Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Housing and Diet
2.2. Experimental Design
2.3. The Animal Model
2.4. Measurement of Systolic Blood Pressure (SBP)
2.5. Bleeding and Clotting Times
2.6. Animal Euthanasia and Blood Samples Collections
2.7. Biochemical Analysis of Serum Glucose, Insulin, Lipids and Liver Enzymes
2.8. Measurement of Endothelin-1 (ET-1), Intercellular Adhesion Molecule-1 (ICAM-1), and Vascular Cell Adhesion Molecule-1 (VCAM-1)
2.9. Measurement of Clotting Parameters
2.10. Estimation of Platelet Count and Aggregation
2.11. Assay of Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT)
2.12. Statistical Analysis
3. Results
3.1. Effect of DHEA on Systolic Blood Pressure (SBP), Body Weight, Weight Gain, Body Mass Index (BMI) and Liver Weight in Orchiectomized Rats
3.2. Effect of DHEA on Fasting Blood Glucose, Insulin, and HOMA-IR Index in Orchiectomized Rats
3.3. Effect of DHEA on Lipid Profile and Markers of Liver Functions in Orchiectomized Rats
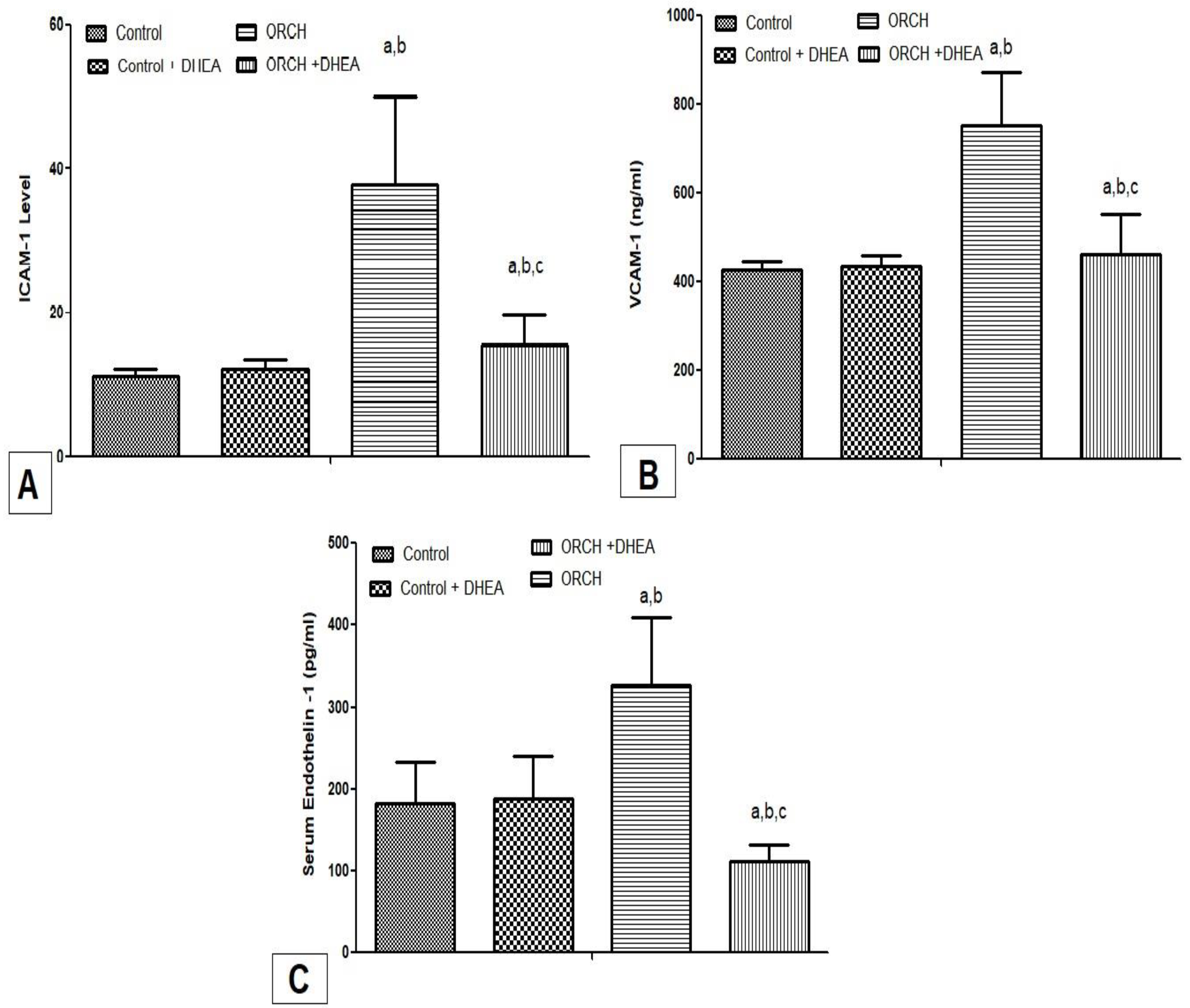
3.4. Effect of DHEA on Inflammatory Cytokines ICAM-1, VCAM-1, and Endothelin-1 in Orchiectomized Rats
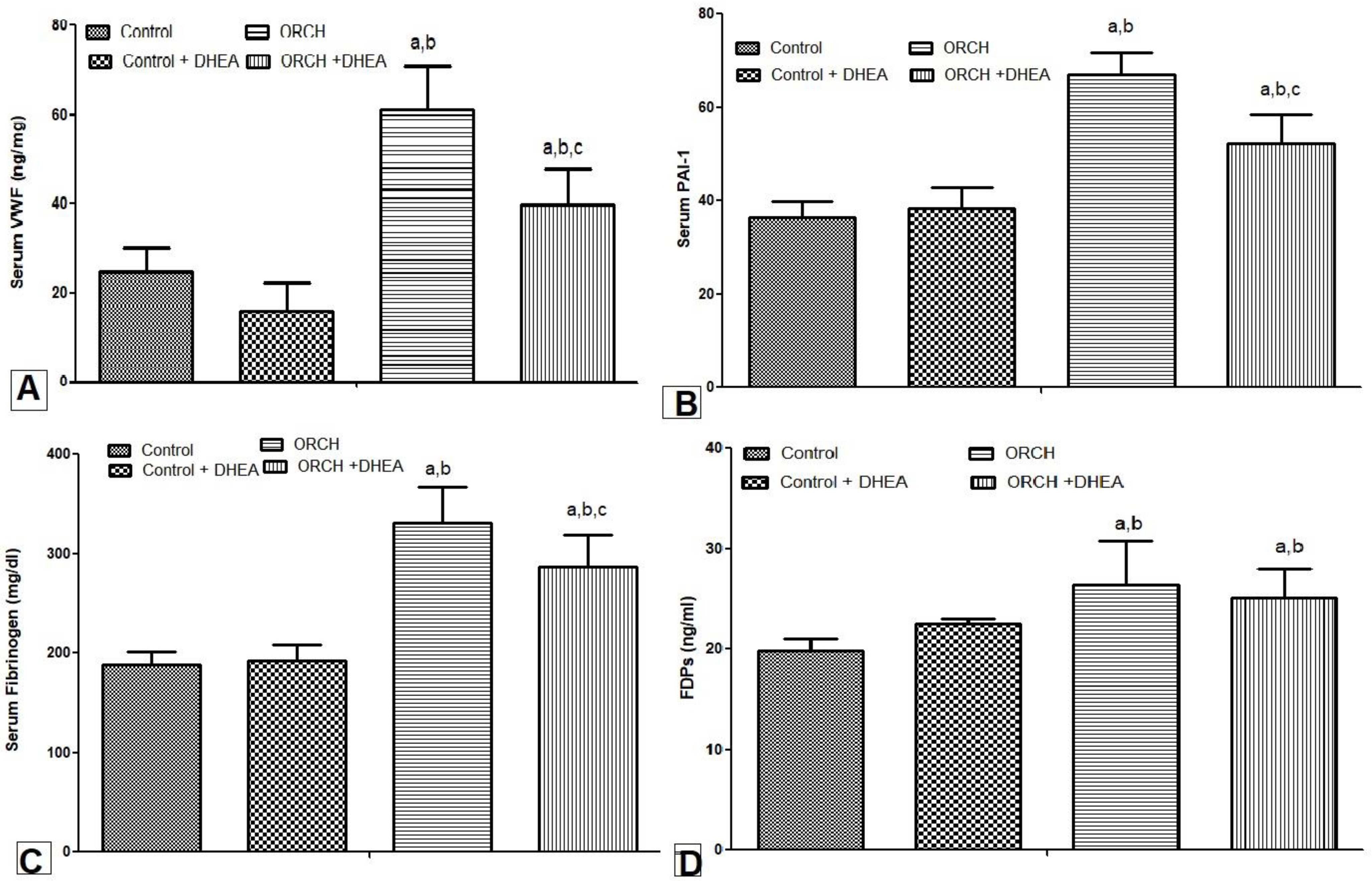
3.5. Effect of DHEA on Serum Levels of vWF, PAI-1, Fibrinogen, and PDFs in Orchiectomized Rats
3.6. Effect of DHEA on Bleeding Time, Clotting Time, PT, APTT, Platelet Count and ADP-Induced Platelet Aggregations in Orchiectomized Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corona, G.; Mannucci, E.; Forti, G.; Maggi, M.; Hypogonadism, E.D. Metabolic syndrome and obesity: A pathological link supporting cardiovascular diseases. Int. J. Androl. 2009, 32, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Eleawa, S.M.; Sakr, H.F.; Hussein, A.; Assiri, A.S.; Bayoumy, N.M.K.; Alkhateeb, M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol. 2013, 209, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Van Der Tweel, I.; Grobbee, D.; Emmelot-Vonk, M.H.; Van Der Schouw, Y.T. Testosterone, sex hormone-binding globulin and the metabolic syndrome: A systematic review and meta-analysis of observational studies. Int. J. Epidemiol. 2010, 40, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and Metabolic Syndrome: A Meta-Analysis Study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef]
- Haring, R.; Völzke, H.; Felix, S.B.; Schipf, S.; Dörr, M.; Rosskopf, D.; Nauck, M.; Schöfl, C.; Wallaschofski, H. Prediction of Metabolic Syndrome by Low Serum Testosterone Levels in Men: Results From the Study of Health in Pomerania. Diabetes 2009, 58, 2027–2031. [Google Scholar] [CrossRef] [Green Version]
- Gevi, F.; Fanelli, G.; Zolla, L. Metabolic patterns in insulin-resistant male hypogonadism. Cell Death Dis. 2018, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transplant. 2006, 12, 523–534. [Google Scholar] [CrossRef]
- Muraleedharan, V.; Jones, T.H. Testosterone and the metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 207–223. [Google Scholar] [CrossRef]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 1–15. [Google Scholar] [CrossRef]
- van Kesteren, P.J.; Kooistra, T.; Lansink, M.; van Kamp, G.J.; Asscheman, H.; Gooren, L.J.; Emeis, J.J.; Vischer, U.M.; Stehouwer, C.D. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb. Haemost. 1998, 79, 1029–1033. [Google Scholar] [CrossRef]
- Webb, C.M.; Elkington, A.G.; Kraidly, M.M.; Keenan, N.; Pennell, D.J.; Collins, P. Effects of Oral Testosterone Treatment on Myocardial Perfusion and Vascular Function in Men With Low Plasma Testosterone and Coronary Heart Disease. Am. J. Cardiol. 2008, 101, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, T.; Doi, K.; Kish, M.; Horiguchi, Y. Sex-related differences in plasminogen activator and plasminogen activator inhibiting activities in young and aged rats. Res. Commun. Mol. Pathol. Pharmacol. 1999, 104, 81–91. [Google Scholar] [PubMed]
- Demirci, I.; Haymana, C.; Demir, O.; Akın, O.; Meriç, C.; Aydoğdu, A.; Sönmez, A. Hematological indices in congenital male hypogonadism and the effects of testosterone replacement therapy: A retrospective study. Gulhane Med. J. 2021, 63, 205–211. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Lanzoni, C.; Genazzani, A.R. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging 2007, 24, 173–185. [Google Scholar] [CrossRef]
- Davis, S.R.; Panjari, M.; Stanczyk, F.Z. Clinical review: DHEA replacement for postmenopausal women. J. Clin. Endocrinol. Metab. 2011, 96, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Erben, R.G.; Eberle, J.; Stahr, K.; Goldberg, M. Androgen deficiency induces high turnover osteopenia in aged male rats: A sequential histomorphometric study. J. Bone Miner. Res. 2000, 15, 1085–1098. [Google Scholar] [CrossRef]
- Wang, C.; Chao, L.; Chao, J. Direct gene delivery of human tissue kallikrein reduces blood pressure in spontaneously hy-pertensive rats. J. Clin. Investig. 1995, 95, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Lemini, C.; Rubio-Poo, C.; Silva, G.; Garcia-Mondragon, J.; Zavala, E. Anticoagulant and estrogenic effects of two new 17 beta-aminoestrogens, butolame [17 beta-(4-hydroxy-1-butylamino)-1,3,5(10)-estratrien-3-ol] and pentolame [17 be-ta-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol]. Steroids 1993, 58, 457–461. [Google Scholar] [CrossRef]
- Mustard, J.F.H.T.; RowSell, H.C. Effects of adenosine nucleotides on platelet aggregation and clotting time. J. Lab. Clin. Med. 1964, 64, 548. [Google Scholar]
- El-Gendy, A.A.; Abbas, A.M. Effect of omega-3 fatty acids on haemostatic functions in urocortin-treated obese rats. J. Physiol. Biochem. 2014, 70, 809–820. [Google Scholar] [CrossRef]
- Rotter, I.; Rył, A.; Grzesiak, K.; Szylińska, A.; Pawlukowska, W.; Lubkowska, A.; Sipak-Szmigiel, O.; Pabisiak, K.; Laszczyńska, M. Cross-Sectional Inverse Associations of Obesity and Fat Accumulation Indicators with Testosterone in Non-Diabetic Aging Men. Int. J. Environ. Res. Public Health 2018, 15, 1207. [Google Scholar] [CrossRef] [Green Version]
- Traish, A.M. Testosterone and weight loss: The evidence. Curr. Opin. Endocrinol. Diabetes. Obes. 2014, 21, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Behre, H.M.; Tammela, T.L.J.; Arver, S.; Tolrá, J.R.; Bonifacio, V.; Lamche, M.; Kelly, J.; Hiemeyer, F.; Giltay, E.J.; Gooren, L.J.; et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012, 15, 198–207. [Google Scholar] [CrossRef]
- Villareal, D.T.; Holloszy, J.O. Effect of DHEA on abdominal fat and insulin action in elderly women and men: A randomized controlled trial. JAMA 2004, 292, 2243–2248. [Google Scholar] [CrossRef]
- Ramamani, A.; Aruldhas, M.M.; Govindarajulu, P. Differential response of rat skeletal muscle glycogen metabolism to tes-tosterone and estradiol. Can. J. Physiol. Pharmacol. 1999, 77, 300–304. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Fujimoto, W.Y.; Hayashi, T.; Kahn, S.E.; Leonetti, D.L.; Boyko, E.J. Increased Visceral Adipose Tissue Is an Independent Predictor for Future Development of Atherogenic Dyslipidemia. J. Clin. Endocrinol. Metab. 2016, 101, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Verkouter, I.; Noordam, R.; Le Cessie, S.; Van Dam, R.M.; Lamb, H.J.; Rosendaal, F.R.; Van Heemst, D.; De Mutsert, R. The Association between Adult Weight Gain and Insulin Resistance at Middle Age: Mediation by Visceral Fat and Liver Fat. J. Clin. Med. 2019, 8, 1559. [Google Scholar] [CrossRef] [Green Version]
- Saad, F.; Haider, A.; Doros, G.; Traish, A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity 2013, 21, 1975–1981. [Google Scholar] [CrossRef]
- Weiss, E.P.; Villareal, D.T.; Fontana, L.; Han, D.-H.; Holloszy, J.O. Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging 2011, 3, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Schafer, K.; Konstantinides, S. Adipokines and thrombosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 864–871. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Trovati, M. Platelet dysfunction in central obesity. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 440–449. [Google Scholar] [CrossRef]
- Basili, S.; Pacini, G.; Guagnano, M.T.; Manigrasso, M.R.; Santilli, F.; Pettinella, C.; Ciabattoni, G.; Patrono, C.; Davì, G. Insulin Resistance as a Determinant of Platelet Activation in Obese Women. J. Am. Coll. Cardiol. 2006, 48, 2531–2538. [Google Scholar] [CrossRef] [Green Version]
- Englyst, N.A.; Taube, J.M.; Aitman, T.J.; Baglin, T.P.; Byrne, C.D. A Novel Role for CD36 in VLDL-Enhanced Platelet Activation. Diabetes 2003, 52, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Korporaal, S.J.; Akkerman, J.W. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol. Haemost. Thromb. 2006, 35, 270–280. [Google Scholar] [CrossRef]
- Akkerman, J.W.N. From low-density lipoprotein to platelet activation. Int. J. Biochem. Cell Biol. 2008, 40, 2374–2378. [Google Scholar] [CrossRef]
- Colas, R.; Sassolas, A.; Guichardant, M.; Cugnet-Anceau, C.; Moret, M.; Moulin, P.; Lagarde, M.; Calzada, C. LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia 2011, 54, 2931–2940. [Google Scholar] [CrossRef] [Green Version]
- Gerrits, A.J.; Gitz, E.; Koekman, C.A.; Visseren, F.L.; van Haeften, T.W.; Akkerman, J.W.N. Induction of insulin resistance by the adipokines resistin, leptin, plasminogen activator inhibitor-1 and retinol binding protein 4 in human megakaryocytes. Haematologica 2012, 97, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Bednarek-Tupikowska, G.; Gosk, I.; Szuba, A.; Bohdanowicz-Pawlak, A.; Kosowska, B.; Bidzińska, B.; Milewicz, A. Influence of dehydroepiandrosterone on platelet aggregation, superoxide dismutase activity and serum lipid peroxide concentrations in rabbits with induced hypercholesterolemia. Med. Sci. Monit. 2001, 6, 40–45. [Google Scholar]
- Bertoni, A.; Rastoldo, A.; Sarasso, C.; Di Vito, C.; Sampietro, S.; Nalin, M.; Bagarotti, A.; Sinigaglia, F. Dehydroepiandrosterone-sulfate inhibits thrombin-induced platelet aggregation. Steroids 2012, 77, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [Green Version]
- Alessi, M.-C.; Bastelica, D.; Mavri, A.; Morange, P.; Berthet, B.; Grino, M.; Juhan-Vague, I. Plasma PAI-1 Levels Are More Strongly Related to Liver Steatosis Than to Adipose Tissue Accumulation. Arter. Thromb. Vasc. Biol. 2003, 23, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Appel, S.J.; Harrell, J.S.; Davenport, M.L. Central Obesity, the Metabolic Syndrome, and Plasminogen Activator Inhibitor-1 in Young Adults. J. Am. Acad. Nurse Pract. 2005, 17, 535–541. [Google Scholar] [CrossRef]
- Abbas, A.M.; Sakr, H.F. Effect of magnesium sulfate and thyroxine on inflammatory markers in a rat model of hypothy-roidism. Can. J. Physiol. Pharmacol. 2016, 94, 426–432. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.-C.; Connelly, P.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Wen, Y.; Yang, G.; Betz, A.L. Increased expression of intercellular adhesion molecule-1 in mouse focal cerebral ischemia model. Chin. Med. J. 2000, 113, 75–79. [Google Scholar]
- van Buul, J.D.; Voermans, C.; van den Berg, V.; Anthony, E.C.; Mul, F.P. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J. Immunol. 2002, 168, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Madonna, R.; Pandolfi, A.; Massaro, M.; Consoli, A.; De Caterina, R. Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated pro-tein-kinase. Diabetologia 2004, 47, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Antoniades, C. Mechanisms of testosterone deficiency-related endothelial dysfunction: Invited com-mentary for the Hellenic Journal of Cardiology on: Tsikas et al. “Associations between asymmetric dimethylarginine, ni-trite-dependent renal carbonic anhydrase activity and plasma testosterone levels in hypogonadal men”. Hell. J. Cardiol. 2018, 59, 207–208. [Google Scholar]
- Formoso, G.; Chen, H.; Kim, J.-A.; Montagnani, M.; Consoli, A.; Quon, M.J. Dehydroepiandrosterone Mimics Acute Actions of Insulin to Stimulate Production of Both Nitric Oxide and Endothelin 1 via Distinct Phosphatidylinositol 3-Kinase- and Mitogen-Activated Protein Kinase-Dependent Pathways in Vascular Endothelium. Mol. Endocrinol. 2006, 20, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; De Vita, F.; Fisichella, A.; Colizzi, E.; Provenzano, S.; Lauretani, F.; Luci, M.; Ceresini, G.; Dall’Aglio, E.; Caffarra, P.; et al. DHEA and cognitive function in the elderly. J. Steroid Biochem. Mol. Biol. 2015, 145, 281–292. [Google Scholar] [CrossRef]
- Rutkowski, K.; Sowa, P.; Rutkowska-Talipska, J.; Kuryliszyn-Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and Hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef]
- De Pergola, G.; De Mitrio, V.; Sciaraffia, M.; Pannacciulli, N.; Minenna, A. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism 1997, 46, 1287–1293. [Google Scholar] [CrossRef]
- Beer, N.A.; Jakubowicz, D.J.; Matt, D.W.; Beer, R.M.; Nestler, J.E. Dehydroepiandrosterone reduces plasma plasminogen ac-tivator inhibitor type 1 and tissue plasminogen activator antigen in men. Am. J. Med. Sci. 1996, 311, 205–210. [Google Scholar] [CrossRef]
- Rabijewski, M.; Zgliczyński, W. Dehydroepiandrosterone therapy in men with angiographically verified coronary heart disease: The effects on plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen activator (tPA) and fibrinogen plasma concentrations. Endokrynol. Pol. 2007, 58, 213–219. [Google Scholar]
- Medraś, M.; Jankowska, E. Testosterone and atherosclerosis in males during andropause. Pol. Merkur. Lekarski. 1999, 6, 205–207. [Google Scholar]
- De Pergola, G.; Pannacciulli, N. Coagulation and fibrinolysis abnormalities in obesity. J. Endocrinol. Investig. 2002, 25, 899–904. [Google Scholar] [CrossRef]

| Con | Con + DHEA | ORCH | ORCH + DHEA | |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 122 ± 5 | 125 ± 9 | 144 ± 8 | 132 ± 4 |
| Body weight (g) | 333.8 ± 4.3 | 345.7 ± 6.7 | 408.5 ± 9.9 ab | 360 ± 8.4 abc |
| Percent of weight gain (%) | 31 ± 4.5 | 29.8 ± 3.7 | 59.5 ± 7.8 ab | 35.4 ± 6.08 abc |
| BMI (g/cm2) | 0.60 ± 0.04 | 0.59 ± 0.07 | 0.75 ± 0.95 ab | 0.63 ± 0.04 abc |
| Liver weight (g) | 10.8 ± 0.48 | 11.1 ± 0.76 | 15.00 ± 0.31 ab | 12.65 ± 0.6 abc |
| Liver weight/body weight (%) | 3.04 ± 0.1 | 3.09 ± 0.2 | 3.55 ± 0.09 ab | 3.25 ± 0.2 abc |
| Con | Con + DHEA | ORCH | ORCH + DHEA | |
|---|---|---|---|---|
| Triglycerides (mg/dL) | 93.1 ± 5.7 | 88.45 ± 6.2 | 166.7 ± 12.8 ab | 133.7 ± 15.58 abc |
| Total Cholesterol (mg/dL) | 88.0 ± 4.51 | 90.45 ±3.41 | 144.7 ± 15.7 ab | 125.7 ± 14.61 ab |
| LDL-C (mg/dL) | 35.6 ± 4.46 | 34.4 ± 5.61 | 77.4 ± 5.2 ab | 60.8 ± 9.44 abc |
| HDL-C (mg/dL) | 48.4 ± 4.82 | 47.7 ± 6.51 | 37.7 ± 4.8 ab | 44.7 ± 3.6 c |
| ALT (U/L) | 50.05 ± 4.34 | 52.1 ± 3.67 | 88.41 ± 9.52 ab | 67.34 ± 6.05 abc |
| AST (U/L) | 58.64 ± 5.04 | 60.45 ± 6.85 | 109.75 ± 11.41 ab | 84.51 ± 7.93 abc |
| ALK. Phosphatase (U/L) | 189.45 ± 12.65 | 200.14 ± 15.7 | 452.68 ± 35.7 ab | 287.45 ± 23.95 abc |
| GGT (U/L) | 0.93 ± 0.13 | 0.86 ± 0.24 | 2.53 ± 0.34 ab | 1.69 ± 0.35 abc |
| Con | Con + DHEA | ORCH | ORCH + DHEA | |
|---|---|---|---|---|
| PT (s) | 24.5 ± 0.5 | 25.1 ± 0.6 | 18.4 ± 0.034 ab | 23.7 ± 0.58 abc |
| aPTT (s) | 19.2 ± 0.6 | 20.1 ± 0.7 | 13.5 ± 0.6 ab | 14.8 ± 0.4 abc |
| Bleeding time (s) | 84.1 ± 2.9 | 85.4 ± 3.5 | 66.7 ± 3.2 ab | 73.2 ± 3.1 abc |
| Clotting time (s) | 123.6 ± 3.8 | 124.6 ± 4.1 | 89.6 ± 3.1 ab | 106.5 ± 2.8 abc |
| Platelet count (×103/µL) | 915 ± 25 | 933 ± 35 | 942 ± 39 | 919 ± 45 |
| ADP-induced platelet aggregation (%) | 45.1 ± 2.9 | 47.4 ± 3.6 | 67.3 ± 3.8 ab | 59.6 ± 4.1 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safwat, S.M.; Hussein, A.M.; Eid, E.A.; Serria, M.S.; Elesawy, B.H.; Sakr, H.F. Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism. Sci. Pharm. 2022, 90, 6. https://doi.org/10.3390/scipharm90010006
Safwat SM, Hussein AM, Eid EA, Serria MS, Elesawy BH, Sakr HF. Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism. Scientia Pharmaceutica. 2022; 90(1):6. https://doi.org/10.3390/scipharm90010006
Chicago/Turabian StyleSafwat, Sally M., Abdelaziz M. Hussein, Elsayed A. Eid, Mohamed S. Serria, Basem H. Elesawy, and Hussein F. Sakr. 2022. "Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism" Scientia Pharmaceutica 90, no. 1: 6. https://doi.org/10.3390/scipharm90010006
APA StyleSafwat, S. M., Hussein, A. M., Eid, E. A., Serria, M. S., Elesawy, B. H., & Sakr, H. F. (2022). Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism. Scientia Pharmaceutica, 90(1), 6. https://doi.org/10.3390/scipharm90010006







