Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant Material
2.2. Sample Preparation
Sample Extraction
2.3. Experimental Animals
2.4. Acute Oral Toxicity Study
2.5. Subacute Toxicity Study
2.5.1. Weekly Body Weight
2.5.2. Mortality and Toxic Signs
2.5.3. Relative Organ Weight
2.5.4. Haematological Parameters
2.5.5. Biochemical Estimations
2.5.6. Histopathology Study
2.6. Statistical Analysis
3. Results
3.1. Acute Oral Toxicity Study
3.2. Subacute Toxicity Test
3.2.1. Weekly Body Weight
3.2.2. Clinical Observation and Mortality
3.2.3. Relative Organ Weight
3.2.4. Haematological Parameters
3.2.5. Biochemical Analysis
3.2.6. Histopathological Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patil, U.H.; Gaikwad, D.K. Phytochemical profile and antibacterial activity of stem bark of Anogeissus latifolia. Pharm. J. 2010, 2, 70–73. [Google Scholar] [CrossRef]
- Dias, F.D.; Takahashi, C.S. Cytogenetic evaluation of aqueous extracts of the medicinal plants Alpiniamutans rose (Zingerberaceae) and Pogostemum hyneanus benth (labitae) on wistar rats and Allium cepa (Liliaceae) root tip cells. Braz. J. Genet. 1994, 17, 175–180. [Google Scholar]
- Nath, P.; Yadav, K.A. Acute and sub-acute oral toxicity assessment of the methanolic extract from leaves of Hibiscus rosa-sinensis L. in mice. J. Intercult. Ethnopharmacol. 2015, 4, 70–73. [Google Scholar]
- Ankush, H.G.; Harimohan, C.; Harisha, C.R.; Shukla, V.J.; Goyal, M.D.; Pandya, P. Pharmacognostical and preliminary physicochemical evaluation of Triphaladi granules—A polyherbal Ayurvedic formulation. Ayu. 2013, 34, 288–293. [Google Scholar]
- Anonymous. Pharmacognosy of Ayurvedic Drugs I (I); Department of Pharmacognosy, Government of Kerala: Trivandrum, India, 1953.
- Hatapakki, B.C.; Hukkeri, V.I. Antipyretic activity of root of Marsdenia tenacissima in rats. J. Nat. Rem. 2011, 11, 98–102. [Google Scholar] [CrossRef]
- Nayak, A.; De, S. Phytopharmacognostic investigation of Marsdenia tenacissima (ROXB) moon. World J. Pharm. Res. 2014, 3, 891–904. [Google Scholar]
- Han, S.Y.; Zhao, M.B.; Zhuang, G.B.; Li, P.P. Marsdenia tenacissima extract restored gefitinib sensitivity in resistant non-small cell lung cancer cells. Lung Cancer 2012, 75, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Kang, L.P.; Fang, X.M.; Zhao, Y.; Yu, H.S.; Han, L.F.; Li, H.T.; Zhang, L.X.; Guo, B.L.; Yu, L.Y.; et al. Polyoxypregnaneglycosides from the roots of Marsdenia tenacissima and their anti-HIV activities. Planta Med. 2017, 83, 126–134. [Google Scholar] [PubMed]
- Xing, W.X.; Cheng, B.; Mi, H.M.; Yang, G.J.; Wu, Y.T. Two new C21 steroidal glycosides from Marsdenia tenacissima. Yao Xue Xue Bao 2004, 39, 272–275. [Google Scholar]
- Wang, S.; Lai, Y.H.; Tian, B.; Yang, L. Two New C21 Steroidal Glycosides from Marsdenia tenacissima (ROXB.) WIGHT et ARN. Chem. Pharm. Bull. 2006, 54, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Xing, W.X.; Mao, S.L.; Lao, A.N.; Uzawa, J.; Yoshida, S.; Fujimoto, Y. Pregnane glycosides from the stems of Marsdenia tenacissima. J. Asian Nat. Prod. Res. 2004, 6, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Q.; Lin, L.Z.; Cordell, G.A.; Xue, L.; Johnson, M.E. Polyoxypregnanes from Marsdenia tenacissima. Phytochem 1993, 34, 1615–1620. [Google Scholar] [PubMed]
- Liwei, H.E.; Tulin, L.U.; Chunqin, M.A.O.; Jingyan, Y. Chemical constituents and anti-tumoractivity of Marsdenia tenacissima. Chin. J. Modern Appl. Pharm. 2014, 7, 821–824. [Google Scholar]
- Ye, B.; Yang, J.; Li, J.; Niu, T.; Wang, S. In vitro and in vivo antitumor activities of tenacissoside C from Marsdenia tenacissima. Planta Med. 2014, 80, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bimbima. Available online: www.bimbima.com/herbs/marsdenia-tenacissima-murva-information-uses-and-more/14/ (accessed on 1 July 2017).
- Organization for Economic Co-operation and Development (OECD). Guidance Document on Acute Oral Toxicity Testing 420; Organization for Economic Co-operation and Development: Paris, France, 2008. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). Guidance Document on Subacute Oral Toxicity Testing 407; Organization for Economic Co-operation and Development: Paris, France, 2008. [Google Scholar]
- World Health Organization (WHO). General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; WHO: Geneva, Switzerland, 2000; p. 35. [Google Scholar]
- Das, N.; Goshwami, D.; Hasan, M.; Sharif, R.; Zahir, S. Evaluation of acute and subacute toxicity induced by methanol extract of Terminalia citrina leaves in Sprague Dawley rats. J. Acute Dis. 2015, 4, 316–321. [Google Scholar] [CrossRef]
- Mobolaji, J.; Muhammad, H.L.; Makun, A.H.; Busari, B.M.; Abdullah, S.A. Acute and subacute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J. Acute Dis. 2016, 5, 62–70. [Google Scholar] [CrossRef]
- Yuet, P.K.; Darah, I.; Chen, Y.; Sreeramanan, S.; Sasidharan, S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomed. Res. Int. 2013, 2013, 182064–182071. [Google Scholar] [PubMed]
- Bigoniya, P.; Sahu, T.; Tiwari, V. Hematological and biochemical effects of sub-chronic artesunate exposure in rats. Toxicol. Rep. 2015, 2, 280–288. [Google Scholar] [CrossRef]
- Eran, B.-A.; Noah, S.; Lee, H.G.; Kamer, M.; Suha, O.; Elad, S. Potential risks associated with traditional herbal medicine use in cancer care: A study of middle eastern oncology health care professionals. Cancer 2016, 122, 598–610. [Google Scholar]
- National Research Council (NRC). Toxicity Testing for Assessing Environmental Agents; Interim Report; National Academics Press: Washington, DC, USA, 2006. [Google Scholar]
- Hilaly, J.; Israili, H.; Lyoussi, B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004, 91, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, W.M. Reanl functions tests as indicators of kidney injury in subacute toxicity studies. Toxicol. Appl. Pharmacol. 1981, 57, 414–424. [Google Scholar] [CrossRef]
- Odeyemi, O.O.; Yakubu, M.T.; Masika, P.J.; Afolayan, A.J. Toxicological evaluation of the essential oil from Menthalongifolia L. subsp. capensis leaves in rats. J. Med. Food. 2009, 12, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Acute and subchronic toxicity studies of methanolic extract of Tulbaghiaviolacea rhizomes in Wistar rats. Afr. J. Biotechnol. 2012, 11, 14934–14940. [Google Scholar]
- Clarice, R.M.P.; Franklin, R.-C.; Rosane, M.T.M.; Sara, V.D.S.; Alessandro, R. Poisoning by Marsdenia hilariana and Marsdenia megalantha (Apocynaceae) in ruminants. Toxicon 2011, 58, 610–613. [Google Scholar] [PubMed]
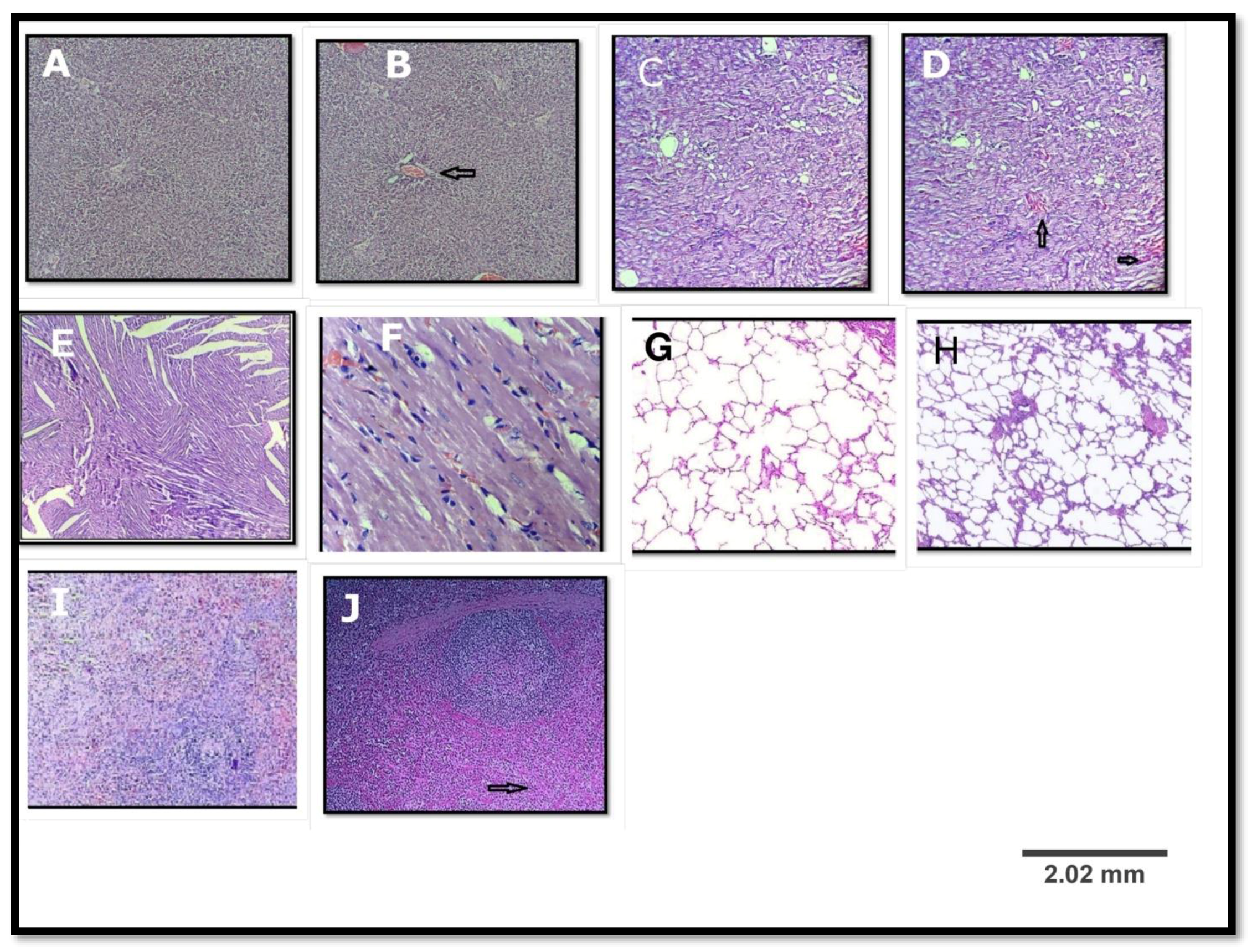
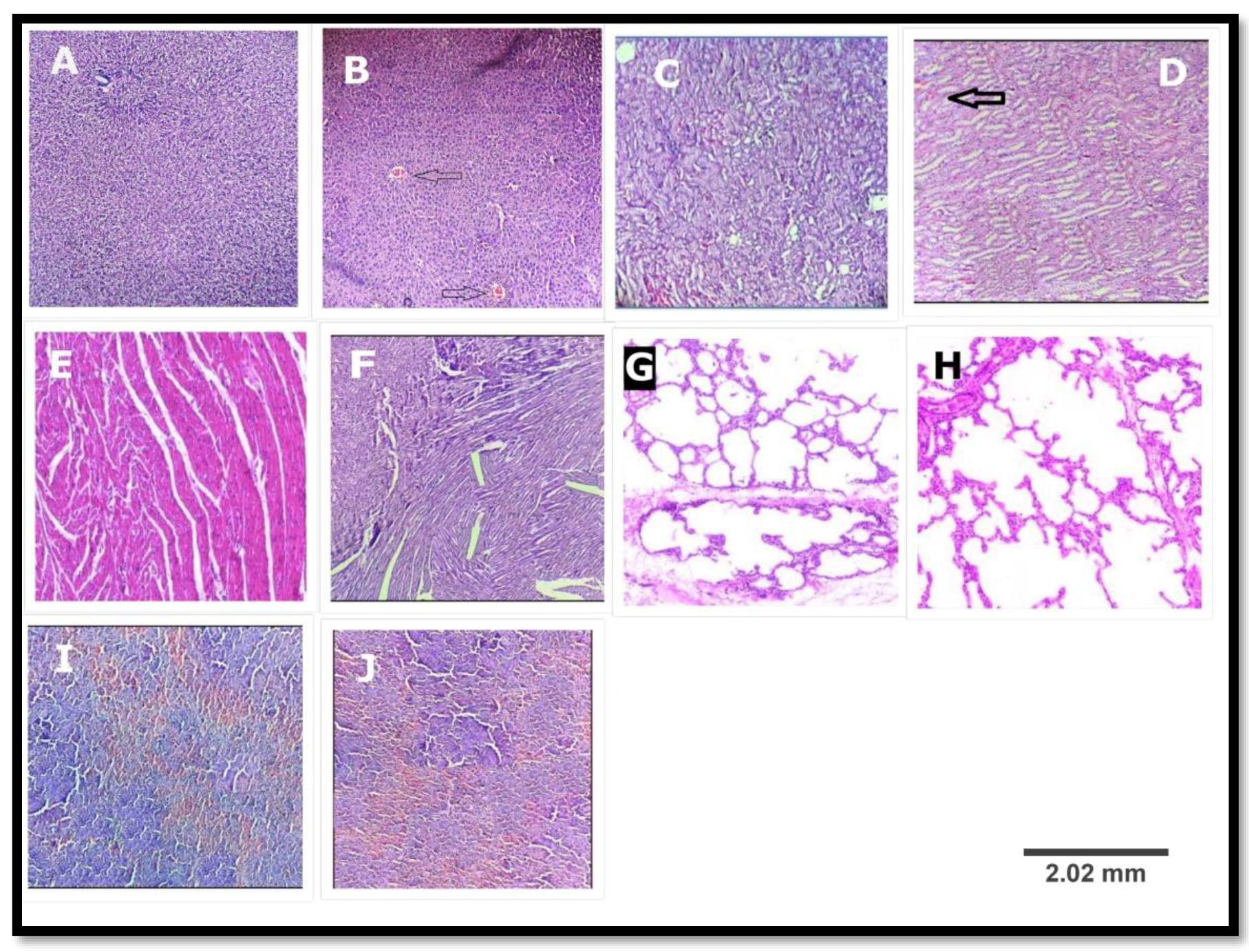
| Organs | Control | MTE (5000 mg/kg) |
|---|---|---|
| Heart | 0.46 ± 0.02 | 0.43 ± 0.01ns |
| Liver | 3.32 ± 0.13 | 3.28 ± 0.11 ns |
| Spleen | 0.31 ± 0.02 | 0.33 ± 0.02 ns |
| Kidney | 0.84 ± 0.03 | 0.86 ± 0.01 ns |
| Lungs | 0.79 ± 0.05 | 0.77 ± 0.02 ns |
| Parameter | Unit | Control | MTE (5000 mg/kg) |
|---|---|---|---|
| Haemoglobin (Hb) | g/L | 140.23 ± 3.11 | 140.77 ± 3.02 ns |
| Total red blood cells (RBC’s) | 1012/L | 7.52 ± 0.23 | 7.31 ± 0.31 ns |
| Packed cell volume (PCV) | L/L | 0.43 ± 0.01 | 0.46 ± 0.00 ns |
| Mean corpuscular volume (MCV) | fL | 54.3 ± 0.00 | 54.21 ± 0.77 ns |
| Mean corpuscular haemoglobin (MCH) | pg | 18.55 ± 0.66 | 18.01 ± 0.42 ns |
| Mean corpuscular haemoglobin concentration (MCHC) | g/L | 328 ± 4.72 | 327.03 ± 4.13 ns |
| Total white blood cells (WBC’s) | 109/L | 7.52 ± 0.22 | 9.00 ± 1.3 ns |
| Neutrophils | % | 12.13 ± 1.03 | 12.07 ± 1.00 ns |
| Lymphocytes | % | 85.41 ± 1.92 | 82.02 ± 2.7 ns |
| Monocytes | % | 2.38 ± 0.00 | 2.31 ± 0.07 ns |
| Eosinophils | % | 1.01± 0.01 | 1.09 ± 0.02 ns |
| Platelet count | 109/L | 870 ± 10.71 | 865.00 ± 17.11 ns |
| Parameter | Unit | Control | MTE (5000 mg/kg) |
|---|---|---|---|
| Sodium | mmol/L | 138.00± 0.20 | 138.20 ± 0.08 ns |
| Potassium | mmol/L | 6.12 ± 0.32 | 6.33 ± 0.35 ns |
| Chloride | mmol/L | 104.00 ± 0.27 | 104.30 ± 0.01 ns |
| Urea | mmol/L | 6.57 ± 0.34 | 6.36 ± 0.20 ns |
| Creatinine | µmol/L | 45.07 ± 0.33 | 45.12± 0.48 ns |
| Uric acid | mmol/L | 0.17 ± 0.03 | 0.19 ± 0.01 ns |
| Total protein | g/L | 69.37 ± 0.21 | 69.53 ± 0.43 ns |
| Albumin | g/L | 36.73 ± 0.67 | 37.13 ± 0.71 ns |
| Globulin | g/L | 32.64 ± 0.47 | 32.40 ± 0.51 ns |
| Albumin/globulin ratio | 1.12 ± 0.04 | 1.09 ± 0.05 ns | |
| Alkaline phosphatase (ALP) | U/L | 135 ± 8.32 | 135.04 ± 9.71 ns |
| Aspartate aminotransferase (AST) | U/L | 74.31 ± 4.31 | 74.03 ± 3.39 ns |
| Alanine aminotranferase (ALT) | U/L | 45.41 ±0.71 | 45.81 ± 0.52 ns |
| Group | Doses | Weight | |||
|---|---|---|---|---|---|
| Initial day | 9th day | 18th day | 28th day | ||
| Control | - | 134.21 ± 4.01 | 139.54 ± 5.24 | 145.56 ± 4.78 | 147.21 ± 4.85 |
| I | 250 mg/kg | 138.23 ± 5.22 | 145.36 ± 5.78 | 149.25 ± 6.21 | 153.54 ± 5.74 |
| II | 500 mg/kg | 142.22 ± 6.24 | 148.26 ± 5.01 | 152.21 ± 5.89 | 155.87 ± 5.81 |
| III | 1000 mg/kg | 140.25 ± 3.22 | 146.28 ± 3.89 | 150.66 ± 4.86 | 153.24 ± 4.01 |
| Organs | Control | MTE (250 mg/kg) | MTE (500 mg/kg) | MTE (1000 mg/kg) |
|---|---|---|---|---|
| Heart | 0.45 ± 0.05 | 0.44 ± 0.01ns | 0.42 ± 0.01 ns | 0.43 ± 0.02 ns |
| Liver | 3.03 ± 0.10 | 3.24 ± 0.15 ns | 3.14 ± 0.04 ns | 3.15 ± 0.08 ns |
| Spleen | 0.33 ± 0.02 | 0.30 ± 0.01 ns | 0.29 ± 0.02 ns | 0.28 ± 0.01 ns |
| Kidney | 0.74 ± 0.02 | 0.77 ± 0.01 ns | 0.73 ± 0.01 ns | 0.74 ± 0.03 ns |
| Lungs | 0.85 ± 0.06 | 0.91 ± 0.09 ns | 0.86 ± 0.02 ns | 0.88 ± 0.01 ns |
| Parameter | Unit | Control | 250 mg/kg | 500 mg/kg | 1000 mg/kg |
|---|---|---|---|---|---|
| Haemoglobin (Hb.) | g/L | 150.33 ± 0.71 | 146.11 ± 0.11 | 147.31 ± 0.27 | 149.52 ± 0.51 |
| Total red blood cells (RBC’s) | 1012/L | 7.52 ± 0.18 | 7.62 ± 0.31 ns | 7.20 ± 0.26 ns | 7.49 ± 0.22 ns |
| Packed cell volume (PCV) | L/L | 0.46 ± 0.01 | 0.43 ± 0.07 ns | 0.43 ± 0.02 ns | 0.42± 0.01 ns |
| Mean corpuscular volume (MCV) | fL | 55.41 ± 0.77 | 54.33 ± 0.21 ns | 54.81 ± 0.11 ns | 55.01 ± 0.03 ns |
| Mean corpuscular haemoglobin (MCH) | pg | 17.67 ± 0.17 | 17.20 ± 0.22 ns | 17.44 ± 0.14 ns | 17.57 ± 0.24 ns |
| Mean corpuscular haemoglobin concentration (MCHC) | g/L | 310.22 ± 0.04 | 313.07 ± 0.14 ns | 314.11 ± 0.23 ns | 311.71 ± 0.44 ns |
| Total white blood cells (WBC’s) | 109/L | 7.23 ± 0.17 | 7.01 ± 0.11 ns | 7.45 ± 0.19 ns | 7.66 ± 0.29 ns |
| Neutrophils | % | 12.71 ± 1.01 | 12.70 ± 1.13 ns | 12.60 ± 1.11 ns | 12.03 ± 1.79 ns |
| Lymphocytes | % | 86.11 ± 2.91 | 86.00 ± 2.01 ns | 86.51 ± 1.13 ns | 86.33 ± 1.00 ns |
| Monocytes | % | 2.10 ± 0.04 | 2.91 ± 0.11 ns | 2.61 ± 0.65 ns | 2.17 ± 0.31 ns |
| Platelet count | 109/L | 833.60 ± 48.20 | 838.51± 42.31 ns | 841.11 ± 41.11 ns | 840.08 ± 53.33 ns |
| Red blood cell distribution unit (RDW) | % | 14.37 ± 1.12 | 13.11 ± 1.93 ns | 13.49 ± 1.10 ns | 13.33 ± 1.09 ns |
| Platelet distribution width (PDW) | fL | 11.5 ± 0.01 | 10.91 ± 0.01 ns | 10.59 ± 0.04 ns | 9.76 ± 0.07 ns |
| Platelet large cell ratio (P-LCR) | % | 14.51 ± 0.11 | 14.70 ± 0.22 ns | 13.01 ± 0.22 ns | 13.71 ± 0.23 ns |
| Mean platelet volume (MPV) | fL | 7.72 ± 0.04 | 7.64 ± 0.08 ns | 7.27±0.01 ns | 7.57 ± 0.01 ns |
| Procalcitonin (PCT) | % | 0.99 ± 0.23 | 0.75 ± 0.37 ns | 0.68 ± 0.41 ns | 0.64 ± 0.44 ns |
| Parameter | Unit | Control | 250 mg/kg | 500 mg/kg | 1000 mg/kg |
|---|---|---|---|---|---|
| Sodium | mmol/L | 137.00 ± 0.44 | 135.20 ± 0.94 ns | 133.70 ± 0.86 ns | 134.30 ± 0.56 ns |
| Potassium | mmol/L | 6.37 ± 0.01 | 6.83 ± 0.09 ns | 6.39 ± 0.04 ns | 6.77 ± 0.13 ns |
| Chloride | mmol/L | 102.83 ± 0.03 | 102.70 ± 0.05 ns | 102.11 ± 0.03 ns | 101.00 ± 0.01 ns |
| Urea | mmol/L | 6.65 ± 0.21 | 6.12 ± 0.17 ns | 6.98 ± 0.18 ns | 6.81 ± 0.10 ns |
| Creatinine | µmol/L | 47.30 ± 0.52 | 44.17 ± 0.69 ns | 46.1 ± 0.61 ns | 46.79 ± 0.49 ns |
| Uric acid | mmol/L | 0.16 ± 0.04 | 0.15 ± 0.01 ns | 0.14 ± 0.09 ns | 0.15 ± 0.02 ns |
| Total protein | g/L | 68.50 ± 0.90 | 68.30 ± 0.86 ns | 69.00 ± 1.14 ns | 68.81 ± 0.90 ns |
| Albumin | g/L | 36.67 ± 0.03 | 37.21 ± 0.03 ns | 38.77 ± 0.01 ns | 38.92 ± 0.03 ns |
| Globulin | g/L | 31.83 ± 0.05 | 31.09 ± 0.09 ns | 30.23 ± 0.09 ns | 29.89 ± 0.07 ns |
| Albumin/globulin ratio | 1.15 ± 0.00 | 1.19 ± 0.01 ns | 1.28 ± 0.01 ns | 1.30 ± 0.01 ns | |
| Alkaline phosphatase (ALP) | U/L | 132.83 ± 6.17 | 133.70 ± 10.12 ns | 134.65 ± 12.92 ns | 135.00 ± 11.33 ns |
| AST | U/L | 71.00 ± 6.91 | 74.00 ± 6.02 ns | 75.21 ± 7.29 ns | 77.00 ± 6.24 ns |
| ALT | U/L | 48.67 ± 1.17 | 44.21 ± 2.42 ns | 48.21 ± 2.71 ns | 50.00 ± 1.23 ns |
| Bilirubin (Total) | mg/dL | 0.35 ± 0.01 | 0.31± 0.00 ns | 0.34 ± 0.01 ns | 0.45 ± 0.02 ns |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porwal, M.; Khan, N.A.; Maheshwari, K.K. Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats. Sci. Pharm. 2017, 85, 29. https://doi.org/10.3390/scipharm85030029
Porwal M, Khan NA, Maheshwari KK. Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats. Scientia Pharmaceutica. 2017; 85(3):29. https://doi.org/10.3390/scipharm85030029
Chicago/Turabian StylePorwal, Mayur, Najam Ali Khan, and Kamal Kishore Maheshwari. 2017. "Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats" Scientia Pharmaceutica 85, no. 3: 29. https://doi.org/10.3390/scipharm85030029
APA StylePorwal, M., Khan, N. A., & Maheshwari, K. K. (2017). Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats. Scientia Pharmaceutica, 85(3), 29. https://doi.org/10.3390/scipharm85030029






