Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparation of Celery Extract and Physicochemical Evaluation
2.3. Chromatography
2.4. Preparation of the Standard Solution: Calibration Standards and Quality Control Sample Preparation
2.5. Preparation of Plasma Sample
2.6. Pharmacokinetic Study
3. Results
3.1. Physico-Chemical Characteristics
3.2. Optimization of LC-MS/MS Parameters
3.3. Calibration Curve and Lower Limit of Quantification (LLOQ)
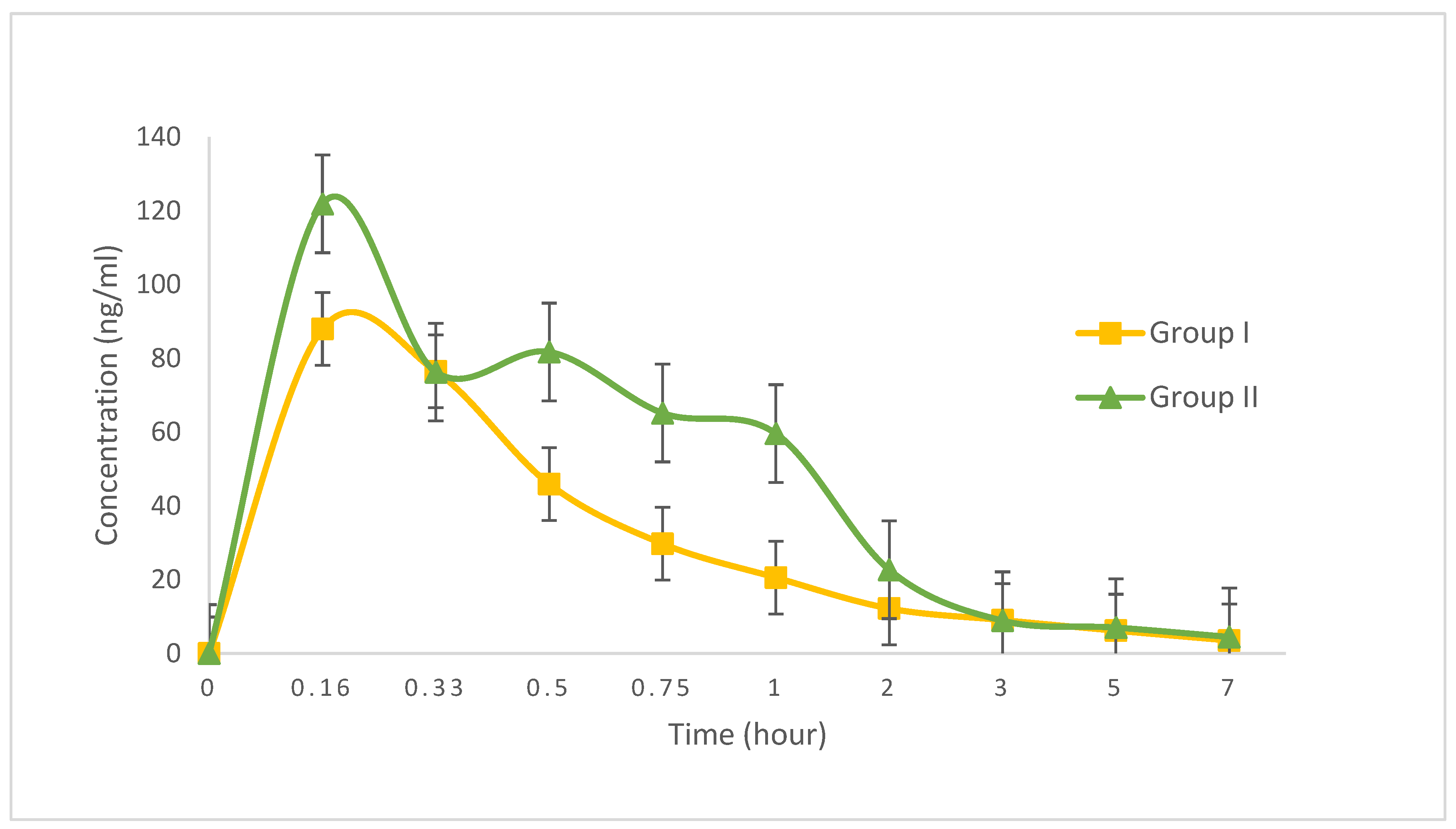
3.4. Pharmacokinetics
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kooti, W.; Ali-Akbari, S.; Asadi-Samani, M.; Ghadery, H.; Ashtary-Larky, D. A Review on medicinal plant of Apium. graveolens. Adv. Herb. Med. 2015, 1, 48–59. [Google Scholar]
- Al-Snafi, A.E. The pharmacology of Apium. graveolens. A review. Int. J. Pharm. Res. Sch. 2014, 3, 671–677. [Google Scholar]
- Kuhn, M.A. Herbal remedies: Drug-herb interactions. Crit. Care Nurse 2002, 22, 22–32. [Google Scholar] [PubMed]
- Patel, B.; Jivani, N.P.; Khodakiya, A. Drug interaction: Can we make them advantageous for a human being. Int. J. Pharm. Res. Dev. 2012, 4, 8–15. [Google Scholar]
- Brunton, L.; Chabner, B.; Knollman, B. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th ed.; Mc Graw Hill: La Jolla, CA, USA, 2011. [Google Scholar]
- Anonymous. Penelitian dan Pengembangan Kesehatan; Directorate General of Drug and Food Supervison; Departement of Health Republik of Indonesia: Jakarta, Indonesia, 2008.
- Tyashapsari, W.E.; dan Zulkarnain, A.K. Penggunaan Obat pada pasien Hipertensi di Instalasi Rawat Inap Rumah Sakit Umum Pusat Dr. Kariadi Semarang. Majalah Farmaseutik. 2012, 8, 145–151. [Google Scholar]
- Gusmira, S. Evaluasi Penggunaan Antihipertensi Konvensional dan Kombinasi Konvensional Bahan Alam pada Pasien Hipertensi di Puskesmas Wilayah Depok. Makara Kesehatan. 2012, 16, 77–83. [Google Scholar]
- Jakovljevic, V.; Raskovic, A.; Popovic, M.; Sabo, J. The effect of celery and parsley juices on pharmacodynamic activity of drugs involving cytochrome P450 in their metabolism. Eur. J. Drug Met. Pharmacokinet. 2002, 27, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Latif, Z.; Gray, A.L. Natural Products Isolation, 2nd ed.; Humana Press: Totowo, NJ, USA, 2006. [Google Scholar]
- Johnson, M.; Kalaiarasi, V.; Sivaraman, A.; Janakiraman, N.; Babu, A.; Narayani, M. Phytochemical and Antibacterial Studies on Aristolochia tagala. Cham. World J. Pharm. Res. 2014, 3, 2172–2178. [Google Scholar]
- World Health Organization. Quality Control. Methods for Medicinal Plant. Materials; WHO Library: Geneva, Switzerland, 1998; pp. 1–115. [Google Scholar]
- Anonymous. Parameter Standar. Umum. Ekstrak. Tumbuhan. Obat; Directorate General of Drug and Food Supervison; Departement of Health Republik of Indonesia: Jakarta, Indonesia, 2000.
- Donáth-Nagy, G.; Vancea, S.; Imre, S. Comparative study of captopril derivatization reaction by LC-UV, LC-MS and CE-UV methods. Croatica. Chem. Acta. 2011, 84, 423–427. [Google Scholar] [CrossRef]
- Vancea, S.; Imre, S.; Muntean, T. Determination of free captopril in human plasma by liquid chromatography with mass spectrometry detection. Talanta 2009, 79, 436–441. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Committee for Medicinal Products for Human Use. In Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011. [Google Scholar]
- Burnett, B.P.; Pillai, L.; Bitto, A.; Squadrito, F.; Levy, R.M. Evaluation of CYP450 inhibitory effects and steady-state pharmacokinetics of genistein in combination with cholecalciferol and citrated zinc bisglycinate in postmenopausal women. Int. J. Women’s Health. 2011, 3, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, A. Drug Interaction, in Pharmacology and Therapeutics. Department of Pharmacology and Therapeutic Faculty of Medicine Universitas Indonesia: Gaya Baru, Jakarta, Indonesia, 2007; pp. 800–801. [Google Scholar]
- Asdaq, S.M.; Inamdar, M.N. Pharmacodynamic interaction of captopril with garlic in isoproterenol-induced myocardial damage in rat. Phytother. Res. 2010, 24, 720–725. [Google Scholar] [CrossRef] [PubMed]
- He, Z.X. Clinical herb-drug interactions as a safety concern in pharmacotherapy. J. Pharmacol. Drug. Metab. 2014, 1, 1–3. [Google Scholar]
| Parameter | Celery Extract |
|---|---|
| Ash values (%) w/w | 6.70% |
| Water content (%) v/w | 8.89% |
| Essential oil (%) v/w | 3.34% |
| Loss on drying (%) w/w | 4.87% |
| Compound | Parent (m/z) | Daughter (m/z) | Cone (V) | Collison (V) | Area |
|---|---|---|---|---|---|
| Captopril | 415 | 216.16 | 35 | 17 | 3.05 x 105 |
| Propranolol | 260 | 183.17 | 42 | 17 | 5.59 x 106 |
| Apigenin | 271.13 | 153.07 | 61 | 31 | 7.03 x 106 |
| Concentration (ng/mL) | Mean Measured Concentration (ng/mL) ± Standard Deviation | (%CV) | (%diff) |
|---|---|---|---|
| 3 | 3.13 ± 0.25 | 7.91 | 4.46 |
| 9 | 8.46 ± 0.71 | 8.37 | −5.82 |
| 40 | 39.36 ± 2.93 | 7.46 | −1.41 |
| 80 | 77.37 ± 5.34 | 6.91 | −3.10 |
| Pharmacokinetic Parameters | Group I (n = 6) (single captopril) | Group II (n = 6) (captopril + celery extract) |
|---|---|---|
| Cmax (ng/mL) | 100.63 ± 28.62 | 127.86 ± 29.30 |
| Tmax (hour) | 0.16 | 0.16 |
| Ke (hour−1) | 0.2391 ± 0.08 | 0.2162 ± 0.07 |
| T1/2 (hour) | 1.8578 ± 3.67 | 4.7029 ± 1.05 |
| AUCtotal | 99.11 ± 13.05 | 158.28 ± 25.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siska, S.; Mun`im, A.; Bahtiar, A.; Suyatna, F.D. Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats. Sci. Pharm. 2018, 86, 6. https://doi.org/10.3390/scipharm86010006
Siska S, Mun`im A, Bahtiar A, Suyatna FD. Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats. Scientia Pharmaceutica. 2018; 86(1):6. https://doi.org/10.3390/scipharm86010006
Chicago/Turabian StyleSiska, Siska, Abdul Mun`im, Anton Bahtiar, and Franciscus D. Suyatna. 2018. "Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats" Scientia Pharmaceutica 86, no. 1: 6. https://doi.org/10.3390/scipharm86010006
APA StyleSiska, S., Mun`im, A., Bahtiar, A., & Suyatna, F. D. (2018). Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats. Scientia Pharmaceutica, 86(1), 6. https://doi.org/10.3390/scipharm86010006




