Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives
Abstract
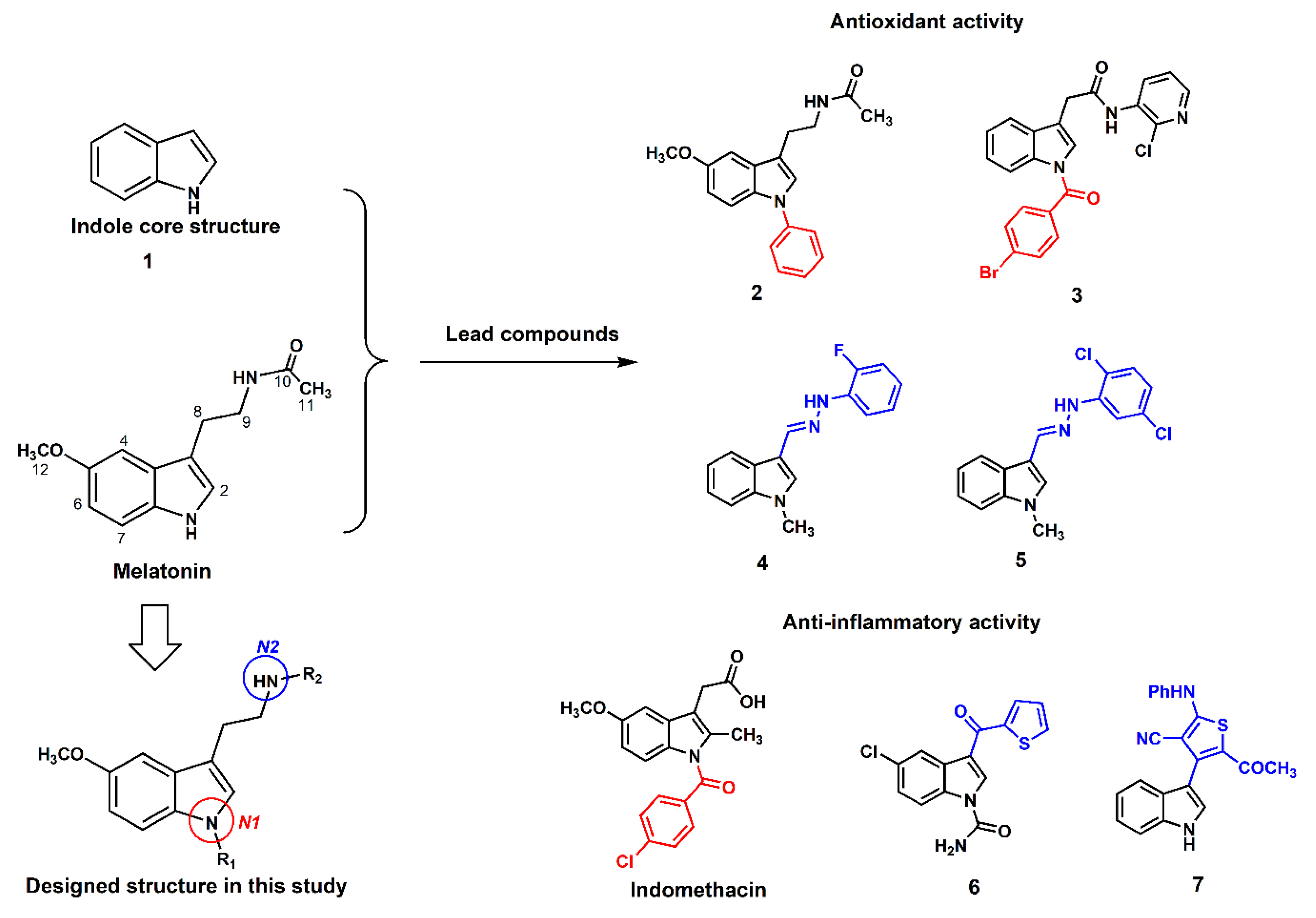
1. Introduction
2. Materials and Methods
2.1. Chemicals
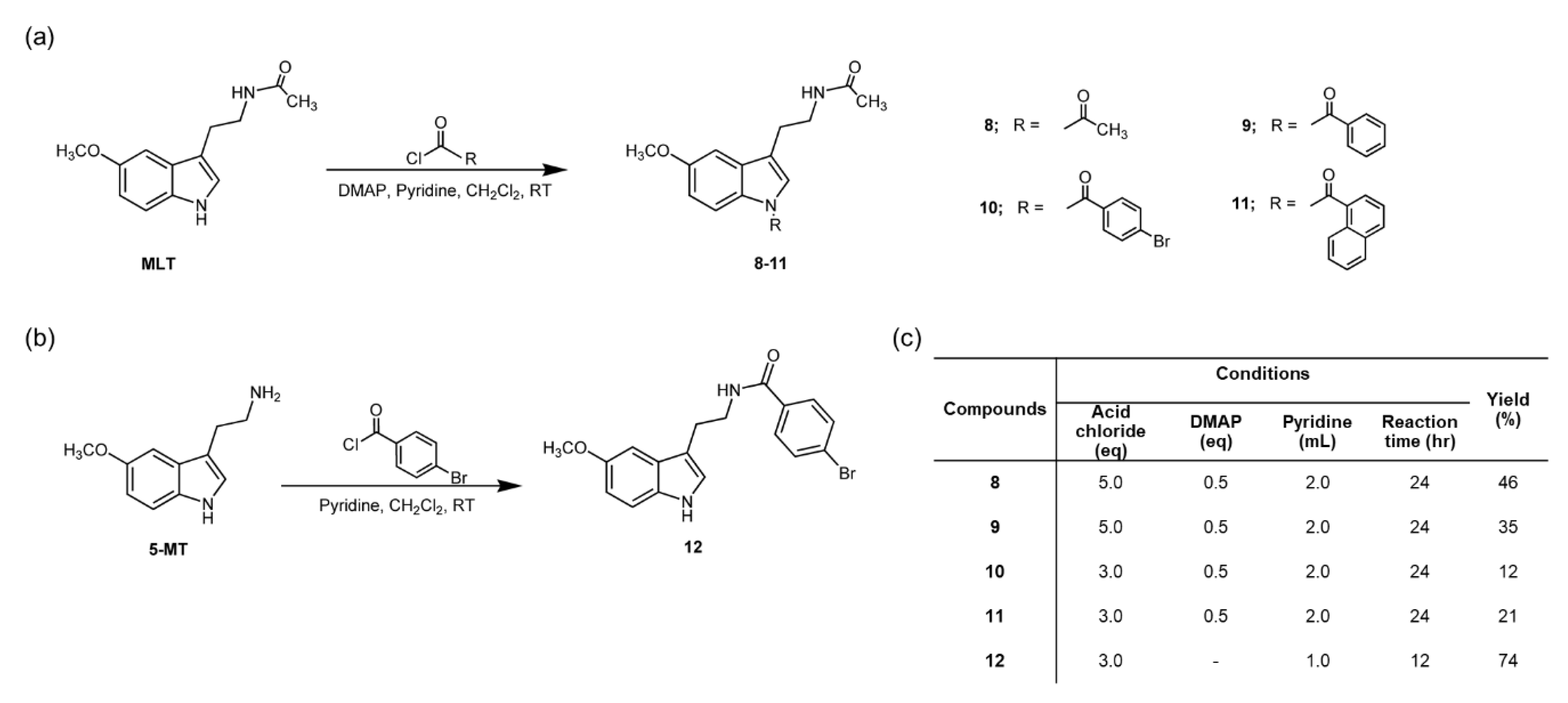
2.2. General Procedure for the Preparation of Compounds 8–12
2.2.1. N-(2-(1-acetyl-5-methoxy-1H-indol-3-yl)ethyl)acetamide (8)
2.2.2. N-(2-(1-benzoyl-5-methoxy-1H-indol-3-yl)ethyl)acetamide (9)
2.2.3. N-(2-(1-4-bromobenzoyl-5-methoxy-1H-indol-3-yl)ethyl)acetamide (10)
2.2.4. N-(2-(1-naphthoyl-5-methoxy-1H-indol-3-yl)ethyl)acetamide (11)
2.2.5. 4-Bromo-N-(2-(5-methoxy-1H-indol-3-yl)ethyl)benzamide (12)
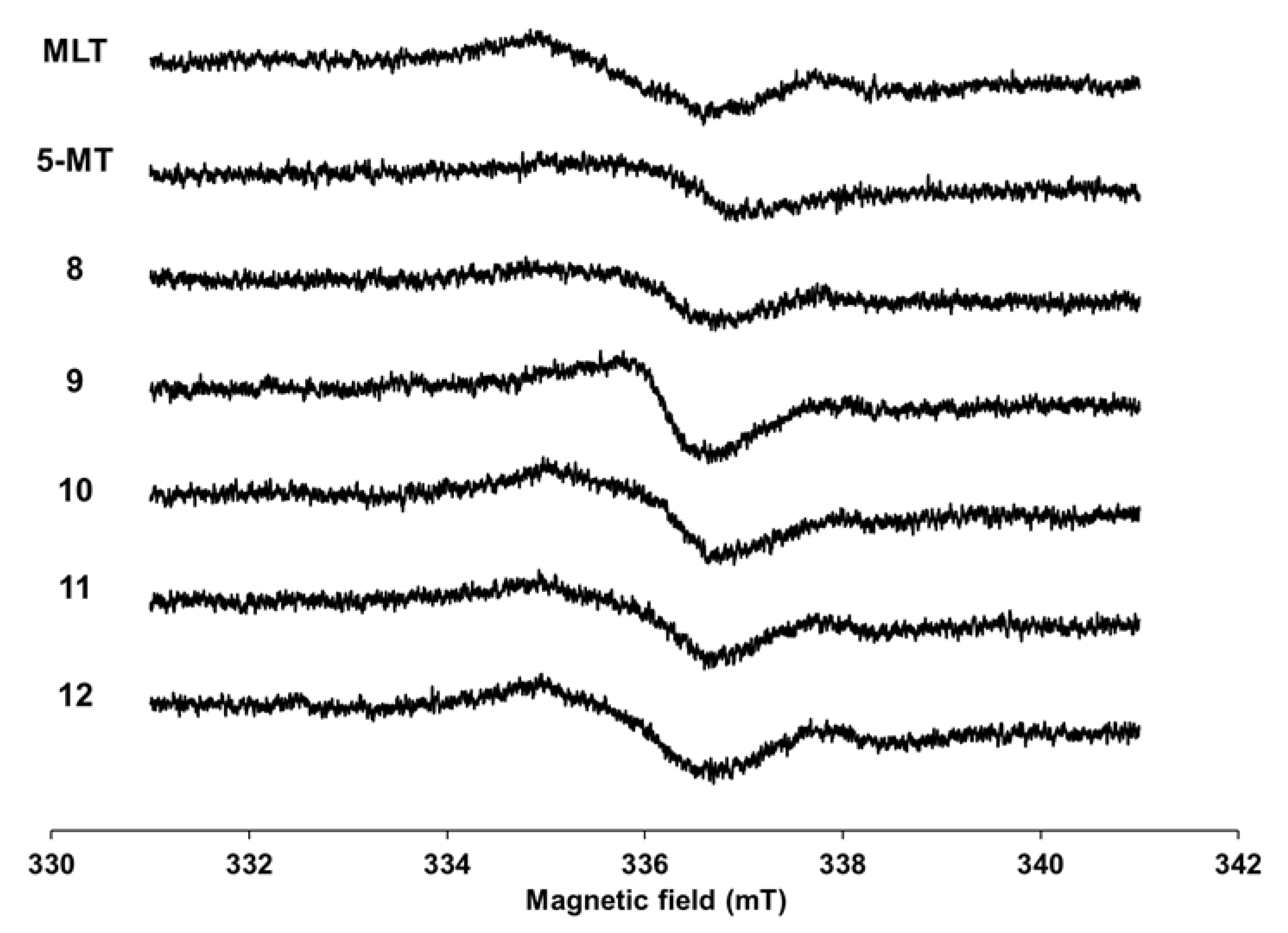
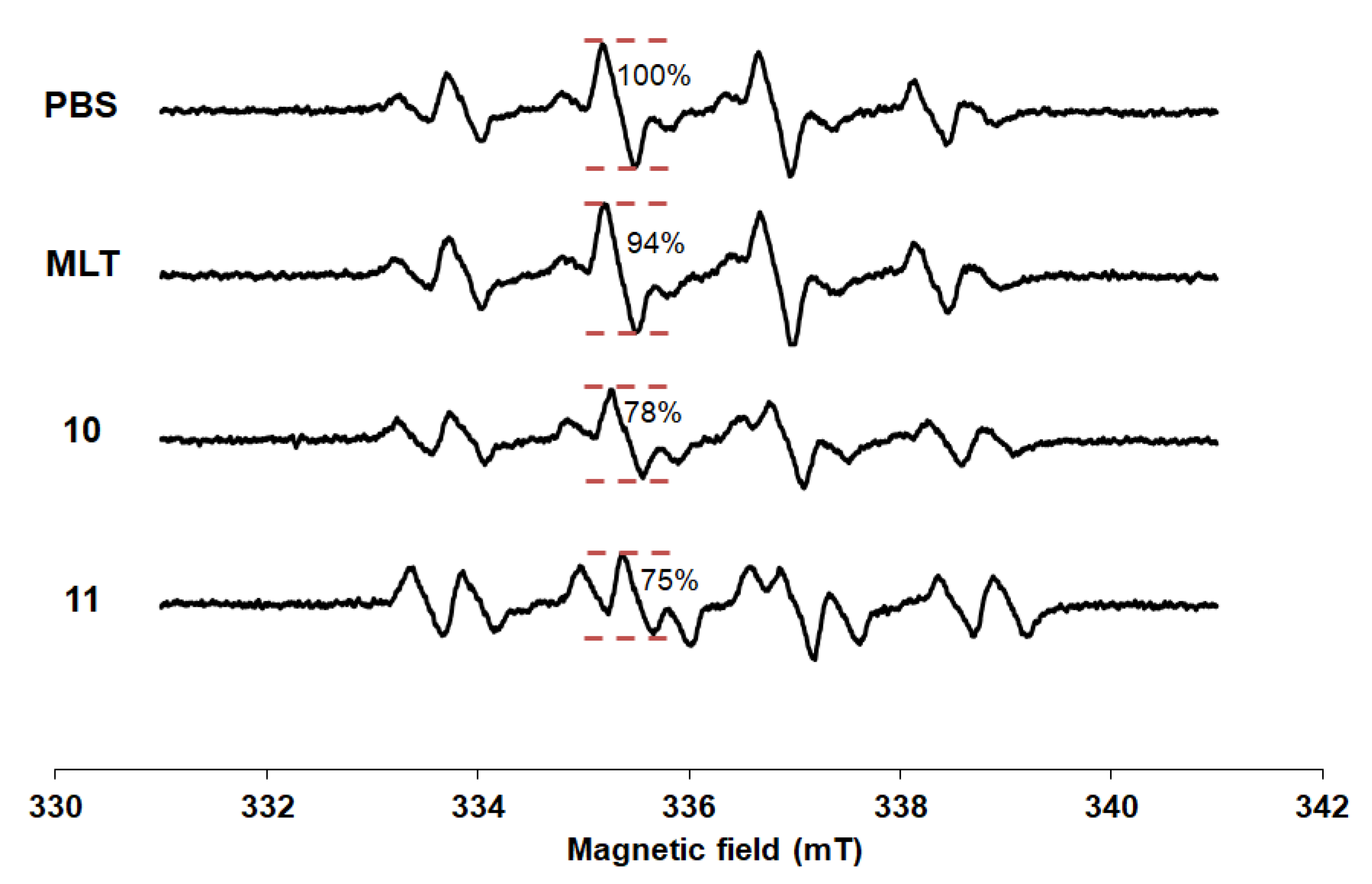
2.3. Electron Spin Resonance (ESR) Study
2.4. Study of Antioxidant Activities
2.4.1. 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) Disodium Salt (ABTS) Assay
2.4.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Electron Spin Resonance (ESR) Study
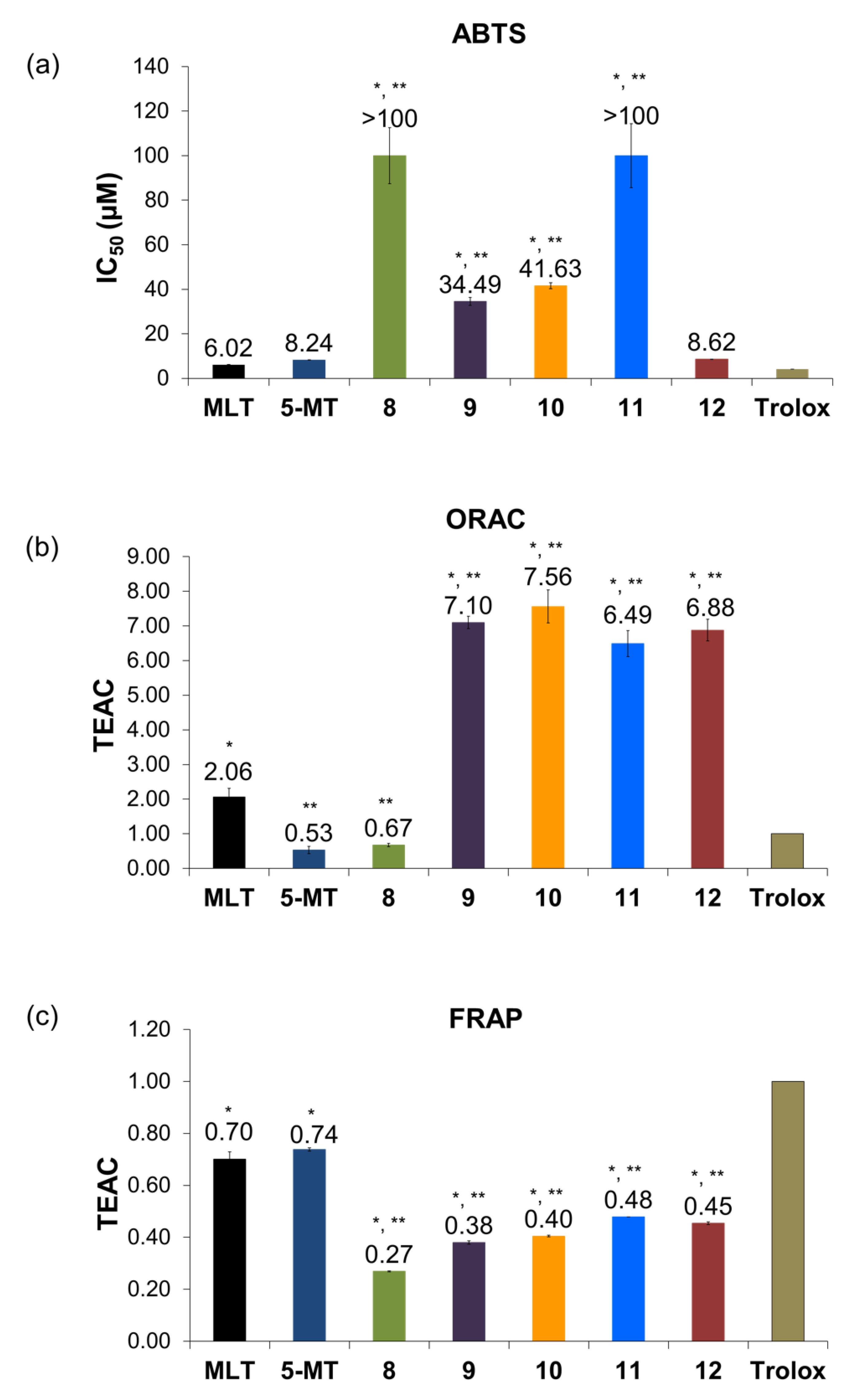
3.3. Study of Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharm. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Jyoti, R.B.; Gupta, S. Free radical pharmacology and its role in various diseases. J. Drug Deliv. 2019, 9, 690–694. [Google Scholar]
- Acuña-Castroviejo, D.; Martín, M.; Macías, M.; Escames, G.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001, 30, 65–74. [Google Scholar] [CrossRef]
- Davies, M.J. Quantification and Mechanisms of Oxidative Stress in Chronic Disease. Proceedings 2019, 11, 18. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Cui, K.; Luo, X.; Xu, K.; Murthy, M.V. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 771–799. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S. Reactive oxygen and nitrogen species and cellular and organismal decline: Amelioration with melatonin. Mech. Ageing Dev. 2002, 123, 1007–1019. [Google Scholar] [CrossRef]
- Sisein, E.A. Biochemistry of free radicals and antioxidants. Sch. Acad. J. Biosci. 2014, 2, 110–118. [Google Scholar]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Tamura, H.; Reiter, R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007, 43, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.; Qin, L.; Reiter, R. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P. Melatonin: Clinical perspectives in neurodegeneration. Front Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, 12514. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Neurosignals 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharm. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Iriti, M. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.H.; Rosenberg, J. Pharmacokinetics of alternative administration routes of melatonin: A systematic review. Drug Res. 2016, 66, 169–173. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Hajialyani, M.; Naseri, R. Melatonin. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 99–105. [Google Scholar]
- Gozzo, A.; Lesieur, D.; Duriez, P.; Fruchart, J.C.; Teissier, E. Structure-activity relationships in a series of melatonin analogues with the low-density lipoprotein oxidation model. Free Radic. Biol. Med. 1999, 26, 1538–1543. [Google Scholar] [CrossRef]
- Mor, M.; Silva, C.; Vacondio, F.; Plazzi, P.V.; Bertoni, S.; Spadoni, G.; Franceschini, D. Indole-based analogs of melatonin: In vitro antioxidant and cytoprotective activities. J. Pineal Res. 2004, 36, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Panyatip, P.; Puthongking, P.; Tadtong, S. Neuroprotective and neuritogenic activities of melatonin and N-acetyl substituent derivative. Isan J. Pharm. Sci. 2015, 11, 14–19. [Google Scholar]
- Oelgen, S.; Çoban, T. Synthesis and antioxidant properties of novel N-Substituted indole-2-carboxamide and indole-3-acetamide derivatives. Arch. Pharm. 2002, 335, 331–338. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Marnett, A.B.; Crews, B.C.; Remmel, R.P.; Marnett, L.J. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 2000, 43, 2860–2870. [Google Scholar] [CrossRef]
- Shirinzadeh, H.; Eren, B.; Gurer-Orhan, H.; Suzen, S.; Özden, S. Novel indole-based analogs of melatonin: Synthesis and in vitro antioxidant activity studies. Molecules 2010, 15, 2187–2202. [Google Scholar] [CrossRef]
- Radwan, M.A.; Ragab, E.A.; Sabry, N.M.; El-Shenawy, S.M. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2007, 15, 3832–3841. [Google Scholar] [CrossRef]
- Nakagawa, K. Electron spin resonance investigation of small spin probes in aqueous and vesicle phases of mixed membranes made from poly (oxyethylene) hydrogenated castor oil and hexadecane. Lipids 2005, 40, 745. [Google Scholar]
- Trouillas, P.; Calliste, C.A.; Allais, D.P.; Simon, A.; Marfak, A.; Delage, C.; Duroux, J.L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Younas, U.; Chan, K.W.; Sarfraz, R.A.; Uddin, M.K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Kroh, L.W. Electron spin resonance-a spectroscopic method for determining the antioxidative activity. Mol. Nutr. Food Res. 2005, 49, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Poeggeler, B.; Lopez-Burillo, S.; Mayo, J.C.; Reiter, R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003, 34, 249–259. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Tan, D.X. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyatip, P.; Pratheepawanit Johns, N.; Priprem, A.; Nakagawa, K.; Puthongking, P. Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives. Sci. Pharm. 2020, 88, 3. https://doi.org/10.3390/scipharm88010003
Panyatip P, Pratheepawanit Johns N, Priprem A, Nakagawa K, Puthongking P. Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives. Scientia Pharmaceutica. 2020; 88(1):3. https://doi.org/10.3390/scipharm88010003
Chicago/Turabian StylePanyatip, Panyada, Nutjaree Pratheepawanit Johns, Aroonsri Priprem, Kouichi Nakagawa, and Ploenthip Puthongking. 2020. "Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives" Scientia Pharmaceutica 88, no. 1: 3. https://doi.org/10.3390/scipharm88010003
APA StylePanyatip, P., Pratheepawanit Johns, N., Priprem, A., Nakagawa, K., & Puthongking, P. (2020). Effect of N-Amide Substitution on Antioxidative Activities of Melatonin Derivatives. Scientia Pharmaceutica, 88(1), 3. https://doi.org/10.3390/scipharm88010003





