BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Inhibition Study on β-Secretase (BACE1) Enzyme
2.2. In Vitro Inhibition Study on Acetylcholinesterase (AChE) Enzyme
2.3. Molecular Modelling
2.4. Neuroprotective Assay
2.5. Neuritogenic Assay
2.6. Statistical Analysis
3. Results
3.1. Inhibitory Effect on BACE1 and AChE
3.2. Molecular Interactions with BACE1 by Molecular Docking
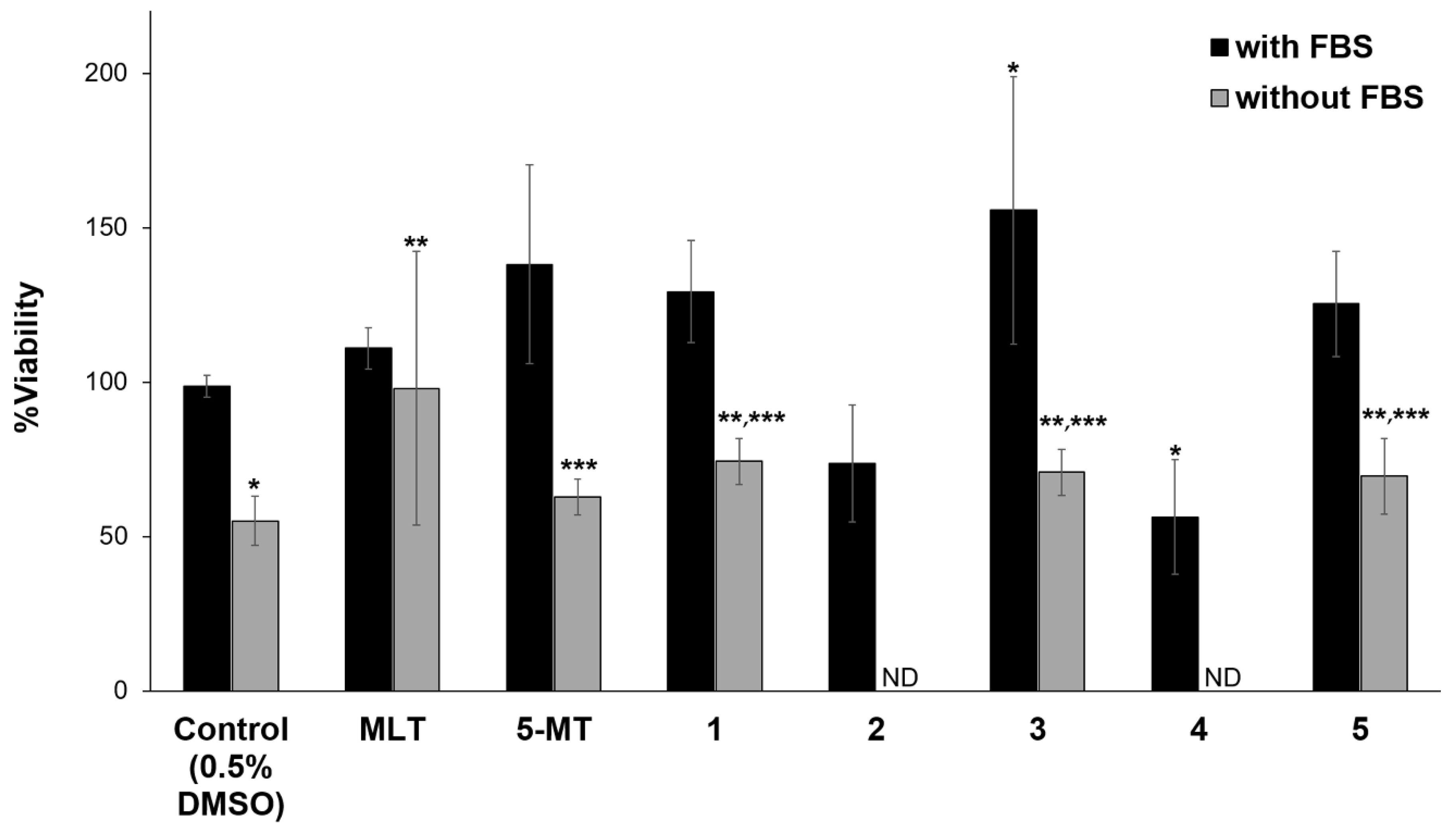
3.3. Neuroprotective and Neuritogenic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanabria-Castro, A.; Alvarado-Echeverria, I.; Monge-Bonilla, C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Perneczky, R. Amyloid Clearance as a Treatment Target against Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 24, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J.F. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Perry, R.H.; Cross, A.J.; Crow, T.J. Neuropathological and Biochemical Observations on the Noradrenergic System in Alzheimer’s Disease. J. Neurol. Sci. 1981, 51, 279–287. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S. Neuropathology and Therapeutic Management of Alzheimer’s Disease—An Update. Drugs Future 2008, 33, 433–446. [Google Scholar] [CrossRef]
- Salomone, S.; Caraci, F.; Leggio, G.M.; Fedotova, J.; Drago, F. New Pharmacological Strategies for Treatment of Alzheimer’s Disease: Focus on Disease Modifying Drugs. Br. J. Clin. Pharmacol. 2012, 73, 504–517. [Google Scholar] [CrossRef]
- Hardy, J. The Amyloid Hypothesis for Alzheimer’s Disease: A Critical Reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Roth, A.D.; Ramirez, G.; Alarcon, R.; Von Bernhardi, R. Oligodendrocytes Damage in Alzheimer’s Disease: Beta Amyloid Toxicity and Inflammation. Biol. Res. 2005, 38, 381–387. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, X. Alzheimer’s Disease Drug Development Based on Computer-Aided Drug Design. Eur. J. Med. Chem. 2016, 121, 851–863. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Silva, C.; Vacondio, F.; Plazzi, P.V.; Bertoni, S.; Spadoni, G.; Franceschini, D. Indole-Based Analogs of Melatonin: In Vitro Antioxidant and Cytoprotective Activities. J. Pineal Res. 2004, 36, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Sos, M.; Omar, R.A.; Bick, R.J.; Hickson-Bick, D.L.; Reiter, R.J.; Robakis, N.K. Melatonin Prevents Death of Neuroblastoma Cells Exposed to the Alzheimer Amyloid Peptide. J. Neurosci. 1997, 17, 1683–1690. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, Z.F.; Wang, Q.; Wang, Y.P.; Wang, J.Z. Melatonin Attenuates Beta-Amyloid-Induced Inhibition of Neurofilament Expression. Acta Pharmacol. Sin. 2004, 25, 447–451. [Google Scholar] [PubMed]
- Wang, X.C.; Zhang, Y.C.; Chatterjie, N.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J.Z. Effect of Melatonin and Melatonylvalpromide on β-Amyloid and Neurofilaments in N2a Cells. Neurochem. Res. 2008, 33, 1138–1144. [Google Scholar] [CrossRef]
- Matsubara, E.; Bryant-Thomas, T.; Pacheco Quinto, J.; Henry, T.L.; Poeggeler, B.; Herbert, D.; Shoji, M. Melatonin Increases Survival and Inhibits Oxidative and Amyloid Pathology in a Transgenic Model of Alzheimer’s Disease. J. Neurochem. 2003, 85, 1101–1108. [Google Scholar] [CrossRef]
- Mukda, S.; Panmanee, J.; Boontem, P.; Govitrapong, P. Melatonin Administration Reverses the Alteration of Amyloid Precursor Protein-Cleaving Secretases Expression in Aged Mouse Hippocampus. Neurosci. Lett. 2016, 621, 39–46. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Brusco, L.I.; Liberczuk, C.; Furio, A.M. The Use of Melatonin in Alzheimer’s Disease. Neuroendocrinol. Lett. 2002, 23, 20–23. [Google Scholar]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Iriti, M. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Ramos, E.; Egea, J.; de Los Rios, C.; Marco-Contelles, J.; Romero, A. Melatonin as a Versatile Molecule to Design Novel Multitarget Hybrids against Neurodegeneration. Future Med. Chem. 2017, 9, 765–780. [Google Scholar] [CrossRef]
- Panyatip, P.; Puthongking, P.; Tadtong, S. Neuroprotective and Neuritogenic Activities of Melatonin and N-Acetyl Substituent Derivative. Indian J. Pharm. Sci. 2015, 11, 14–19. [Google Scholar]
- Panyatip, P.; Johns, N.P.; Priprem, A.; Nakagawa, K.; Puthongking, P. Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives. Sci. Pharm. 2020, 88, 3. [Google Scholar] [CrossRef]
- Jiaranaikulwanitch, J.; Tadtong, S.; Govitrapong, P.; Fokin, V.V.; Vajragupta, O. Neuritogenic Activity of Bi-Functional Bis-Tryptoline Triazole. Bioorg. Med. Chem. 2017, 25, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Petrachaianan, T.; Chaiyasirisuwan, S.; Athikomkulchai, S.; Sareedenchai, V. Screening of Acetylcholinesterase Inhibitory Activity in Essential Oil from Myrtaceae. Turk. J. Pharm. Sci. 2019, 43, 63–68. [Google Scholar]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Gupta, S.; Parihar, D.; Shah, M.; Yadav, S. Computational Screening of Promising Beta-Secretase 1 Inhibitors through Multi-Step Molecular Docking and Molecular Dynamics Simulations-Pharmacoinformatics Approach. J. Mol. Struct. 2020, 1205, 127660. [Google Scholar] [CrossRef]
- Mashhadi, H.R.; Shanechi, H.M.; Lucas, C. A New Genetic Algorithm with Lamarckian Individual Learning for Generation Scheduling. IEEE Trans. Power Syst. 2003, 18, 1181–1186. [Google Scholar] [CrossRef]
- Jones-Villeneuve, E.M.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic Acid Induces Embryonal Carcinoma Cells to Differentiate into Neurons and Glial Cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef]
- MacPherson, P.A.; McBurney, M.W. P19 Embryonal Carcinoma Cells: A Source of Cultured Neurons Amenable to Genetic Manipulation. Methods 1995, 7, 238–252. [Google Scholar] [CrossRef]
- Iacovitti, L.; Stull, N.D.; Johnston, K. Melatonin Rescues Dopamine Neurons from Cell Death in Tissue Culture Models of Oxidative Stress. Brain Res. 1997, 768, 317–326. [Google Scholar] [CrossRef]
- Lopez-Maderuelo, M.D.; Fernandez-Renart, M.; Moratilla, C.; Renart, J. Opposite Effects of the Hsp90 Inhibitor Geldanamycin: Induction of Apoptosis in PC12, and Differentiation in N2A Cells. FEBS Lett. 2001, 490, 23–27. [Google Scholar] [CrossRef]
- Tadtong, S.; Kanlayavattanakul, M.; Lourith, N. Neuritogenic and Neuroprotective Activities of Fruit Residues. Nat. Prod. Commun. 2013, 8, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roy, S.; Tripathi, S.; Sharma, A. Molecular Docking Based Virtual Screening of Natural Compounds as Potential BACE1 Inhibitors: 3D QSAR Pharmacophore Mapping and Molecular Dynamics Analysis. J. Biomol. Struct. Dyn. 2016, 34, 239–249. [Google Scholar] [CrossRef]
- Guevara, J.; Zahran, M. Ensemble Docking of Potential BACE1 Inhibitors for Alzheimer’s Disease. FASEB J. 2019, 33, 642–644. [Google Scholar]
- Xu, Y.; Li, M.J.; Greenblatt, H.; Chen, W.; Paz, A.; Dym, O.; Jiang, H. Flexibility of the Flap in the Active Site of BACE1 as Revealed by Crystal Structures and Molecular Dynamics Simulations. Acta Crystallogr. Sect. D 2012, 68, 13–25. [Google Scholar] [CrossRef]
- Feng, Z.; Chang, Y.; Cheng, Y.; Zhang, B.L.; Qu, Z.W.; Qin, C.; Zhang, J.T. Melatonin Alleviates Behavioral Deficits Associated with Apoptosis and Cholinergic System Dysfunction in the APP 695 Transgenic Mouse Model of Alzheimer’s Disease. J. Pineal Res. 2004, 37, 129–136. [Google Scholar] [CrossRef]
- Vassar, R. BACE1 Inhibitor Drugs in Clinical Trials for Alzheimer’s Disease. Alzheimer’s Res. Ther. 2014, 6, 89. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Sousa, N.; Palha, J.A. Blood-Brain-Barriers in Aging and in Alzheimer’s Disease. Mol. Neurodegener. 2013, 8, 38. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 2717. [Google Scholar] [CrossRef]
- Zhao, X.J.; Gong, D.M.; Jiang, Y.R.; Guo, D.; Zhu, Y.; Deng, Y.C. Multipotent AChE and BACE-1 Inhibitors for the Treatment of Alzheimer’s Disease: Design, Synthesis and Bio-Analysis of 7-Amino-1, 4-Dihydro-2H-Isoquilin-3-one Derivates. Eur. J. Med. Chem. 2017, 138, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Halima, S.B.; Mishra, S.; Raja, K.M.P.; Willem, M.; Baici, A.; Simons, K.; Rajendran, L. Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein. Cell Rep. 2016, 14, 2127–2141. [Google Scholar] [PubMed]
- Seong, S.H.; Ali, M.Y.; Kim, H.R.; Jung, H.A.; Choi, J.S. BACE1 Inhibitory Activity and Molecular Docking Analysis of Meroterpenoids from Sargassum Serratifolium. Bioorg. Med. Chem. 2017, 25, 3964–3970. [Google Scholar] [CrossRef] [PubMed]
- Parnas, D.; Linial, M. Cholinergic Properties of Neurons Differentiated from an Embryonal Carcinoma Cell-Line (P19). Int. J. Dev. Neurosci. 1995, 13, 767–781. [Google Scholar] [CrossRef]
- Atabay, C.; Cagnoli, C.M.; Kharlamov, E.; Ikonomovic, M.D.; Manev, H. Removal of Serum from Primary Cultures of Cerebellar Granule Neurons Induces Oxidative Stress and DNA Fragmentation: Protection with Antioxidants and Glutamate Receptor Antagonists. J. Neurosci. Res. 1996, 43, 465–475. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Lv, Q.; Chen, X.; Deng, W.; Shi, K.; Pan, L. Effects and Mechanisms of Melatonin on the Proliferation and Neural Differentiation of PC12 Cells. Biochem. Biophys. Res. Commun. 2016, 478, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Rajendran, L.; Zetterberg, H.; Gustavsson, M.; Andreasson, U.; Olsson, M.; Neumann, U. BACE1 Inhibition Induces a Specific Cerebrospinal Fluid β-Amyloid Pattern that Identifies Drug Effects in the Central Nervous System. PLoS ONE 2012, 7, e31084. [Google Scholar] [CrossRef]
- Hasebe, N.; Fujita, Y.; Ueno, M.; Yoshimura, K.; Fujino, Y.; Yamashita, T. Soluble β-amyloid precursor protein alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PLoS ONE 2013, 8, e82321. [Google Scholar] [CrossRef]
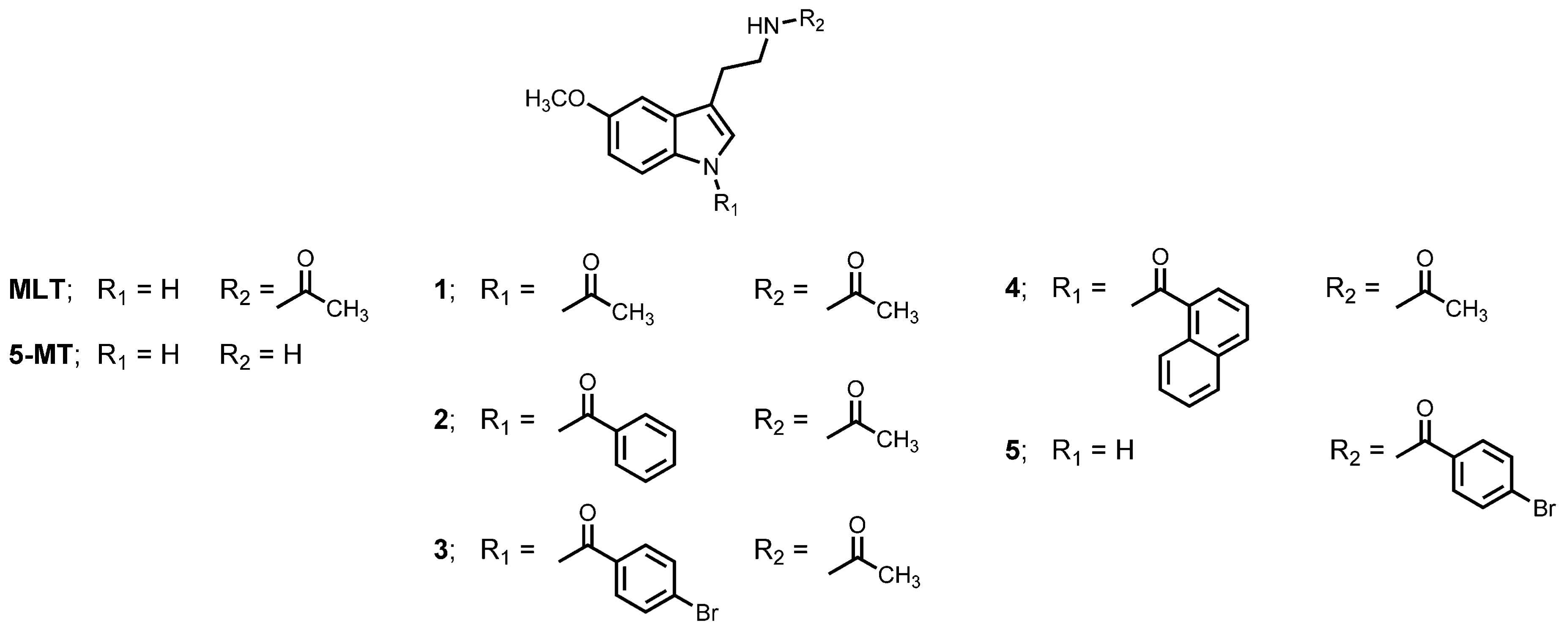

| Compounds | %Inhibition | |
|---|---|---|
| AChE ± SD at 100 µM | BACE1 ± SD at 5 µM | |
| MLT | 4.82 ± 3.13 | 83.23 ± 1.93 * |
| 5-MT | 4.44 ± 3.73 | 66.94 ± 2.31 *,** |
| 1 | 6.57 ± 3.04 | 87.74 ± 1.14 * |
| 2 | 5.76 ± 3.45 | 84.70 ± 3.79 * |
| 3 | 5.31 ± 2.37 | 79.56 ± 3.42 * |
| 4 | 6.84 ± 4.75 | 76.53 ± 3.40 * |
| 5 | 6.43 ± 2.67 | 86.92 ± 1.60 * |
| Quercetin | ND a | 48.46 ± 1.02 |
| Galantamine (35 µM) | 97.24 ± 0.54 | ND a |
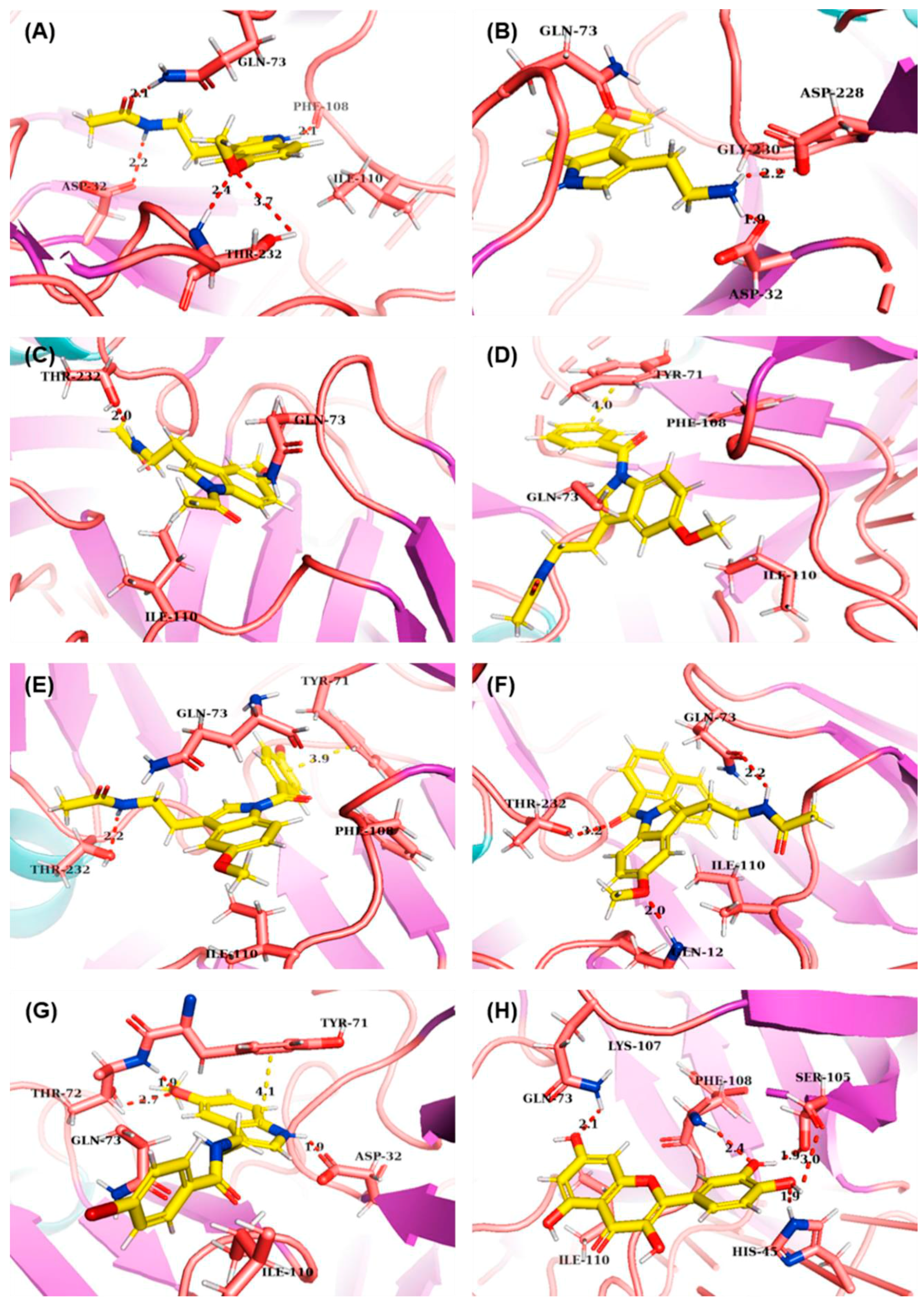
| Compounds | ΔG (kCal/mol) | Binding Amino Acids | ||
|---|---|---|---|---|
| H-Bond | π-π | van der Waals | ||
| MLT | −6.81 | Asp32, Gln73, Phe108, Thr232 | - | Gln73, Phe108, Ile110 |
| 5-MT | −9.97 | Asp32, Asp228 | - | Gln73, Gly230 |
| 1 | −6.72 | Thr232 | - | Gln73, Ile110 |
| 2 | −7.88 | - | Tyr71 | Tyr71, Gln73, Phe108, Ile110 |
| 3 | −8.48 | Thr232 | Tyr71 | Tyr71, Gln73, Phe108, Ile110 |
| 4 | −7.93 | Gln12, Gln73, Thr232 | - | Gln73, Ile110 |
| 5 | −7.66 | Asp32, Tyr71 | Tyr71 | Tyr71, Gln73, Ile110 |
| Quercetin | −6.63 | His45, Gln73, Ser105, Phe108 | - | Lys107, Ile110 |
| Compounds | Number of Neurites | Neurite Length | ||
|---|---|---|---|---|
| Average ± SD | %Increase | Average ± SD | %Increase | |
| Control (0.5% DMSO) | 1.50 ± 0.51 | 0.00 | 70.42 ± 54.40 | 0.00 |
| Quercetin | 2.80 ± 1.00 | 86.67 | 85.34 ± 53.31 | 21.19 |
| MLT | 2.90 ± 0.71 * | 93.33 | 63.94 ± 31.91 | −9.20 |
| 5-MT | 3.63 ± 1.22 *,**,*** | 142.00 | 69.23 ± 40.83 | −1.69 |
| 1 | 3.20 ± 1.16 * | 113.33 | 97.96 ± 65.67 *** | 39.11 |
| 3 | 4.60 ± 1.57 *,**,*** | 206.67 | 95.00 ± 70.35 *** | 34.90 |
| 5 | 3.73 ± 1.14 *,**,*** | 148.67 | 102.87 ± 78.83 *,*** | 46.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyatip, P.; Tadtong, S.; Sousa, E.; Puthongking, P. BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives. Sci. Pharm. 2020, 88, 58. https://doi.org/10.3390/scipharm88040058
Panyatip P, Tadtong S, Sousa E, Puthongking P. BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives. Scientia Pharmaceutica. 2020; 88(4):58. https://doi.org/10.3390/scipharm88040058
Chicago/Turabian StylePanyatip, Panyada, Sarin Tadtong, Emília Sousa, and Ploenthip Puthongking. 2020. "BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives" Scientia Pharmaceutica 88, no. 4: 58. https://doi.org/10.3390/scipharm88040058
APA StylePanyatip, P., Tadtong, S., Sousa, E., & Puthongking, P. (2020). BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives. Scientia Pharmaceutica, 88(4), 58. https://doi.org/10.3390/scipharm88040058






