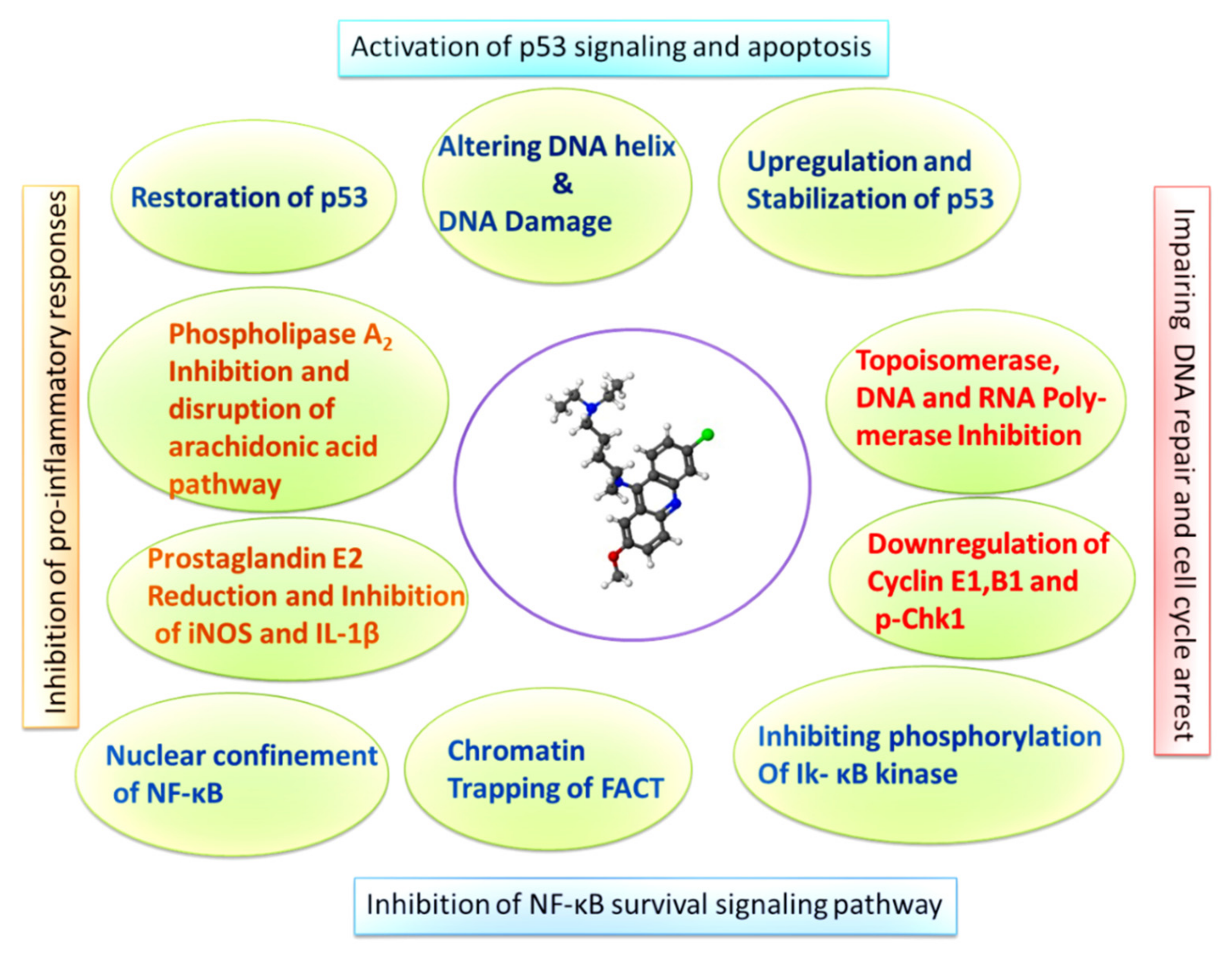
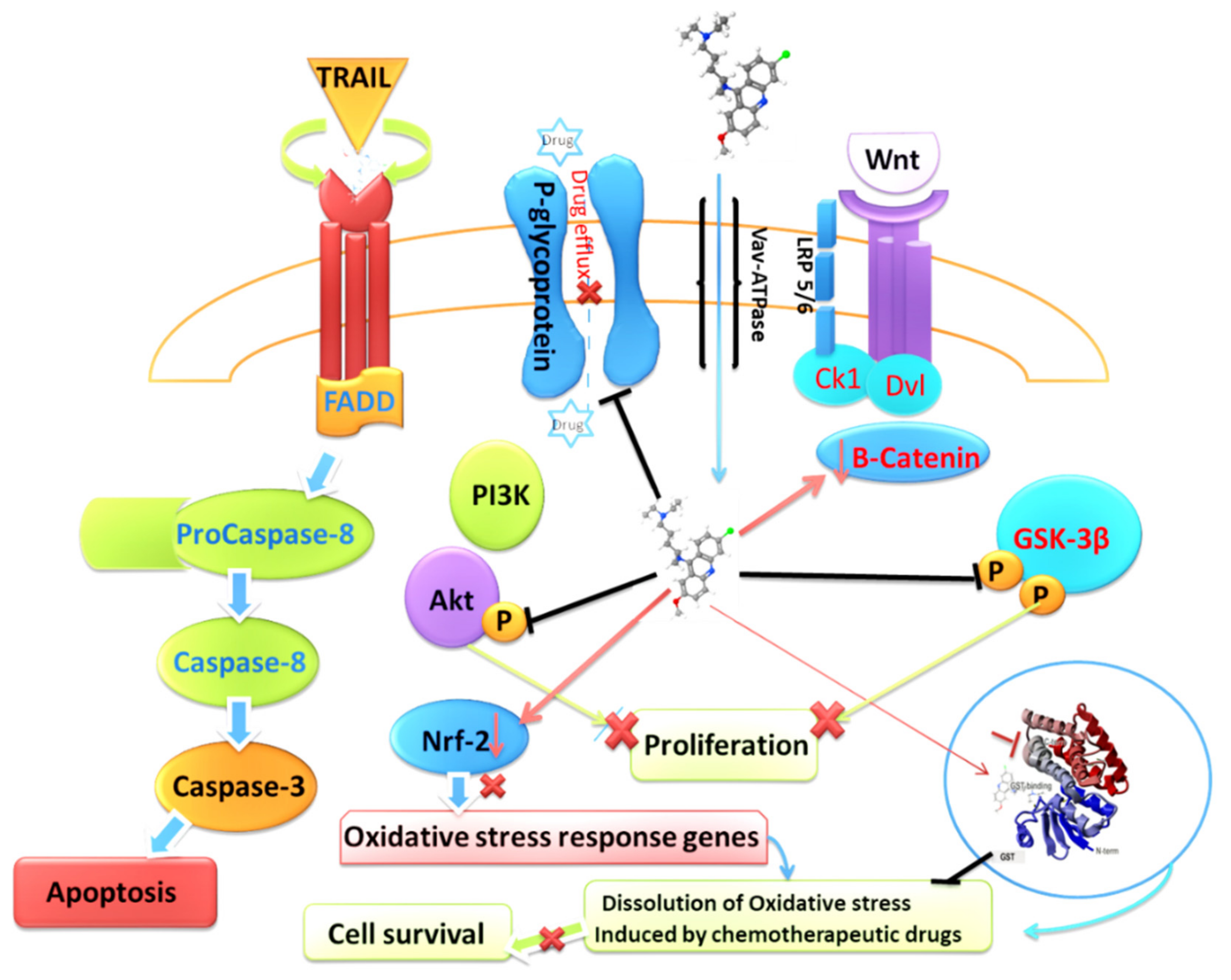
Abstract
Quinacrine (QC), a synthetic drug belonging to the 9-aminoacridine family, has been used extensively to treat malaria and multiple ailments over the past several decades. Following its discovery in the 1920s and extensive use for the treatment of malaria for nearly two decades, numerous studies have explored its antineoplastic potential in both preclinical and clinical settings. Multiple studies spanning over seven decades have examined a wide range of QC anticancer activities across various types of cancers, along with the underlying mechanisms. Many of these mechanisms, including activation of the p53 signaling cascade and simultaneous NF-κB signaling inhibition, have been reported in various studies, bringing QC to a unique polypharmacological category drug possessing the potential to treat a wide variety of diseases, including cancer. This article summarizes most of the research conducted over several decades to uncover new molecular mechanisms activated or inactivated and directly correlate with antineoplastic activity QC.
1. Introduction
Quinacrine (IUPAC name: 4-N-(6-chloro-2-methoxyacridin-9-yl)-1-N,1-N-diethylpentane-1,4-diamine) is a synthetic drug belonging to the family of acridine-based synthetic compounds and the 9-aminoacridine subfamily. The chemical structure of QC is composed of three heterocyclics with acridine, which was designed and produced by the pharmaceutical organization Bayer in 1928 during the post-World War I period and was actively used for four years during World War II to treat soldiers infected with malaria [1]. According to a U.S. Surgeon General Office report, more than three million soldiers had reportedly taken QC during this period, and they were routinely monitored for safety and efficacy, making QC one of the most extensively studied synthetic drugs in the history of modern medicine [2,3]. Low concentrations of QC have been shown to induce redistribution of Ca2+ ions, leading to disruption of IP3 dependent Ca2+ oscillation, hampering the growth of Plasmodium falciparum, as they exhibit stage-specific Ca2+ oscillations in the ring form during the trophozoite stage, which is a key requirement for the maturation of the parasite inside erythrocytes. Disruption of these oscillations obstructs intra-erythrocytic maturation, resulting in the death of P. falciparum parasites [4]. Chloroquine eventually replaced QC, which has a similar structure, but a quinolone replaced the central ring. Since the World War II era, QC has been explored and utilized for treating various infections and other ailments, including giardiasis, taeniasis, helminthic infections, prion diseases, and lupus erythematosus, and as anti-inflammatory drugs for rheumatoid arthritis [5,6,7,8]. QC has shown significant clinical efficacy in treating all these aliments, especially lupus and rheumatoid disease. Exploring QC activity and usage beyond treating the conditions and infections mentioned earlier started when its DNA-binding and fluorescent properties were discovered in the early 1950s. Owing to its fluorescent and DNA-binding properties, QC is also routinely used in laboratories to stain chromosomes and study their patterns. This technique is explicitly called “Q-banding” [9,10,11]. In addition, QC was extensively utilized as a sterilizing agent in the 1980s [12,13].
2. Methods
A systemic search with the title “Quinacrine and cancer research” was conducted in PubMed and the relevant articles were saved onto the drive, thoroughly analyzed for quality and key findings. The saved articles were indexed and stored for future study.
3. Internalization and Pharmacokinetics of QC
Quinacrine is prescribed as oral tablets to be taken with water after a meal; however, other administration routes, such as intravenous, intramuscular, rectal, transcervical, and interstitial, have also been used to deliver the drug [8,14,15]. QC is rapidly absorbed following oral administration, and its serum plasma concentration peaks approximately 12 h post-ingestion [3]. The plasma concentration of QC increases during the first week of administration and equilibrates at 94% around the fourth week of treatment. During standard treatment cycles, peak plasma concentrations of up to 140 ng/mL (0.32 µM) have been reported during its utilization to treat malaria [16]. Nearly 80–90% of the drug present in the plasma is bound to plasma proteins, and the half-life of the drug inside the body is roughly around fourteen days. QC is evenly distributed throughout the body, with the liver, kidney, lungs, and spleen having the highest concentrations. In contrast, the heart, brain, and skeletal muscle show the lowest QC concentrations. The primary route of eliminating QC is through the renal system, which can be altered depending on clinical requirements, that is, enhanced through acidification or reduced through alkalinization [17].
Quinacrine is mainly internalized through P-glycoprotein ABC transport pumps and vacuolar ATPases (Vav-ATPases) [18]. Continuous pumping by Vav-ATPases maintains the drug in a non-diffusible and cationic form in large vacuoles. Concentrations as low as 25 nM have been shown to be readily taken up by cells from 30 min to 3 h. The maximum uptake of QC varies depending on cell type. The smooth muscle cells showed maximum uptake at 5 µM concentration over two hours. The concentration-dependent 30 min uptake of QC follows hyperbolic kinetics, with a Km of 8.7 µM in umbilical smooth muscle cells, 1.14 µM in peripheral mono nucleated leukocytes (PMLNs), and 6.32 µM in lymphocytes [19]. Upon internalization, QC is readily metabolized to monomethyl quinacrine by cytochrome P450 isoform CYP3A4/5 [20]. A study of the tissue distribution of QC analogs and hydroxychloroquine showed that QC exhibited a higher concentration in A549 induced tumors than HCQ and its analog VATG-027. The mean tumor concentration for QC after the 28-day treatment was found to be approximately 1200 ng/mL and reached statistical significance. They also demonstrated that QC drug retention in lung tissue was significantly higher (2600 ng/mL ± 580 ng/mL), only behind that in the liver and kidney tissues [21].
Reported Toxicities of Quinacrine
QC is one of the most extensively used drugs and possesses certain advantages in terms of drug tolerance and toxicity in a great number of patients, unlike any other medicine. QC is generally well tolerated and considered safe at clinical dosages of 400 mg daily. However, a few notable toxicities have been observed in a smaller fraction of patients and those treated with high-dose regimens. Dermatological toxicity is one of the most prominent and visible side effects in patients on a standard drug regimen as it accumulates in the skin, producing yellowish-appearing stains and blue and black rashes surrounding the stained areas, presumably due to its melanin-binding properties. However, the generation of rashes has been found in a significantly lower percentage of the population (1.6%), based on a study conducted on 120,000 Australian soldiers during World War II [22]. The most threatening toxic side effect of QC is anaplastic anemia, which occurs in a significantly lower percentage (0.003%) of patients at higher dosages than recommended and with less frequent blood counts during long periods of treatment [23,24,25]. However, in modern clinical studies on QC, a dose of 300 mg has been found to be well tolerated, and no incidences of anaplastic anemia have been reported. In rare cases, patients administered a dose of over 500 mg might experience drug-induced hypersensitivity in the cornea, which is primarily reversible [26,27]. Toxicities in the central nervous system have also been reported in rare cases [28,29]. However, they occurred in sporadic cases (0.1–0.4%), as only twenty-eight out of nearly 300,000 soldiers treated with QC experienced psychotic effects [30]. Apart from these side effects, some general side effects, including mild headache and gastrointestinal problems such as diarrhea, nausea and abdominal cramps, can be experienced and are primarily minor and reversible upon dose reduction.
4. Quinacrine and Cancer
The first study that tested the efficacy of QC in cancer was published in 1958 [31], and Vassey et al. reported a significant increase in the survival of carcinoma tumor-bearing C57BL mice following QC administration. Another study conducted by Hill and colleagues tested QC for breast cancer in laboratory and animal models. It demonstrated a similar growth inhibition pattern in cancer cells in both in vitro and in vivo models [32]. A few clinical studies on QC for the treatment of malignant pleural effusions were conducted on smaller subsets of patients (<100) over the next two decades [14,33,34,35]. QC was reported to be a better alternative to bleomycin in one of the studies owing to its better response and fewer side effects displayed by the patients enrolled in the study. These studies paved the way for exploring the antineoplastic potential of this antimalarial drug. Interest in understanding the molecular mechanisms involved in the antineoplastic effect of QC rose in the late 1990s. Since then, many new QC mechanisms have been reported over a wide range of cancers, demonstrating its multi-spectrum effectivity and propensity to modulate the expression of multiple signaling molecules that regulate one or more oncogenic signaling networks. Numerous preclinical studies have demonstrated a significant reduction in cancer cell viability owing to QC exposure in both in vitro and in vivo models across the spectrum of various cancer types. The reported LD50 values have been shown to vary from t2 to 10 µM depending on the type of cancer cells [36,37]. Many new mechanisms of QC activity that have a direct impact on cancer cell proliferation and spread have been uncovered (Table 1). These studies have highlighted the activity and role of QC in regulating various cellular phenomena such as DNA replication, epigenetic regulation including reversal of gene expression of methylated or silenced genes, cell cycle progression, and interference with signaling pathways leading to cell proliferation. Accumulation of these molecular effects contributes to the pharmacological activity of QC. The effects of QC on these processes are described in detail in the following subsection.

Table 1.
Table summarizing the effect of QC studied on various types of cancers. NSCLC—Non-small Cell Lung Cancer, CC—Colon Cancer, ATC—Anaplastic Thyroid Cancer, GBM—Glioblastoma Multiforme, BC—Breast Cancer, AML—Acute Myeloid Leukemia, HCC—Hepatocellular Carcinoma, OC—Ovarian Cancer, RCC—Renal Cell Carcinoma, HNSCC—Head and Neck Squamous Cell Carcinoma, GIST—Gastrointestinal Tumor, ALL—Acute Lymphocytic Leukemia, BER—Base Excision Repair.
4.1. Quinacrine and DNA Intercalation
The discovery of QC’s DNA binding properties and the multi-disease affectivity of this particular drug drew the attention of cancer researchers in the 1970s, which led to an exploration of QC’s anti-cancer potential DNA-binding cytotoxic drugs as the standard therapeutics used for cancer treatment during that time. QC exhibits significant DNA intercalation properties similar to all other acridine backbone-based drugs, with a planar structure that allows them to intercalate through stacking between the bases. However, intercalation is not the only way QC interacts with DNA; it also interacts with the minor groove of DNA through its side diaminobutyl chain, further stabilizing the track. DNA intercalation is thought to be the primary mode of QC anti-cancer activity. Few studies have shown nuclear fragmentation as a direct result of QC exposure in breast, gastric, and lung cancer cells, suggesting that QC may induce single-strand DNA damage [36,37,38]. However, this property has been proven insufficient for inducing cell death, as QC has been shown to interact with DNA through a nine-amino acid framework, but lacks an alkyl substitute to confer the additional damage necessary for triggering cell death. [62,63]. The intercalation of QC with DNA has also been reported to interfere with the methylation activity of the DNA methyl transferase enzyme DNMT1, preventing its binding to the promoter regions of multiple genes, including CDH13, E-cadherin, p16, secreted frizzle-related proteins (SRFPs), and subsequent silencing of gene expression through methylation [64].
4.2. Quinacrine Mediated Induction of P53 Signaling and Inhibition of NF-κB
Multiple studies investigating the molecular mechanism of cell death induced by QC have pointed out the activation of p53 signaling and simultaneous inhibition of NF-κB, independent of its DNA-damaging ability [56,63]. QC has also been shown to induce p53 stabilization in a manner different from DNA-damage-independent p53 stabilization [56,65]. QC has also been reported to restore p53 deficiency in wild-type head and neck squamous cell carcinoma cells that under express TP53 compared to normal human keratinocytes [57].
Another mechanism by which QC activates p53 is the suppression of NF-κB. The QC-mediated inhibition of NF-κB is a multicentric and multistep process. For one, its mode of action has been experimentally shown as mediating through Phospholipase A2. PL A2 plays an important role in the generation of platelet activation factors and eicosanoids, which are believed to play a role in NF-κB activation [66,67,68]. Studies have provided evidence of a link between NF-κB activation and arachidonic acid metabolites [69]. QC has been reported to block lysophosphatidic acid (LPA)-induced activation of NF-κB and transcription of its downstream targets ICAM-1 and IL-8 [70]. Another mechanism through which QC suppresses NF-κB activity is the prevention of phosphorylation of IκB kinase, which is responsible for the activation of NF-κB and its transcriptional activity [41]. Furthermore, the detailed underlying molecular mechanisms demonstrated that QC exposure confines the p65 subunit of NF-κB to the nucleus [64]. Furthermore, studies have shown that QC prevents the binding of the p65 subunit of NF-κB to the promoter region of its downstream targets, such as ICAM-1, which is necessary for the activation of NF-κB signaling [39]. QC has also been reported to primarily inhibit NF-κB activity by downregulating its phosphorylation at position 536 via IKkα. This was further confirmed by partial inhibition of H2O2 induced phosphorylation of the p65 subunit by quinacrine and depletion of the levels of the active subunit of facilitates chromatin transcription (FACT), which binds to the minor groove of DNA and to bent and cruciform DNA structures through its HMG domain. QC treatment has been shown to alter the DNA helix and create multiple binding sites for FACT, trapping FACT onto the chromatin, thus indicating phosphorylation of p53 and preventing the activation of NF-κB signaling due to unavailability of free active FACT [39,40,71,72]. These studies cumulatively suggest that the mechanism of QC-induced inhibition of NF-κB occurs at multiple levels and is more effective than selective inhibitors of NF-κB, which primarily inhibit activity through sequestration of the protein into the cytoplasm (Figure 1).
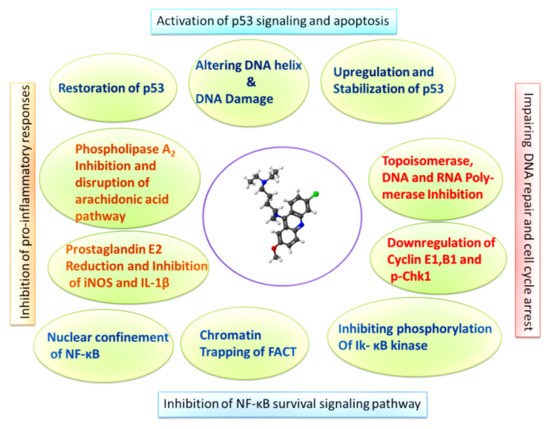
Figure 1.
Graphic describing the molecular mechanisms of NF-κB inhibition, p53 induction, and cell cycle arrest induced by QC in cancer cells.
4.3. Quinacrine and Inhibition of DNA Replication Enzymes
DNA replication is central to proliferation and plays a vital role in oncogenesis. Numerous studies have provided evidence for the promiscuous nature of QC, owing to its ability to inhibit the activities of multiple enzymes that are part of DNA replication machinery such as topoisomerases, DNA polymerases, RNA polymerases, and telomerases. Numerous studies have reported the inhibition of DNA damage repair, including single-strand breaks (SSB) and base excision repair (BER) by QC, which is similar to topoisomerase inhibitors, as topoisomerases are predominantly involved in this process [73,74,75,76,77]. Most of these studies have demonstrated significant DNA damage repair mechanism inhibition, enhancing the toxic potential of radiation, such as UV and X-rays, or chemical inhibitors of topoisomerase II, such as etoposide. Further, the direct inhibition of topoisomerase activity occurred on breast cancer cells which showed supercoiling of plasmid DNA mixed with breast cancer cell lysate when treated with 10 and 15 µM concentrations of QC, similar to topoisomerase inhibitor etoposide, used as a positive control.
Telomerases are a family of enzymes responsible for replicating telomeres and have been associated with aggressive tumor growth and malignant growth in various types of cancer. Deregulation of its expression has been observed in many cancer types, enabling them to override replicative cell death and achieve replicative immortality. QC has been reported to inhibit purified telomerase activity in humans, T. thermophila, and E. adiculetaus, at 50 µM concentration, which is much higher than the LD50 observed in various cancer cells [78]. However, the exact mechanism underlying this phenomenon remains unclear. Furthermore, another possible mechanism through which QC could inhibit telomere replication has been shown through stabilization of G-quadruplex structures in the telomere region, which are well known to inhibit telomeric replication in in vitro and in vivo studies [79,80].
Another aspect of QC’s action on controlling DNA replication through the inhibition of DNA polymerase activity has been reported in a few studies. These studies further showed almost complete inhibition of DNA synthesis at a concentration of 32 µM QC [81,82]. Although these reported IC50 values are much higher than the LD50 value of the drug on cancer cells, they found their relevance in the context of cancer in experiments reported in another independent study, demonstrating a significant preference for QC in inhibiting DNA polymerases from malignant cells compared to those from normal non-transformed cells. They demonstrated that the IC50 concentrations for DNA polymerases α, δ, and ε from Novikoff’s hepatoma were 15.2, 22.6, and 11.4, respectively, whereas the same IC50 concentrations of 92.5, 200, and 146 µM for DNA polymerases α, δ, and ε were isolated from normal rat liver [59]. The exact reasons for this selective preference are yet to be understood and could be one of the reasons why cancer cells are multifold more sensitive to QC than normal non-malignant cells. Furthermore, a few studies have also presented evidence suggesting that quinacrine inhibits ribosome biogenesis (RBG) by suppressing the activity of the RPA194 catalytic subunit of RNA polymerase-I and nucleolar RBG nucleostemin (NS/GNL3), inducing nucleolar stress, which leads to a reduction in RAD51 recruitment to the DNA damage site and eventually causes disruption of the homologous recombination repair mechanism [49,51].
4.4. Quinacrine Induced Autophagy and Cell Cycle Arrest
In addition to inducing apoptotic cell death, QC has also been reported to trigger autophagic signaling and disrupt the cell cycle, independent of DNA damage and repair activity. Studies have linked the autophagy-promoting activity of QC to its p53 upregulation effect and through downregulation of the S-phase kinase-associated protein 2 (Skp2)–p62/SQSTM axis, which is directly involved in the degradation of the tumor suppressor proteins p21 and p27. The downregulation of these autophagosome cargos triggers the accumulation of LC3B-II chains in autophagic vacuoles and initiates p21/p27 mediated apoptotic signaling [42,52,53]. Aberrant regulation of the cell cycle is a prominent promoter of uncontrolled proliferation, which is exploited by cancer cells for rapid growth. Several studies have pointed out the effect of QC on the progression of the cell cycle in breast and gastric cancer cells by altering the DNA structure, genome fragmentation, and arresting cells at the S-phase stage, presumably through multiple mechanisms, including the inhibition of DNA and RNA polymerases and topoisomerases. A few studies have also highlighted the direct downregulation of cyclin D1, E, and B1, and DNA polymerase subunit PCNA, thereby imparting cell cycle machinery. In addition, QC has been reported to promote the degradation of phosphorylated checkpoint kinase 1 (p-Chk1) and downregulate replication protein A (RPA), impairing base excision repair (BER) machinery. Another key event initiated by QC treatment is the phosphorylation of eIF2α at the Ser51 position, causing the shutdown of global protein synthesis. All these mechanisms cumulatively prevent the cell from proceeding through the stages of division [38,43,48,83,84] (Table 2).

Table 2.
Table demonstrating the reported molecular targets of QC and its downstream effect on cellular cascades. Molecular targets of Quinacrine (QC) impacting cancer progression.
4.5. Quinacrine and TRAIL Sensitivity
TRAIL and its receptors TRAIL1/2 (also called death receptors DR4 and DR5) play vital roles in apoptotic signaling. Loss of cellular sensitivity to TRAIL, either through mutations in its downstream molecule c-FLIP or mutations in the TRAIL receptor death domain, has been shown to be one of the mechanisms through which cancer cells possibly evade cell death, and numerous studies have reported QC restoring TRAIL sensitivity in ovarian cancer, breast cancer, hepatocellular carcinoma, and glioblastoma [45,50,54,85]. QC has been demonstrated to significantly upregulate DR5 protein levels and prevent its degradation through lipid raft sequestration and autophagy. A combination of QC and TRAIL ligand at 10–40 ng/mL has been reported to induce death in over two-thirds of cells that were previously resistant to even very high concentrations of TRAIL (256 ng/mL). Another interesting mechanism through which QC facilitates TRAIL-induced apoptotic signaling is the formation of a functional bridge between TRAIL and DR5 receptors, as reported by Das et al. [86]. In addition, QC induced upregulation of p53 and p21, eradication of pro-survival Bcl family protein Mcl-1, and generation of high amounts of ROS and nitric oxide (NO) cumulatively enhanced the apoptotic effect multifold when used in combination with TRAIL.
4.6. Quinacrine and Chemoresistance
Chemoresistance is a major obstacle to cancer treatment. Cancer cells have been shown to evade the effects of therapeutics through various means, including effective pumping of the drug from the cell through ATP-dependent efflux pumps, such as P-glycoproteins [87], and activating alternative signaling pathways, including FGFR1, PI3K/mTOR, and Wnt-TCF, to substitute for targeted signaling blockade by therapeutics [88,89]. One of the key aspects of QC activity that has emerged over the years of extensive research is the restoration of chemosensitivity of chemotherapeutic drugs to which cancer cells have acquired resistance. Numerous studies have reported this game-changing phenomenon in which QC has restored and sensitized cancer cells to various therapeutics currently being used for the treatment of cancer. QC has been reported to reverse cisplatin chemoresistance in head and neck squamous cell carcinoma by restoring p53 deficiency [57]. One report also demonstrated that QC restored vincristine sensitivity by preventing the efflux (outward transport) of drugs by cancer cells when exposed to vincristine [90]. Another study pointed out the restoration of sensitivity by QC through simultaneous upregulation of cathepsin L and downregulation of p62 sequestosome apparatus [55]. A few more studies have also reported the reversal of resistance to other chemotherapeutic drugs, including cytarabine in ALL xenografts, MAPK pathway inhibitors in NRAS and BRAF mutant melanoma, TRAIL in glioblastoma and hepatocellular carcinoma cells mostly through simultaneous downregulation of p62 and NF-κB, upregulation of p53, mitochondrial damage, and subsequent activation of caspases [54,85,91,92]. In addition to its aforementioned effects, QC has also been reported as an inhibitor of P-glycoproteins, thereby impairing its own efflux, as well as the combinational therapeutic administered with it [93,94]. In addition, QC has been reported to downregulate canonical Wnt signaling by depleting β-catenin and phosphorylated GSK3β levels [95]. QC has also been reported to inhibit Akt activity by preventing its phosphorylation at Ser437 and interrupting the positive feedback loop between Akt and mTOR, thereby inhibiting the Akt-mediated proliferative signaling cascade [96]. Additionally, QC has been shown to downregulate and promote JNK1 mediated degradation of nuclear factor E2-related factor 2 (Nrf2), which is known to upregulate the expression of oxidative stress-responsive genes, prevent the increase in double-stranded DNA breaks as a consequence of oxidative and electrophilic stress, and restore sensitivity to chemotherapeutics such as 5-FluoroUracil and cisplatin in colorectal and lung cancer cells [97,98]. Furthermore, recent findings from our group regarding its direct role in binding to the G-site of glutathione-S transferase subtypeA1 (GSTA1) and preventing conjugation of reduced glutathione with electrophilic compounds have added another interesting aspect of its potential for the reversal of chemoresistance [38] (Figure 2).
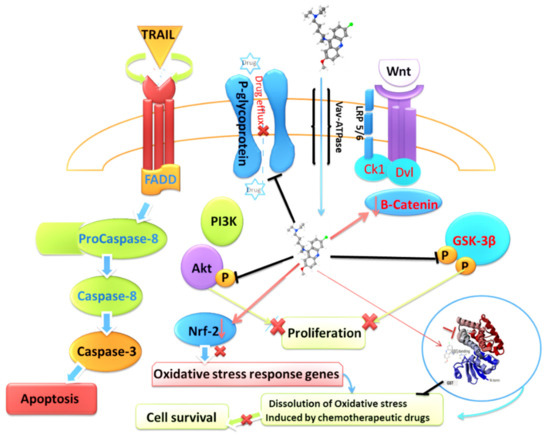
Figure 2.
Graphic describing the effect of QC on chemoresistance pathways enacted and utilized by cancer cells.
4.7. Synergistic Effects of Quinacrine with Other Chemotherapeutic and Targeted Therapies
QC has been studied in combination with various clinically approved therapeutics to explore the possibility of combinational therapeutic strategies. Every study conducted so far has demonstrated QC’s good synergy with cytotoxic, microtubule-targeting, and targeted therapeutic TKIs, which are unique to all chemical therapeutics. QC has shown its potential to enhance the therapeutic effect by many times and achieve the desired anticancer effect at much lower concentrations than the specific drug alone in both preclinical and clinical studies. In laboratory models, QC has shown good synergy with vorinostat (SAHA, an HDAC inhibitor), paclitaxel, 5-Fluorouracil, daunorubicin, cisplatin, cytarabine, erlotinib, and sorafenib in various types of cancer cells [44,58,60,61,99,100,101,102,103] (Table 3). QC has also been reported to synergize with the cytotoxic drugs carboplatin in ovarian cancer [52], temozolomide, SGK kinase inhibitor drug SI113 Glioblastoma multiforme (GBM) [46,104], and the PARP inhibitor drug Rucaparib in ovarian cancer cells [51]. QC has also been reported to enhance the effects of imatinib in GIST tumors through autophagy inhibition and to reverse the resistance to imatinib in both IV vitro and xenograft models [47]. These studies have demonstrated the remarkable effect of quinacrine in enhancing the potential of other drugs. They have opened new frontiers for exploration of even more permutations and combinations of QC with promising new targeted therapeutics, including therapeutic mAbs and immunotherapies.

Table 3.
Table describing the synergistic potential of QC with numerous clinically approved therapies. Synergy of QC with clinically approved therapies.
5. Quinacrine Nanoparticles in Cancer Treatment
Nanoparticle formulations of clinical therapeutics possess certain advantages, such as enhanced permeabilization due to their smaller size, thereby reducing the concentrations of effective dosage by multifold. Several studies have explored the aspect of assessing the formulation of QC nanoparticles (NPs), both standalone and in combination with other therapeutics, for their impact on various cancer cells. Hybrid gold and silver-based nanoparticles of QC have been reported to induce cell viability reduction, cell cycle arrest, and apoptosis at very low concentrations of 0.5 µg/mL in SCC-09 cells [107]. Another study from the same research group showed the effectiveness of silver and gold-based hybrid nanoparticles in downregulating cancer stem cell-specific markers by 40–60%. Loss of DNA repair activity due to downregulation of the BER mechanism was also observed due to QC NPs [108]. Quinacrine-loaded Undaria pinnatifida fucoidan nanoparticles have been shown to enhance the effect of QC by nearly 5.7-fold and induced 68% tumor reduction in xenograft models compared to 20% with liquid drug alone in pancreatic cancer cells [109]. Another study also reported disruption of hedgehog signaling by QC NPs and induction of apoptosis at lower concentrations than the liquid formulation of the drug. They have provided evidence for QC NP-mediated inhibition of the hedgehog-GLI cascade through binding of QC NPs to the consensus sequences (5 GACCACCCA3) of GLI1 and destabilizing the cascade [110]. Nano formulations of QC and erlotinib have also been recently reported to exhibit much better synergy than their plain drug solutions in NSCLC three-dimensional models. Multifold-enhanced antiproliferative and cytotoxic effects were observed in the nano form of both drugs [111]. Another study explored the development of inhalable bovine serum albumin-coated QC NPs for the treatment of NSCLC. The nanoparticles showed good aerosolization potential, with less than 5 µm median diameter. These aerosolized NPs were found to be much more effective on different NSCLC cells in three-dimensional culture models. They observed lower Ic50 values and prominent cell cycle arrest, leading to apoptotic death due to aerosolized NPs compared to the standard drug exposed in liquid form [112]. All these studies have laid a solid foundation for further exploration of QC NPs, both as a standalone and in combination with other therapeutics in xenografts as well as early phase clinical studies.
6. Clinical Research Studies of Quinacrine in Cancer Treatment
The earliest hints about the possibility of utilizing QC as a potential clinical anti-neoplastic drug came from a series of clinical studies exploring QC for the treatment of malignant pleural effusions as multiple cancers, including breast, lung, ovarian, and lymphoma, develop malignant effusions during the course of their disease. All these reports suggested a significant response of QC, leading to noticeable improvement in OS of the patients and a remarkable regression in reaccumulating of pleural fluid for months following QC treatment [14,38,113]. These studies reported that 68–72% of patients treated with a single dose or multiple oral dosages showed significant responses, and the average duration of response was approximately 20 weeks in patients without lung metastases. Taylor et al. also reported longer survival (<1 year) in 28% of the patients without recurrent effusion. Later, another study exploring QC combination and tetracycline in a randomized trial was conducted by Bayly et al. [114]. Six out of ten patients who received QC demonstrated a complete response to the therapy, with OS survival ranging from 3 to 6 months, and three other patients showed a partial response to the treatment.
Later, a few studies presented evidence of a decrease in fibrinolytic activity, evident by decreased levels of D-dimer, and fibrin lysis was observed after six hours of QC treatment. The levels of regulators of fibrinolysis, plasminogen activator inhibitor PAI-1, thrombin, fibrinopeptide A(FPA), and beta-thromoglobulin markedly increased after QC treatment. High levels of these markers, especially FPA and thrombin, are indicative of active fibrin formation and play an important role in arresting the pleural exudation process [115,116]. A single-agent quinacrine phase II clinical trial for androgen-dependent prostate cancer was conducted at the Cleveland Clinic, USA (clinical trial identifier NCT00417274). The patients were administered 100 mg of the drug daily and monitored for 18 months. Only three out of 31 patients showed serious adverse side effects (one back pain, one sepsis, and one thrombosis). The other details of this study are yet to be elucidated. Two other phase I clinical studies explored QC’s efficacy as a combinational therapeutic approach. A phase I dose-escalation clinical trial of a QC and erlotinib combination in advanced stage metastatic NSCLC (clinical trial identifier NCT0183995) recommended a dose of 50 mg QC every other day with 150 mg erlotinib for further phase II studies, which are currently in the planning stage. The dose was well tolerated with mild and manageable side effects. One of the six patients showed stable disease (SD) for eight months, and one patient showed a partial response (PR) for six months duration [105]. However, all of the patients selected for this study either had EGFR-negative adenocarcinoma or squamous cell carcinoma, which technically could limit the effects of erlotinib, as shown in various earlier phase II/III studies of erlotinib in EGFR negative and SCC patients. Another phase Ib clinical study completed this year assessed the combination of quinacrine and capecitabine in metastatic colon cancer patients (clinical trial identifier NCT01844076) [106]. Three patients showed SD and one showed PR in the dose-finding cohort of the study, while two out of the seven patients in the expansion cohort showed SD on 100 mg/kg QC. The ORR for the entire cohort was 5.9%, the disease control rate (CR + PR + SD) was 35.4%, and the OS in the heavily pretreated overall cohort was approximately 5.22 months. Interestingly, four of the six patients with responsive disease exhibited mutations in the K-Ras gene. The study concluded that a safe and well-tolerated dose of 100 mg QC and 1000 mg/m2 capecitabine was required. Although both Phase I studies have shown promising results, no Phase II studies have been reported yet.
7. Conclusions
These recent preclinical and clinical studies have put QC into the spotlight for exploring combinational therapeutic regimens that are more effective, less prone to chemoresistance, and have overall tolerable toxicity profiles. Additionally, a new generation of drugs, such as CBL 0137, that are modelled on QC have been designed and found effective in pre-clinical studies and currently have entered phase I clinical studies (NCT04870944). More clinical studies continuing this trend in NSCLC and other cancers are warranted. Moreover, the polypharmacological potential of quinacrine, which is able to simultaneously target multiple signaling cascades and cellular processes that are implicated in promoting carcinogenesis, is indeed a beneficial factor, adding a unique aspect to its scope of utility for cancer treatment. Simultaneously, more research is required to further elucidate the role of QC in cancer regression, and the molecular mechanisms involved and their synergy with new innovative targeted therapies are required to fully utilize the polypharmacological potential of this small molecule in combatting cancer. Numerous studies on nanoformulations of QC have opened a new frontier for further increasing the bioavailability of the drug and have further reduced the concentrations required to achieve remarkable antineoplastic effects. Further intensive exploration of new formulations and combinations of QC NPs with nanoformulations of other clinical therapeutics will provide less toxicity and better new promising therapeutic formulations and strategies in the future.
Author Contributions
Conceptualization, A.S. and M.K.; methodology, M.K.; formal analysis, M.K.; data curation, M.K.; writing—original draft preparation, M.K.; writing—review and editing, A.S.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was received for this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors thank Goa Cancer Society, Goa, India and DBT, Govt. of India for financial support. M.K. acknowledges the UGC and the Govt. of India for his fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenwood, D. Conflicts of interest: The genesis of synthetic antimalarial agents in peace and war. J. Antimicrob. Chemother. 1995, 36, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Office of the Surgeon General UCln. The drug treatment of malaria, suppressive and clinical. J. Am. Med. Assoc. 1943, 123, 205–208. [Google Scholar] [CrossRef]
- Joint Report of the Armored Medical Research Laboratory and Commission Tropical Diseases AEB, Preventative Medicine Service, Office of the Surgeon General, US Army, Plasma quinacrine concentration as a function of dosage and environment. Arch Intern. Med. 1946, 78, 64–107. [CrossRef] [PubMed]
- Lee, Y.Q.; Goh, A.S.; Ch’ng, J.H.; Nosten, F.H.; Preiser, P.R.; Pervaiz, S.; Yadav, S.K.; Tan, K.S. A high-content phenotypic screen reveals the disruptive potency of quinacrine and 3′, 4′-dichlorobenzamil on the digestive vacuole of Plasmodium falciparum. Antimicrob. Agents Chemother. 2014, 58, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C. Treatment of human taeniasis in the Philippines: A review. Southeast Asian J. Trop. Med. Public Health 1991, 22, 271–274. [Google Scholar] [PubMed]
- Lerman, S.J.; Walker, R.A. Treatment of giardiasis: Literature review and recommendations. Clin. Pediatrics 1982, 21, 409–414. [Google Scholar] [CrossRef]
- Feldmann, R.; Salomon, D.; Saurat, J.H. The association of the two antimalarials chloroquine and quinacrine for treatment-resistant chronic and subacute cutaneous lupus erythematosus. Dermatology 1994, 189, 425–427. [Google Scholar] [CrossRef]
- Wallace, D.J. The use of quinacrine (Atabrine) in rheumatic diseases: A reexamination. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 1989; Volume 18, pp. 282–296. [Google Scholar]
- Weisblum, B.; De Haseth, P.L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylate-rich regions in DNA. Proc. Natl. Acad. Sci. USA 1972, 69, 629–632. [Google Scholar] [CrossRef]
- Latt, S.A. Fluorescent probes of chromosome structure and replication. Can. J. Genet. Cytol. 1977, 19, 603–623. [Google Scholar] [CrossRef]
- Sahar, E.; Latt, S.A. Enhancement of banding patterns in human metaphase chromosomes by energy transfer. Proc. Natl. Acad. Sci. USA 1978, 75, 5650–5654. [Google Scholar] [CrossRef]
- Zipper, J.; Trujillo, V. 25 years of quinacrine sterilization experience in Chile: Review of 2592 cases. Int. J. Gynecol. Obstet. 2003, 83, S23–S29. [Google Scholar] [CrossRef]
- Lippes, J. Quinacrine sterilization (QS): Time for reconsideration. Contraception 2015, 92, 91–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, S.A.; Hooton, N.S.; Macarthur, A.M. Quinacrine in the management of malignant pleural effusion. J. Br. Surg. 1977, 64, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Kessel, E. Advances in contraception. Adv. Contracept. 1996, 12, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.A.; Earle, D.P.; Brodie, B.B.; Taggart, J.V.; Berliner, R.W. The pharmacological basis for the rational use of atabrine in the treatment of malaria. J. Pharmacol. Exp. Ther. 1944, 81, 307–330. [Google Scholar]
- Goodman, L.S. Goodman and Gilman’s the Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Marceau, F.; Bawolak, M.T.; Bouthillier, J.; Morissette, G. Vacuolar ATPase-mediated cellular concentration and retention of quinacrine: A model for the distribution of lipophilic cationic drugs to autophagic vacuoles. Drug Metab. Dispos. 2009, 37, 2271–2274. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Gagné, V.; Fernandes, M.J.; Marceau, F. High affinity capture and concentration of quinacrine in polymorphonuclear neutrophils via vacuolar ATPase-mediated ion trapping: Comparison with other peripheral blood leukocytes and implications for the distribution of cationic drugs. Toxicol. Appl. Pharmacol. 2013, 270, 77–86. [Google Scholar] [CrossRef]
- Huang, Y.; Okochi, H.; May, B.C.; Legname, G.; Prusiner, S.B.; Benet, L.Z.; Guglielmo, B.J.; Lin, E.T. Quinacrine is mainly metabolized to mono-desethyl quinacrine by CYP3A4/5 and its brain accumulation is limited by P-glycoprotein. Drug Metab. Dispos. 2006, 34, 1136–1144. [Google Scholar] [CrossRef]
- Solitro, A.R.; MacKeigan, J.P. Tissue distribution and tumor concentrations of hydroxychloroquine and quinacrine analogs in mice. bioRxiv 2018, 496018. [Google Scholar]
- Bauer, F. Quinacrine hydrochloride drug eruption (tropical lichenoid dermatitis): Its early and late sequelae and its malignant potential: A review. J. Am. Acad. Dermatol. 1981, 4, 239–248. [Google Scholar] [CrossRef]
- Custer, R.P. Aplastic anemia in soldiers treated with atabrine (quinacrine). Am. J. Med. Sci. 1946, 212, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.P.; Kinsman, J.M. Hypoplastic anemia due to atabrine. Blood 1949, 4, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Paton, M.D.; Riddell, M.J.; Strong, J.A. Aplastic anaemia following mepacrine treatment of lupus erythematosus. Lancet 1955, 265, 281–282. [Google Scholar] [CrossRef]
- Carr, R.E.; Henkind, P.; Rothfield, N.; Siegel, I.M. Ocular toxicity of antimalarial drugs: Long-term follow-up. Am. J. Ophthalmol. 1968, 66, 738–744. [Google Scholar] [CrossRef]
- Ansdell, V.E.; Common, J.D. Corneal changes induced by mepacrinc. J. Trop. Med. Hyg. 1979, 82, 206–207. [Google Scholar]
- Engel, G.L. Quinacrine effects on the central nervous system. JAMA 1966, 197, 515. [Google Scholar] [CrossRef]
- Lindenmayer, J.P.; Vargas, P. Toxic psychosis following use of quinacrine. J. Clin. Psychiatry 1981, 42, 162–164. [Google Scholar]
- Theodore, T.; Kahn, R.L. Toxicity of quinacrine (atabrine) for the central nervous system: III. An experimental study on human subjects. Arch. Neurol. Psychiatry 1946, 56, 284–299. [Google Scholar] [CrossRef]
- Vassey, J.W.; Edmonds, J.; Irvin, J.L.; Green, J.A.; Irvin, E.M. Studies on the administration of quinacrine to tumor-bearing mice. Cancer Res. 1955, 15, 573–578. [Google Scholar]
- Hiller, R.I. A study of quinacrine dihydrochloride in the human breast in vitro and in vivo. Am. J. Surg. 1970, 119, 317–321. [Google Scholar] [CrossRef]
- Borja, E.R.; Pugh, R.P. Single-dose quinacrine (atabrine) and thoracostomy in the control of pleural effusions in patients with neoplastic diseases. Cancer 1973, 31, 899–902. [Google Scholar] [CrossRef]
- Denny, W.A.; Baguley, B.C.; Cain, B.F.; Waring, M.J. Antitumour acridines. In Molecular Aspects of Anti-Cancer Drug Action; Palgrave: London, UK, 1983; pp. 1–34. [Google Scholar]
- Koldsland, S.; Svennevig, J.L.; Lehne, G.; Johnson, E. Chemical pleurodesis in malignant pleural effusions: A randomised prospective study of mepacrine versus bleomycin. Thorax 1993, 48, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Preet, R.; Mohapatra, P.; Mohanty, S.; Sahu, S.K.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Quinacrine has anticancer activity in breast cancer cells through inhibition of topoisomerase activity. Int. J. Cancer 2012, 130, 1660–1670. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wang, H.; Wang, Q.; Wang, L.; Miao, J.; Cui, F.; Wang, J. Quinacrine inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. Curr. Ther. Res. 2012, 73, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Martin, A.; Nirgude, S.; Chaudhary, B.; Mondal, S.; Sarkar, A. Quinacrine inhibits GSTA1 activity and induces apoptosis through G1/S arrest and generation of ROS in human non-small cell lung cancer cell lines. Oncotarget 2020, 11, 1603. [Google Scholar] [CrossRef]
- Harada, M.; Morimoto, K.; Kondo, T.; Hiramatsu, R.; Okina, Y.; Muko, R.; Matsuda, I.; Kataoka, T. Quinacrine inhibits ICAM-1 transcription by blocking DNA binding of the NF-κB subunit p65 and sensitizes human lung adenocarcinoma A549 cells to TNF-α and the Fas ligand. Int. J. Mol. Sci. 2017, 18, 2603. [Google Scholar] [CrossRef]
- Dermawan, J.K.; Gurova, K.; Pink, J.; Dowlati, A.; De, S.; Narla, G.; Sharma, N.; Stark, G.R. Quinacrine Overcomes Resistance to Erlotinib by Inhibiting FACT, NF-κB, and Cell-Cycle Progression in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2014, 13, 2203–2214. [Google Scholar] [CrossRef]
- Jani, T.S.; DeVecchio, J.; Mazumdar, T.; Agyeman, A.; Houghton, J.A. Inhibition of NF-κB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or oxaliplatin. J. Biol. Chem. 2010, 285, 19162–19172. [Google Scholar] [CrossRef]
- Mohapatra, P.; Preet, R.; Das, D.; Satapathy, S.R.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Quinacrine-mediated autophagy and apoptosis in colon cancer cells is through a p53-and p21-dependent mechanism. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2012, 20, 81–91. [Google Scholar] [CrossRef]
- Park, S.; Oh, A.Y.; Cho, J.H.; Yoon, M.H.; Woo, T.G.; Kang, S.M.; Lee, H.Y.; Jung, Y.J.; Park, B.J. Therapeutic effect of quinacrine, an antiprotozoan drug, by selective suppression of p-CHK1/2 in p53-negative malignant cancers. Mol. Cancer Res. 2018, 16, 935–946. [Google Scholar] [CrossRef]
- Abdulghani, J.; Gokare, P.; Gallant, J.N.; Dicker, D.; Whitcomb, T.; Cooper, T.; Liao, J.; Derr, J.; Liu, J.; Goldenberg, D.; et al. Sorafenib and quinacrine target anti-apoptotic protein MCL1: A poor prognostic marker in anaplastic thyroid cancer (ATC). Clin. Cancer Res. 2016, 22, 6192–6203. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Cingöz, A.; Bagci-Onder, T.; Kizilel, S. Quinacrine Mediated Sensitization of Glioblastoma (GBM) Cells to TRAIL through MMP-Sensitive PEG Hydrogel Carriers. Macromol. Biosci. 2017, 17, 1600267. [Google Scholar] [CrossRef]
- Matteoni, S.; Abbruzzese, C.; Matarrese, P.; De Luca, G.; Mileo, A.M.; Miccadei, S.; Schenone, S.; Musumeci, F.; Haas, T.L.; Sette, G.; et al. The kinase inhibitor SI113 induces autophagy and synergizes with quinacrine in hindering the growth of human glioblastoma multiforme cells. J. Exp. Clin. Cancer Res. 2019, 38, 202. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Roy, S.; Lazar, A.J.; Wang, W.L.; McAuliffe, J.C.; Reynoso, D.; McMahon, J.; Taguchi, T.; Floris, G.; Debiec-Rychter, M.; et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc. Natl. Acad. Sci. USA 2010, 107, 14333–14338. [Google Scholar] [CrossRef]
- Preet, R.; Siddharth, S.; Satapathy, S.R.; Das, S.; Nayak, A.; Das, D.; Wyatt, M.D.; Kundu, C.N. Chk1 inhibitor synergizes quinacrine mediated apoptosis in breast cancer cells by compromising the base excision repair cascade. Biochem. Pharmacol. 2016, 105, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Österroos, A.; Hassan, S.; Gullbo, J.; Rickardson, L.; Jarvius, M.; Nygren, P.; Fryknäs, M.; Höglund, M.; Larsson, R. Drug screen in patient cells suggests quinacrine to be repositioned for treatment of acute myeloid leukemia. Blood Cancer J. 2015, 5, e307. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Katz, S.I.; Abdulghani, J.; Dicker, D.; El-Deiry, W.S. Quinacrine sensitizes hepatocellular carcinoma cells to TRAIL via up-regulating DR5 and eradicating MCL-1. Cancer Res. 2010, 70, 680. [Google Scholar]
- Oien, D.B.; Ray, U.; Pathoulas, C.L.; Jin, L.; Thirusangu, P.; Jung, D.; Kumka, J.E.; Xiao, Y.; Sarkar Bhattacharya, S.; Montoya, D.; et al. Quinacrine Induces Nucleolar Stress in Treatment-Refractory Ovarian Cancer Cell Lines. Cancers 2021, 13, 4645. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Roy, D.; Kalogera, E.; Mondal, S.; Wen, X.; He, X.; Dowdy, S.; Shridhar, V. Quinacrine promotes autophagic cell death and chemosensitivity in ovarian cancer and attenuates tumor growth. Oncotarget 2015, 6, 36354. [Google Scholar] [CrossRef]
- Jung, D.; Khurana, A.; Roy, D.; Kalogera, E.; Bakkum-Gamez, J.; Chien, J.; Shridhar, V. Quinacrine upregulates p21/p27 independent of p53 through autophagy-mediated downregulation of p62-Skp2 axis in ovarian cancer. Sci. Rep. 2018, 8, 2487. [Google Scholar] [CrossRef]
- Liang, R.; Yao, Y.; Wang, G.; Yue, E.; Yang, G.; Qi, X.; Wang, Y.; Zhao, L.; Zheng, T.; Zhang, Y.; et al. Repositioning quinacrine toward treatment of ovarian cancer by rational combination with TRAIL. Front. Oncol. 2020, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Thirusangu, P.; Pathoulas, C.L.; Ray, U.; Xiao, Y.; Staub, J.; Jin, L.; Khurana, A.; Shridhar, V. Quinacrine-Induced Autophagy in Ovarian Cancer Triggers Cathepsin-L Mediated Lysosomal/Mitochondrial Membrane Permeabilization and Cell Death. Cancers 2021, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Gurova, K.V.; Hill, J.E.; Guo, C.; Prokvolit, A.; Burdelya, L.G.; Samoylova, E.; Khodyakova, A.V.; Ganapathi, R.; Ganapathi, M.; Tararova, N.D.; et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-κB-dependent mechanism of p53 suppression in tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 17448–17453. [Google Scholar] [CrossRef]
- Friedman, J.; Nottingham, L.; Duggal, P.; Pernas, F.G.; Yan, B.; Yang, X.P.; Chen, Z.; Van Waes, C. Deficient TP53 expression, function, and cisplatin sensitivity are restored by quinacrine in head and neck cancer. Clin. Cancer Res. 2007, 13, 6568–6578. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Batis, N.; Franke, A.C.; Clancey, G.; Hartley, M.; Ryan, G.; Brooks, J.; Southam, A.D.; Barnes, N.; Parish, J.; et al. Repurposed quinacrine synergizes with cisplatin, reducing the effective dose required for treatment of head and neck squamous cell carcinoma. Oncotarget 2019, 10, 5229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fox, G.; Popanda, O.; Edler, L.; Thielmann, H.W. Preferential inhibition of DNA polymerases α, δ, and ε from Novikoff hepatoma cells by inhibitors of cell proliferation. J. Cancer Res. Clin. Oncol. 1996, 122, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, Z.; Wang, L.; Peng, D.; Belkhiri, A.; Lockhart, A.C.; El-Rifai, W. A combination of SAHA and Quinacrine is effective in inducing cancer cell death in upper gastrointestinal cancers. Clin. Cancer Res. 2018, 24, 1905–1916. [Google Scholar] [CrossRef]
- Jing, B.; Jin, J.; Xiang, R.; Liu, M.; Yang, L.; Tong, Y.; Xiao, X.; Lei, H.; Liu, W.; Xu, H.; et al. Vorinostat and quinacrine have synergistic effects in T-cell acute lymphoblastic leukemia through reactive oxygen species increase and mitophagy inhibition. Cell Death Dis. 2018, 9, 589. [Google Scholar] [CrossRef]
- Charmantray, F.; Martelli, A. Interest of acridine derivatives in the anticancer chemotherapy. Curr. Pharm. Des. 2001, 7, 1703–1724. [Google Scholar]
- Gurova, K. New hopes from old drugs: Revisiting DNA-binding small molecules as anticancer agents. Future Oncol. 2009, 5, 1685–1704. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Healey, M.A.; Lee, C.; Poh, W.; Yerram, S.R.; Patel, K.; Azad, N.S.; Herman, J.G.; Kern, S.E. DNA-intercalators causing rapid re-expression of methylated and silenced genes in cancer cells. Oncotarget 2013, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ho, W.C.; Dicker, D.T.; MacKinnon, C.; Winkler, J.D.; Marmorstein, R.; El-Deiry, W.S. Acridine derivatives activate p53 and induce tumor cell death through Bax. Cancer Biol. Ther. 2005, 4, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Ehsanian, R.; Van Waes, C.; Feller, S.M. Beyond DNA binding-a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun. Signal. 2011, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Klein, C.L.; van Kooten, T.G.; Kirkpatrick, C.J. Mechanisms of cell activation by heavy metal ions. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 42, 443–452. [Google Scholar] [CrossRef]
- König, W.; Schönfeld, W.; Raulf, M.; Köller, M.; Knöller, J.; Scheffer, J.; Brom, J. The neutrophil and leukotrienes-role in health and disease. Eicosanoids 1990, 3, 1–22. [Google Scholar] [PubMed]
- Stuhlmeier, K.M.; Kao, J.J.; Bach, F.H. Arachidonic acid influences proinflammatory gene induction by stabilizing the inhibitor-κBα/nuclear factor-κB (NF-κB) complex, thus suppressing the nuclear translocation of NF-κB. J. Biol. Chem. 1997, 272, 24679–24683. [Google Scholar] [CrossRef]
- Palmetshofer, A.; Robson, S.C.; Nehls, V. Lysophosphatidic acid activates nuclear factor kappa B and induces proinflammatory gene expression in endothelial cells. Thromb. Haemost. 1999, 82, 1532–1537. [Google Scholar] [PubMed]
- Nesher, E.; Safina, A.; Aljahdali, I.; Portwood, S.; Wang, E.S.; Koman, I.; Wang, J.; Gurova, K.V. Role of chromatin damage and chromatin trapping of FACT in mediating the anticancer cytotoxicity of DNA-binding small-molecule drugs. Cancer Res. 2018, 78, 1431–1443. [Google Scholar] [CrossRef]
- Na, S.I.; Lee, M.Y.; Heo, J.S.; Han, H.J. Hydrogen Peroxide Increases [3H]-2-Deoxyglucose uptake via MAPKs, cPLA2, and NF-κB Signaling Pathways in Mouse Embryonic Stem Cells. Cell. Physiol. Biochem. 2007, 20, 1007–1018. [Google Scholar] [CrossRef]
- Langer, S.W.; Schmidt, G.; Sørensen, M.; Sehested, M.; Jensen, P.B. Inhibitors of topoisomerase II as pH-dependent modulators of etoposide-mediated cytotoxicity. Clin. Cancer Res. 1999, 5, 2899–2907. [Google Scholar]
- Popanda, O.; Thielmann, H.W. The function of DNA topoisomerases in UV-induced DNA excision repair: Studies with specific inhibitors in permeabilized human fibroblasts. Carcinogenesis 1992, 13, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Fuks, Z.; Smith, K.C. Effect of quinacrine on x-ray sensitivity and the repair of damaged DNA in Escherichia coli K-12. Radiat. Res. 1971, 48, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Voiculetz, N.; Smith, K.C.; Kaplan, H.S. Effect of Quinacrine on survival and DNA repair in X-irradiated Chinese hamster cells. Cancer Res. 1974, 34, 1038–1044. [Google Scholar]
- Werbovetz, K.A.; Lehnert, E.K.; Macdonald, T.L.; Pearson, R.D. Cytotoxicity of acridine compounds for Leishmania promastigotes in vitro. Antimicrob. Agents Chemother. 1992, 36, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Dominick, P.K.; Keppler, B.R.; Legassie, J.D.; Moon, I.K.; Jarstfer, M.B. Nucleic acid-binding ligands identify new mechanisms to inhibit telomerase. Bioorganic Med. Chem. Lett. 2004, 14, 3467–3471. [Google Scholar] [CrossRef]
- Harrison, R.J.; Gowan, S.M.; Kelland, L.R.; Neidle, S. Human telomerase inhibition by substituted acridine derivatives. Bioorganic Med. Chem. Lett. 1999, 9, 2463–2468. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, J.; Li, Q.; Liu, Y.; Li, L.; Shang, Q.; Xu, G.; Tang, Y. Recognize three different human telomeric G-quadruplex conformations by quinacrine. Analyst 2012, 137, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.C.; Ramanathan, S. Quinacrine: Site of inhibition of synchronized cell division in Tetrahymena. Life Sci. 1968, 7, 1053–1062. [Google Scholar] [CrossRef]
- Van Dyke, K.; Lantz, C.; Szustkiewicz, C. Quinacrine: Mechanisms of antimalarial action. Science 1970, 169, 492–493. [Google Scholar] [CrossRef]
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. 2018, 126, 450–464. [Google Scholar] [CrossRef]
- Oien, D.B.; Pathoulas, C.L.; Ray, U.; Thirusangu, P.; Kalogera, E.; Shridhar, V. Repurposing quinacrine for treatment-refractory cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 68, pp. 21–30. [Google Scholar]
- Das, S.; Nayak, A.; Siddharth, S.; Nayak, D.; Narayan, S.; Kundu, C.N. TRAIL enhances quinacrine-mediated apoptosis in breast cancer cells through induction of autophagy via modulation of p21 and DR5 interactions. Cell. Oncol. 2017, 40, 593–607. [Google Scholar] [CrossRef]
- Das, S.; Tripathi, N.; Preet, R.; Siddharth, S.; Nayak, A.; Bharatam, P.V.; Kundu, C.N. Quinacrine induces apoptosis in cancer cells by forming a functional bridge between TRAIL-DR5 complex and modulating the mitochondrial intrinsic cascade. Oncotarget 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Van Bambeke, F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 2011, 12, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Virshup, D.M. Wnt signaling and drug resistance in cancer. Mol. Pharmacol. 2020, 97, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Maruyama, E. Reversal of resistance to vincristine in P388 leukemia by various polycyclic clinical drugs, with a special emphasis on quinacrine. Cancer Res. 1988, 48, 2064–2067. [Google Scholar]
- Wu, L.; Chatla, S.; Lin, Q.; Chowdhury, F.A.; Geldenhuys, W.; Du, W. Quinacrine-CASIN combination overcomes chemoresistance in human acute lymphoid leukemia. Nat. Commun. 2021, 12, 6936. [Google Scholar] [CrossRef]
- Carotenuto, P.; Romano, A.; Barbato, A.; Quadrano, P.; Brillante, S.; Volpe, M.; Ferrante, L.; Tammaro, R.; Morleo, M.; De Cegli, R.; et al. Drug Repurposing to Target the Apoptosome in MAPKi-Resistant Melanoma. Cell Rep. 2021. [Google Scholar] [CrossRef]
- Tiberghien, F.; Loor, F. Ranking of P-glycoprotein substrates and inhibitors by a calcein-AM fluorometry screening assay. Anti-Cancer Drugs 1996, 7, 568–578. [Google Scholar] [CrossRef]
- Leader, J.P.; O’Donnell, M.J. Transepithelial transport of fluorescent p-glycoprotein and MRP2 substrates by insect Malpighian tubules: Confocal microscopic analysis of secreted fluid droplets. J. Exp. Biol. 2005, 208, 4363–4376. [Google Scholar] [CrossRef]
- Preet, R.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis 2013, 34, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Gasparian, A.V.; Zhuang, Z.; Bosykh, D.A.; Komar, A.A.; Gudkov, A.V.; Gurova, K.V. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-κB and p53 pathways. Oncogene 2009, 28, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, C.W.; Lee, D.H.; Lee, J.S.; Oh, E.T.; Park, H.J. Quinacrine-mediated inhibition of Nrf2 reverses hypoxia-induced 5-fluorouracil resistance in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 4366. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Sabzichi, M.; Rashidi, M.; Mohammadian, J.; Mahmoudi, S.; Maroufi, N.F.; Ramezani, F.; Ghorbani, M.; Mohammadi, M.; Pirouzpanah, M.; et al. Sensitization of A-549 lung cancer cells to Cisplatin by Quinacrine-loaded lipidic nanoparticles via suppressing Nrf2 mediated defense mechanism. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.L.; Castillo, M.; Myers, C.E. Enhancement of paclitaxel activity against hormone-refractory prostate cancer cells in vitro and in vivo by quinacrine. Br. J. Cancer 1997, 75, 1593–1600. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.J.; Yu, Y.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Sun, M.G.; Shi, J.F.; Lu, W.L. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials 2012, 33, 565–582. [Google Scholar] [CrossRef]
- Gallant, J.N.; Allen, J.E.; Smith, C.D.; Dicker, D.T.; Wang, W.; Dolloff, N.G.; Navaraj, A.; El-Deiry, W.S. Quinacrine synergizes with 5-fluorouracil and other therapies in colorectal cancer. Cancer Biol. Ther. 2011, 12, 239–251. [Google Scholar] [CrossRef]
- Eriksson, A.; Chantzi, E.; Fryknäs, M.; Gullbo, J.; Nygren, P.; Gustafsson, M.; Höglund, M.; Larsson, R. Towards repositioning of quinacrine for treatment of acute myeloid leukemia–Promising synergies and in vivo effects. Leuk. Res. 2017, 63, 41–46. [Google Scholar] [CrossRef]
- Kalogera, E.; Roy, D.; Khurana, A.; Mondal, S.; Weaver, A.L.; He, X.; Dowdy, S.C.; Shridhar, V. Quinacrine in endometrial cancer: Repurposing an old antimalarial drug. Gynecol. Oncol. 2017, 146, 187–195. [Google Scholar] [CrossRef]
- Zhang, P.; Li, N.; Kiang, K.M.; Zhu, Z.; Leung, G.W.; Cheng, S.Y.; Leung, G.K. Quinacrine enhances temozolomide cytotoxicity in temozolomide-sensitive and-resistant glioblastoma cells. Glioma 2018, 1, 175. [Google Scholar]
- Bhateja, P.; Dowlati, A.; Sharma, N. Phase I study of the combination of quinacrine and erlotinib in patients with locally advanced or metastatic non small cell lung cancer. Investig. New Drugs 2018, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Denlinger, C.S.; Vijayvergia, N.; Cohen, S.J.; Astaturov, I.; Dotan, E.; Gallant, J.N.; Wang, E.W.; Kunkel, M.; Lim, B.; et al. First-in-Human Phase 1b Trial of Quinacrine Plus Capecitabine in Patients With Refractory Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2021, 20, e43–e52. [Google Scholar] [CrossRef]
- Hembram, K.C.; Chatterjee, S.; Sethy, C.; Nayak, D.; Pradhan, R.; Molla, S.; Bindhani, B.K.; Kundu, C.N. Comparative and mechanistic study on the anticancer activity of quinacrine-based silver and gold hybrid nanoparticles in head and neck cancer. Mol. Pharm. 2019, 16, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Hembram, K.C.; Dash, S.R.; Das, B.; Sethy, C.; Chatterjee, S.; Bindhani, B.K.; Kundu, C.N. Quinacrine based gold hybrid nanoparticles caused apoptosis through modulating replication fork in oral cancer stem cells. Mol. Pharm. 2020, 17, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Etman, S.M.; Mehanna, R.A.; Bary, A.A.; Elnaggar, Y.S.; Abdallah, O.Y. Undaria pinnatifida fucoidan nanoparticles loaded with quinacrine attenuate growth and metastasis of pancreatic cancer. Int. J. Biol. Macromol. 2021, 170, 284–297. [Google Scholar] [CrossRef]
- Nayak, A.; Satapathy, S.R.; Das, D.; Siddharth, S.; Tripathi, N.; Bharatam, P.V.; Kundu, C. Nanoquinacrine induced apoptosis in cervical cancer stem cells through the inhibition of hedgehog-GLI1 cascade: Role of GLI-1. Sci. Rep. 2016, 6, 20600. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Vaidya, B.; Gupta, V. Nano-synergistic combination of Erlotinib and Quinacrine for non-small cell lung cancer (NSCLC) therapeutics–Evaluation in biologically relevant in-vitro models. Mater. Sci. Eng. C 2021, 121, 111891. [Google Scholar] [CrossRef]
- Vaidya, B.; Kulkarni, N.S.; Shukla, S.K.; Parvathaneni, V.; Chauhan, G.; Damon, J.K.; Sarode, A.; Garcia, J.V.; Kunda, N.; Mitragotri, S.; et al. Development of inhalable quinacrine loaded bovine serum albumin modified cationic nanoparticles: Repurposing quinacrine for lung cancer therapeutics. Int. J. Pharm. 2020, 577, 118995. [Google Scholar] [CrossRef]
- Hickman, J.A.; Jones, M.C. Treatment of neoplastic pleural effusions with local instillations of quinacrine (mepacrine) hydrochloride. Thorax 1970, 25, 226–229. [Google Scholar] [CrossRef][Green Version]
- Bayly, T.C.; Kisner, D.L.; Sybert, A.; Macdonald, J.S.; Tsou, E.; Schein, P.S. Tetracycline and quinacrine in the control of malignant pleural effusions. A randomized trial. Cancer 1978, 41, 1188–1192. [Google Scholar] [CrossRef]
- Agrenius, V.; Chmielewska, J.; Widstrom, O.; Blomback, M. Increased coagulation activity of the pleura after tube drainage and quinacrine instillation in malignant pleural effusion. Eur. Respir. J. 1991, 4, 1135–1139. [Google Scholar] [PubMed]
- Agrenius, V.; Chmielewska, J.; Widstrom, O.L.; Blomback, M. Pleural fibrinolytic activity is decreased in inflammation as demonstrated in quinacrine pleurodesis treatment of malignant pleural effusion. Am. Rev. Respir. Dis. 1989, 140, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).