Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology" Scientia Pharmaceutica 90, no. 4: 62. https://doi.org/10.3390/scipharm90040062
APA StylePakbin, B., Allahyari, S., Dibazar, S. P., Brück, W. M., Vahidi, R., Mahmoudi, R., & Khanjari, A. (2022). Production of Bovine Collagen Hydrolysate with Antioxidant Activity; Optimized by Response Surface Methodology. Scientia Pharmaceutica, 90(4), 62. https://doi.org/10.3390/scipharm90040062







