Insect Diversity in the Coastal Pinewood and Marsh at Schinias, Marathon, Greece: Impact of Management Decisions on a Degraded Biotope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Insect and Vegetation Sampling
2.3. Similarity Analysis of Plots
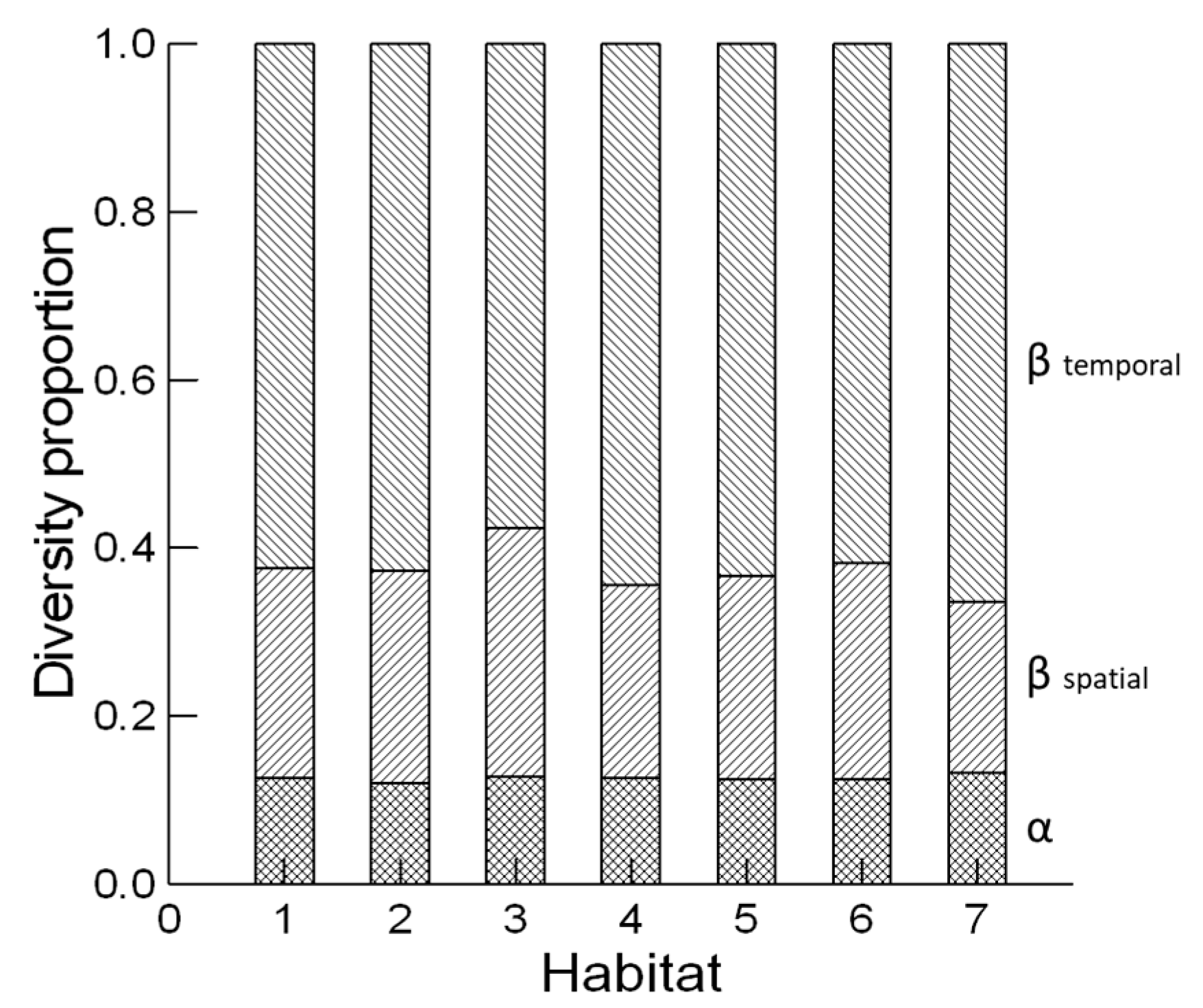
2.4. Diversity Analysis
2.5. Nestedness
2.6. Habitat Specificity Analysis
2.7. Indicator Species
2.8. Functional Roles of Insect Species
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Site Group | Insect Species | Indicator Value (Higher for ‘Good’ Indicator) | P | Functional Role | |
|---|---|---|---|---|---|
| 1 | Grypotes staurus | 0.695 | 0.02 | * | S |
| Tomicus piniperda | 0.693 | 0.043 | * | CPW | |
| Lionychus (Lionychus) quadrillum | 0.617 | 0.021 | * | P | |
| Chrysopa perla | 0.573 | 0.038 | * | PL | |
| Bracon variegator | 0.524 | 0.002 | ** | PP | |
| 2 | Eupelmus urozonus | 0.618 | 0.045 | * | PP |
| 3 | Acupalpus (Acupalpus) maculatus | 1.000 | 0.001 | *** | P |
| Rhagium (Rhagium) inquisitor | 1.000 | 0.001 | *** | CPW | |
| Pissodes (Pissodes) gyllenhali | 1.000 | 0.001 | *** | CPW | |
| Raphidia (Raphidia) ophiopsis mediterranea | 1.000 | 0.001 | *** | P | |
| Mantis religiosa | 1.000 | 0.001 | *** | P | |
| Ulrike attica | 0.8 | 0.008 | ** | P | |
| Acanthocinus reticulatus | 0.798 | 0.001 | *** | CPW | |
| Orthotomicus erosus | 0.758 | 0.029 | * | CPW | |
| Trechus (Trechus) subnotatus | 0.73 | 0.001 | *** | Ps | |
| Bacillus atticus | 0.727 | 0.02 | * | Lc | |
| Panorpa germanica graeca | 0.72 | 0.015 | * | P | |
| Dromius (Dromius) agilis | 0.688 | 0.001 | *** | P | |
| Melanophila cuspidata | 0.682 | 0.036 | * | CPW | |
| Anthaxia (Melanthaxia) corinthia | 0.632 | 0.039 | * | WPo | |
| 4 | Brachymeria tibialis | 1.000 | 0.001 | *** | PP |
| Clanoptilus (Clanoptilus) elegans | 0.828 | 0.001 | *** | Po | |
| Urophora cardui | 0.806 | 0.001 | *** | G | |
| Tentyria rotundata | 0.764 | 0.001 | *** | Ss | |
| Dolichomitus sericeus | 0.714 | 0.023 | * | PP | |
| Bombus terrestris | 0.702 | 0.023 | * | Po | |
| Chorthippus (Glyptobothrus) brunneus brunneus | 0.696 | 0.015 | * | Lc | |
| Scaeva albomaculata | 0.69 | 0.012 | * | PL | |
| Lygaeus pandurus ssp. pandurus | 0.687 | 0.016 | * | FS | |
| Philaenus spumarius | 0.675 | 0.01 | ** | S | |
| Ancyrosoma leucogrammes | 0.664 | 0.007 | ** | S | |
| Loxocnemis dentator | 0.659 | 0.044 | * | S | |
| Centrocoris spiniger | 0.657 | 0.048 | * | S | |
| Staria lunata | 0.651 | 0.049 | * | S | |
| Messsor sp. | 0.621 | 0.029 | * | S | |
| Oedemera (Oedemera) flavipes | 0.618 | 0.048 | * | Po | |
| Hypophloeus linearis | 0.585 | 0.039 | * | P | |
| Calathus (Calathus) fuscipes graecus | 0.537 | 0.015 | * | P | |
| 5 | Lestes barbarus | 1.000 | 0.001 | *** | P |
| Sympetrum striolatum | 1.000 | 0.001 | *** | P | |
| Libellula depressa | 1.000 | 0.001 | *** | P | |
| Chironomus sp. | 1.000 | 0.001 | *** | PM | |
| Sympetrum meridionale | 0.787 | 0.001 | *** | P | |
| Lissonota (Loxonota) histrio | 0.726 | 0.001 | *** | PP | |
| Sympetrum fonscolombii | 0.723 | 0.001 | *** | P | |
| Dasypogon melanopterus | 0.715 | 0.001 | *** | P | |
| Hypera sp. | 0.68 | 0.023 | * | Lc | |
| Harpalus (Artabas) dispar splendens | 0.576 | 0.022 | * | O | |
| Pimelia (Camphonota) subglobosa | 0.545 | 0.037 | * | Sss | |
| 6 | Syromastus rhombeus | 0.872 | 0.001 | *** | F/Se |
| Henestaris halophilus | 0.842 | 0.001 | *** | Se | |
| Aphaenogaster subterranea | 0.838 | 0.001 | *** | O | |
| Trichograma sp. | 0.815 | 0.001 | *** | PP | |
| Paragus tibialis | 0.727 | 0.004 | ** | PP-WPo | |
| Diadegma trochanteratum | 0.679 | 0.027 | * | PP | |
| Ischnura elegans | 0.632 | 0.036 | * | P | |
| 7 | Anax imperator | 1.000 | 0.001 | *** | P |
| Opsius stactogalus | 1.000 | 0.001 | *** | S | |
| Tuponia tamaricicola | 1.000 | 0.001 | *** | S | |
| Notonecta (Notonecta) maculata | 1.000 | 0.001 | *** | P | |
| Myopa buccata | 1.000 | 0.001 | *** | PP-WPo | |
| Coniatus (Coniatus) tamarisci | 0.988 | 0.001 | *** | F/Se | |
| Loboptera decipiens | 0.954 | 0.001 | *** | Ss | |
| Sigara (Subsigara) falleni | 0.948 | 0.001 | *** | P | |
| Calomera littoralis nemoralis | 0.87 | 0.001 | *** | P | |
| Dociostaurus maroccanus | 0.866 | 0.001 | *** | Lc | |
| Hesperocorixa sahlbergi | 0.853 | 0.001 | *** | P | |
| Gerris (Gerris) costae fieberi | 0.812 | 0.001 | *** | P | |
| Empusa fasciata | 0.81 | 0.001 | *** | P | |
| Neides aduncus | 0.746 | 0.001 | *** | S | |
| Acinopus (Acinopus) subquadratus | 0.745 | 0.001 | *** | P | |
| Cephalota (Taenidia) circumdata circumdata | 0.735 | 0.001 | *** | P | |
| Libelloides macaronius | 0.72 | 0.001 | *** | P | |
| Blaps mucronata | 0.692 | 0.001 | *** | Ss | |
| Aciura coryli | 0.689 | 0.001 | *** | F | |
| Carabus (Tomocarabus) convexus | 0.647 | 0.001 | *** | P | |
| Ocypus (Ocypus) olens | 0.604 | 0.036 | * | P | |
| Quedius sp. | 0.592 | 0.023 | * | P | |
| Cybister (Scaphinectes) lateralimarginalis | 0.545 | 0.048 | * | P | |
| Rhyparochromus consors | 0.518 | 0.001 | *** | F/Se-S |


References
- Martínez, J.; Psuty, N.P. (Eds.) Coastal Dunes: Ecology and Conservation; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Everard, M.; Jones, L.; Watts, B. Have We Neglected the Societal Importance of Sand Dunes? An Ecosystem Services Perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 476–487. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Fabiano, M. Ecosystem Level Analysis of Sandy Beaches Using Thermodynamic and Network Analyses: A Study Case in the NW Mediterranean Sea. Ecol. Indic. 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Rust, I.C.; Illenberger, W.K. Coastal dunes: Sensitive or not? Landsc. Urban Plan. 1996, 34, 165–169. [Google Scholar] [CrossRef]
- Sýkora, K.; van den Bogert, J.C.J.M.; Berendse, F. Changes in soil and vegetation during dune slack succession. J. Veg. Sci. 2004, 15, 209–218. [Google Scholar] [CrossRef]
- Spanou, S.; Verroios, G.; Dimitrellos, G.; Tiniakou, A.; Georgiadis, T. Willdenowia, Bd. 36, H. 1; Special Issue: Festschrift Werner; Botanic Garden and Botanical Museum: Berlin, Germany, 2016; pp. 235–246. [Google Scholar]
- Acosta, A.; Carranza, M.L.; Izzi, C.F. Community types and alien species distribution in Italian coastal dunes. In Biological Invasions: From Ecology to Conservation; Rabitsch, W., Essl, F., Klingenstein, F., Eds.; Pensof Publishers: Sofia, Bulgaria, 2007; pp. 96–104. [Google Scholar]
- Clarke, D.; Sanitwong Na Ayutthaya, S. Predicted Effects of Climate Change, Vegetation and Tree Cover on Dune Slack Habitats at Ainsdale on the Sefton Coast, UK. J. Coast. Conserv. 2010, 14, 115–125. [Google Scholar] [CrossRef]
- Psuty, N.P.; Silveira, T.M. Global Climate Change: An Opportunity for Coastal Dunes? J. Coast. Conserv. 2010, 14, 153–160. [Google Scholar] [CrossRef]
- Weeda, E.J. The Role of Archaeophytes and Neophytes in the Dutch Coastal Dunes. J. Coast. Conserv. 2010, 14, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Diogo, A.C.; Vogler, A.P.; Gimenez, A.; Gallego, D.; Galian, J. Conservation genetics of Cicindeladeserticoloides, an endangered tiger beetle endemic to southeastern Spain. J. Insect Conserv. 1999, 3, 117–123. [Google Scholar] [CrossRef]
- Maes, D.; Ghesquiere, A.; Logie, M.; Bonte, D. Habitat Use and Mobility of Two Threatened Coastal Dune Insects: Implications for Conservation. J. Insect Conserv. 2006, 10, 105–115. [Google Scholar] [CrossRef]
- Salz, A.; Fartmann, T. Coastal Dunes as Important Strongholds for the Survival of the Rare Niobe Fritillary (Argynnis niobe). J. Insect Conserv. 2009, 13, 643–654. [Google Scholar] [CrossRef]
- Rooney, P. Changing Perspectives in Coastal Dune Management. J. Coast. Conserv. 2010, 14, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Howe, M.A.; Knight, G.T.; Clee, C. The importance of coastal sand dunes for terrestrial invertebrates in Wales and in the UK, with particular reference to aculeate Hymenoptera (bees, wasps & ants). J. Coast. Conserv. 2010, 14, 91–102. [Google Scholar]
- Wünsch, Y.; Schirmel, J.; Fartmann, T. Conservation Management of Coastal Dunes for Orthoptera Has to Consider Oviposition and Nymphal Preferences. J. Insect Conserv. 2012, 16, 501–510. [Google Scholar] [CrossRef]
- Choi, Y.D.; Temperton, V.M.; Allen, E.B.; Grootjans, A.P.; Halassy, M.; Hobbs, R.J.; Naeth, M.A.; Torok, K. Ecological Restoration for Future Sustainability in a Changing Environment. Ecoscience 2008, 15, 53–64. [Google Scholar] [CrossRef]
- Economidou, E. The Attic Landscape throughout the Centuries and Its Human Degradation. Landsc. Urban Plan. 1993, 24, 33–37. [Google Scholar] [CrossRef]
- Dafis, S.; Papastergiadou, K.; Georghiou, D.; Babalonas, T.; Georgiadis, T.; Papageorgiou, M.; Lazaridou, T.; Tsiaousi, V. Directive 92/43/EEC The Greek “Habitat” Project NATURA 2000: An overview. In Life Contract B4-3200/94/756, Commission of the European Communities DG XI; The Goulandris Natural History Museum-Greek Biotope/Wetland Centre: Athens, Greece, 1996. [Google Scholar]
- Stewart, A.J.A.; New, T.R. Insect Conservation in temperate biomes; issues, progress and prospects. In Insect Conservation Biology; Stewart, A.J.A., New, T.R., Lewis, O.T., Eds.; Proceedings of the Royal Entomological Society: London, UK, 2007; pp. 1–33. [Google Scholar]
- Schaffers, A.P.; Raemakers, I.P.; Sykora, K.V.; ter Braak, C.J.F. Arthropod Assemblages Are Best Predicted By Plant. Ecology 2008, 89, 782–794. [Google Scholar] [CrossRef]
- Speight, M.R.; Hunter, M.D.; Watt, A.D. Ecology of Insects: Concepts and Applications; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Feest, A.; Eyre, M.D.; Aldred, T.D. Measuring carabid beetle biodiversity quality: An example of setting baseline biodiversity indices. Curr. Trends Ecol. 2011, 2, 11–23. [Google Scholar]
- Carvalho, J.C.; Cadoso, P.; Cespo, L.C.; Henriques, S.; Carvalho, R.; Gomes, P. Biogeogrphic paterns of spiders in coastal dunes along a gradient of mediterraneity. Biodivers. Conserv. 2011, 20, 873–894. [Google Scholar] [CrossRef]
- Spanos, K.A.; Petrakis, P.V.; Daskalakou, E. Salient points on the assessment and monitoring of forest biodiversity. Bioremedation Biodivers. Bioavailab. 2010, 4, 1–7. [Google Scholar]
- Tylianakis, J.M. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 2005, 86, 3296–3302. [Google Scholar] [CrossRef] [Green Version]
- Pavlopoulos, K.; Karkanas, P.; Triantaphyllou, M.; Karymbalis, E.; Tsourou, T.; Palyvos, N. Paleoenvironmental Evolution of the Coastal Plain of Marathon, Greece, during the Late Holocene: Depositional Environment, Climate, and Sea Level Changes. J. Coast. Res. 2006, 22, 424–438. [Google Scholar] [CrossRef]
- Petrakis, P.V. Plant–Heteroptera Associations in an East -Mediterranean Ecosystem: An Analysis of Structure, Specificity and Dynamics. Ph.D. Thesis, University of Cardiff, Wales, UK, 1991. [Google Scholar]
- Petrakis, P.V.; Spanos, K.; Feest, A. Insect Biodiversity Reduction of Pinewoods in Southern Greece Caused by the Pine Scale (Marchalina hellenica). For. Syst. 2011, 20, 27. [Google Scholar] [CrossRef] [Green Version]
- Knott, P.; Warren, A. Aeolian processes. In Geomorphological Techniques; Goudie, A., Ed.; George Allen and Unwin: London, UK, 1981; pp. 226–246. [Google Scholar]
- Diapoulis, C. The Hellenic Flora: Identification Keys of Greek Plants; University of Athens: Athens, Greece, 1949. (In Greek) [Google Scholar]
- Economidis, P.S. Check List of Freshwater Fishes of Greece; Hellenic Society for the Protection of Nature: Athens, Greece, 1991. [Google Scholar]
- Gaitlich, M.; Dragoumis, F. The Ecological Value of Small Wetlands; Bulletin of the Hellenic Ornithological Society: Athens, Greece, 1985; Issue 2. (In Greek) [Google Scholar]
- Spanou, S.; Tiniakou, A.; Nikolaidis, V.; Georgiadis, T. Comparative study of protected areas in Greece: The case-study of three littoral Pinus pinea (stone pine) forests. Fresenius Env. Bull 2007, 16, 1335–1344. [Google Scholar]
- Alinvi, O.; Ball, J.P.; Danell, K.; Hjältén, J.; Pettersson, R.B. Sampling Saproxylic Beetle Assemblages in Dead Wood Logs: Comparing Window and Eclector Traps to Traditional Bark Sieving and a Refinement. J. Insect Conserv. 2007, 11, 99–112. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods: With Particular Reference to the Study of Insect Populations; Blackwell Science: Oxford, UK, 2000. [Google Scholar]
- Floren, A.; Gogala, A. Heteroptera from beech (Fagus sylvatica) and silver fir (Abies alba) trees of the primary forest reserve RajhenavskiRog, Slovenia. Acta Entomololica Slov. 2002, 10, 25–32. [Google Scholar]
- Ozanne, C.M.P. Techniques and methods for sampling canopy insects. In Insect Sampling in Forest Ecosystems; Leather, S., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 146–167. [Google Scholar]
- Novotný, V.; Basset, Y. Rare species in communities of tropical insect herbivores: Pondering the mystery of singletons. Oikos 2000, 89, 564–572. [Google Scholar] [CrossRef] [Green Version]
- De Jong, Y.S.D.M. (Ed.) Fauna Europaea Version 2.4.Web Service. Available online: http://www.faunaeur.org/ (accessed on 15 October 2011).
- Aber, J.D. A Method for Estimating Foliage-Height Profiles in Broad-Leaved Forests. J. Ecol. 1979, 67, 35–40. [Google Scholar] [CrossRef]
- Orloci, L.; Rao, C.R.; Stiteler, W.M. (Eds.) Multivariate Methods in Ecological Work, Statistical Ecology; ICPH: Fairland, MD, USA, 1979; Volume 7. [Google Scholar]
- Wilkinson, L. SYSTAT 12: The System for Statistics; SYSTAT Software Inc.: Evanston, IL, USA, 2007. [Google Scholar]
- Kruskal, J.B.; Wish, M. Multidimensional Scaling; Sage Publ.: Beverly Hills, CA, USA, 1978. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical design and analysis of a biological effects study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On Resemblance Measures for Ecological Studies, Including Taxonomic Dissimilarities and a Zero-Adjusted Bray-Curtis Coefficient for Denuded Assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Lasiak, T. Influence of taxonomic resolution, biological attributes and data transformations on multivariate comparisons of rocky macrofaunal assemblages. Mar. Ecol. Prog. Ser. 2003, 250, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’: Community Ecology Package. Version 2.0-0. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 18 December 2011).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 18 December 2011).
- Magurran, A.E. Measuring Biological Diversity. Blackwell, Oxford, UK Management Board of Schinias Marathon National Park. 2021. Technical Report. Installation of a Pilot Network of Experimental Surfaces and Regeneration Centers for the restoration of the Pine Forest in the Schinias—Marathon National Park through the Enrichment of Gaps and the Enhancement of Biodiversity (flora), and the Natural Regeneration with Various Methods. 2004, p. 48. Available online: https://www.greekflora.gr/el/Default.aspx (accessed on 12 December 2022).
- Lambshead, P.J.D.; Platt, H.M. Analysis of disturbance with the Ewens/Caswell neutral model: Theoretical review and practical assessment. Mar. Ecol. Prog. Ser. 1988, 43, 31–41. [Google Scholar] [CrossRef]
- Karakassis, I.; Smith, C.J.; Eleftheriou, A. Performance of neutral model analysis in a spatiotemporal series of macrobenthic replicates. Mar. Ecol. Prog. Ser. 1996, 137, 173–179. [Google Scholar] [CrossRef]
- McAleece, N. BioDiversity Professional. The Natural History Museum and The Scottish Association for Marine Science 1997. Available online: http://www.nhm.ac.uk/zoology/bdpro/ (accessed on 10 February 2012).
- Ulrich, W. NODF—A FORTRAN Program for Nestedness Analysis, Version 1.0; Nicolaus Copernicus University: Torun, Poland, 2010. [Google Scholar]
- Wright, D.H.; Reeves, J.H. On the meaning and measurement of nestedness of species assemblages. Oecologia 1992, 92, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.T.; Sugar, A.; Packer, L. Indicator Taxa, Rapid Biodiversity Assessment, and Nestedness in an Endangered Ecosystem. Conserv. Biol. 2000, 14, 1726–1734. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimaraes, P.; Guimaraes, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2007, 117, 1227–1239. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Ulrich, W. A Straightforward Computational Approach for Measuring Nestedness Using Quantitative Matrices. Environ. Model. Softw. 2011, 26, 173–178. [Google Scholar] [CrossRef]
- Stone, L.; Roberts, A. The checkerboard score and species distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving Indicator Species Analysis by Combining Groups of Sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Roberts, D.W. Ordination and Multivariate Analysis for Ecology, Version 1.4-1. Available online: https://cran.r-project.org/web/packages/labdsv/index.html (accessed on 18 December 2011).
- Machado, A. An index of naturalness. Nat. Conserv. 2004, 12, 95–110. [Google Scholar] [CrossRef]
- Hill, M.O.; Roy, D.B.; Thompson, K. Hemeroby, Urbanity and Ruderality: Bioindicators of Disturbance and Human Impact. J. Appl. Ecol. 2002, 39, 708–720. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multi species responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Maroukian, H.; Zamani, A.; Pavlopoulos, K. Coastal retreat in plain of Marathon (East Attica), Greece: Causes and effects. Geol. Balc. 1993, 23, 67–71. [Google Scholar] [CrossRef]
- Margonis, S. The Holocene Evolution of Theplain of Marathon, Attica, Greece. Ph.D. Thesis, Aristotelian University of Thessaloniki, Thessaloniki, Greece, 2006. (In Greek). [Google Scholar]
- Carboni, M.; Carranza, M.L.; Acosta, A. Assessing Conservation Status on Coastal Dunes: A Multiscale Approach. Landsc. Urban Plan. 2009, 91, 17–25. [Google Scholar] [CrossRef]
- Lepart, J.; Dubussche, M. Invasion Processes as Related to Succession and Disturbance. In Biogeography of Mediterranean Invasions; Cambritge University Press: Victoria, Australia, 1991; pp. 159–177. [Google Scholar] [CrossRef]
- Tapias, R.; Climent, J.; Pardos, J.A.; Gil, L. Life Histories of Mediterranean Pines. Plant Ecol. 2011, 171, 53–68. [Google Scholar] [CrossRef]
- Rouget, M.; Richardson, D.M.; Milton, S.J.; Polakow, D. Predicting Invasion Dynamics of Four Alien Pinus Species in a Highly Fragmented Semi- Arid Shrubland in South Africa. Plant Ecol. 2016, 152, 79–92. [Google Scholar] [CrossRef]
- Mita, E.; Tsitsimpikou, C.; Tziveleka, L.; Petrakis, P.V.; Ortiz, A.; Vagias, K.; Roussis, V. Seasonal variation of oleoresin terpenoids from Pinushalepensis and P. pinea and host selection of the scale insect Marchalinahellenica (Gennadius) (Homoptera, Coccoidea, Margarodidae, Coelostonidiinae). Zeischrift Fuer Holzforsch. 2001, 56, 572–578. [Google Scholar] [CrossRef]
- Rigolot, E. Predicting Postfire Mortality of Pinus halepensis Mill. and Pinus pinea L. Plant Ecol. 2004, 171, 139–151. [Google Scholar] [CrossRef]
- Teobaldelli, M.; Mencuccini, M.; Piussi, P. Water table salinity, rainfall and water use by umbrella pine trees (Pinus pinea L.). Plant Ecol. 2004, 171, 23–33. [Google Scholar] [CrossRef]
- Torma, A.; Császár, P. Species richness and composition patterns across trophic levels of true bugs (Heteroptera) in the agricultural landscape of the lower reach of the Tisza River Basin. J. Insect Conserv. 2013, 17, 35–51. [Google Scholar] [CrossRef]
- Samways, M.J. Insect Conservation: A Synthetic Management Approach. Annu. Rev. Entomol. 2007, 52, 465–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collinge, S.K. Effects of Grassland Fragmentation on Insect Species Loss, Colonization, and Movement Patterns. Ecology 2000, 81, 2211–2226. [Google Scholar] [CrossRef]
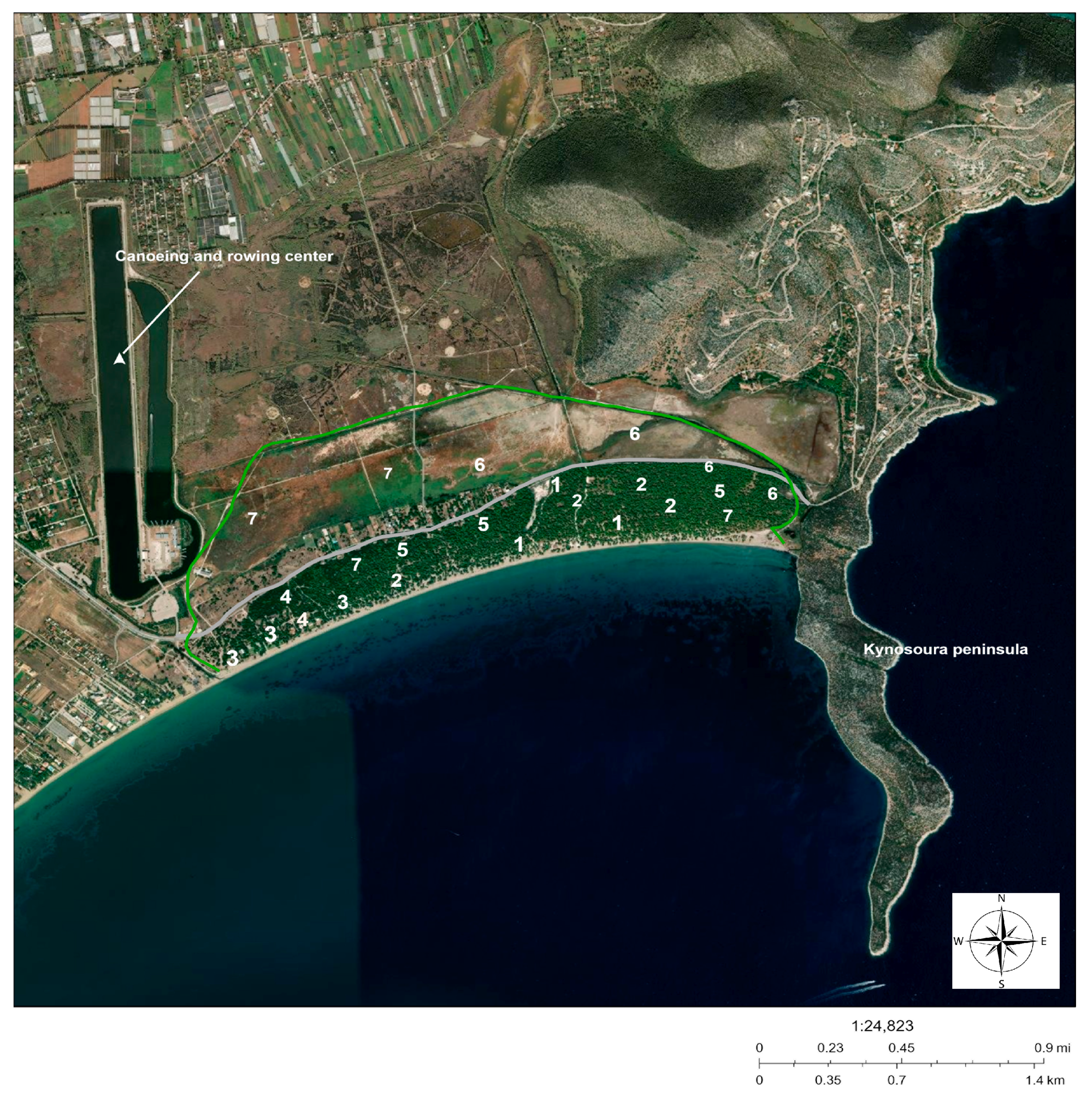


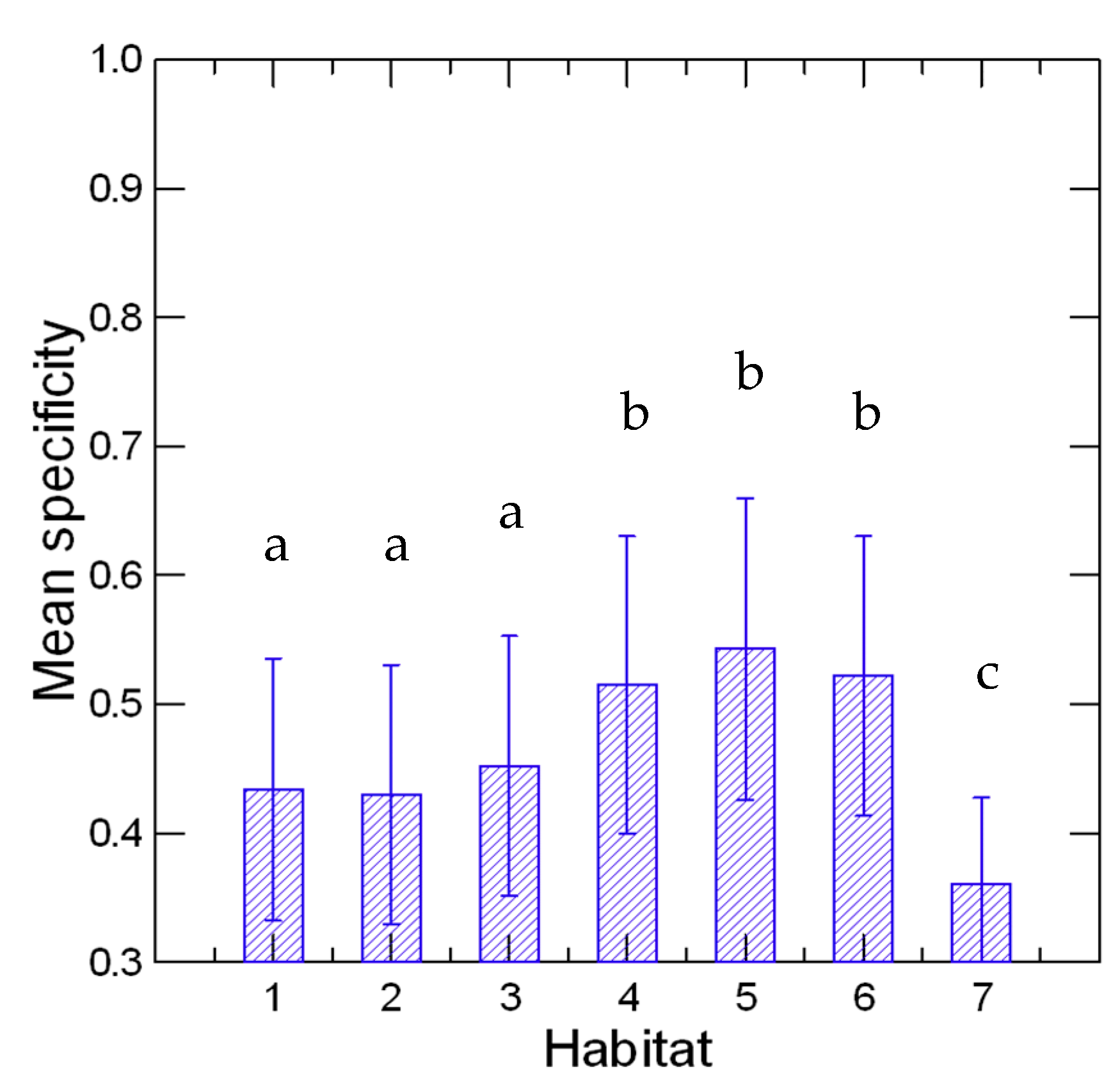
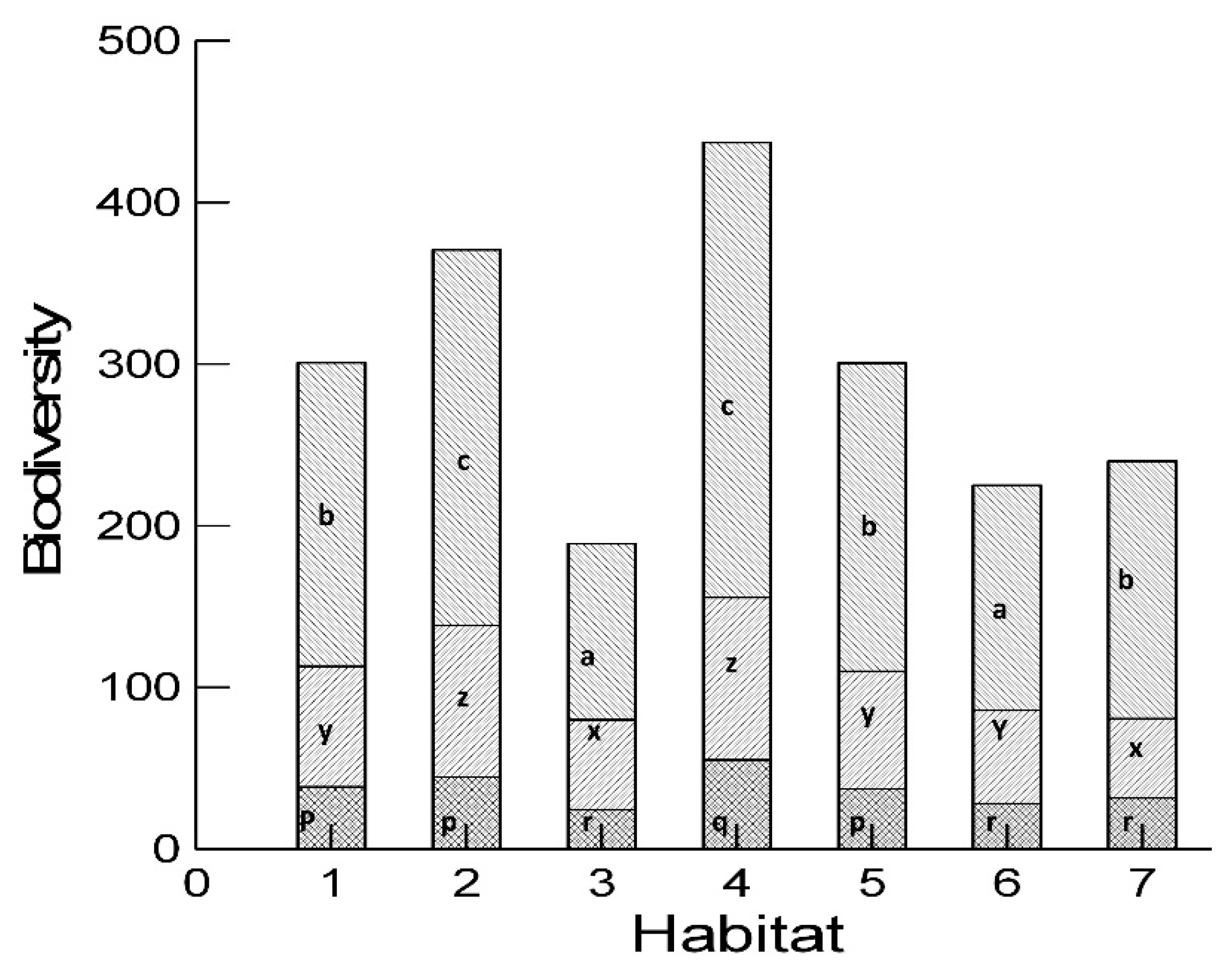
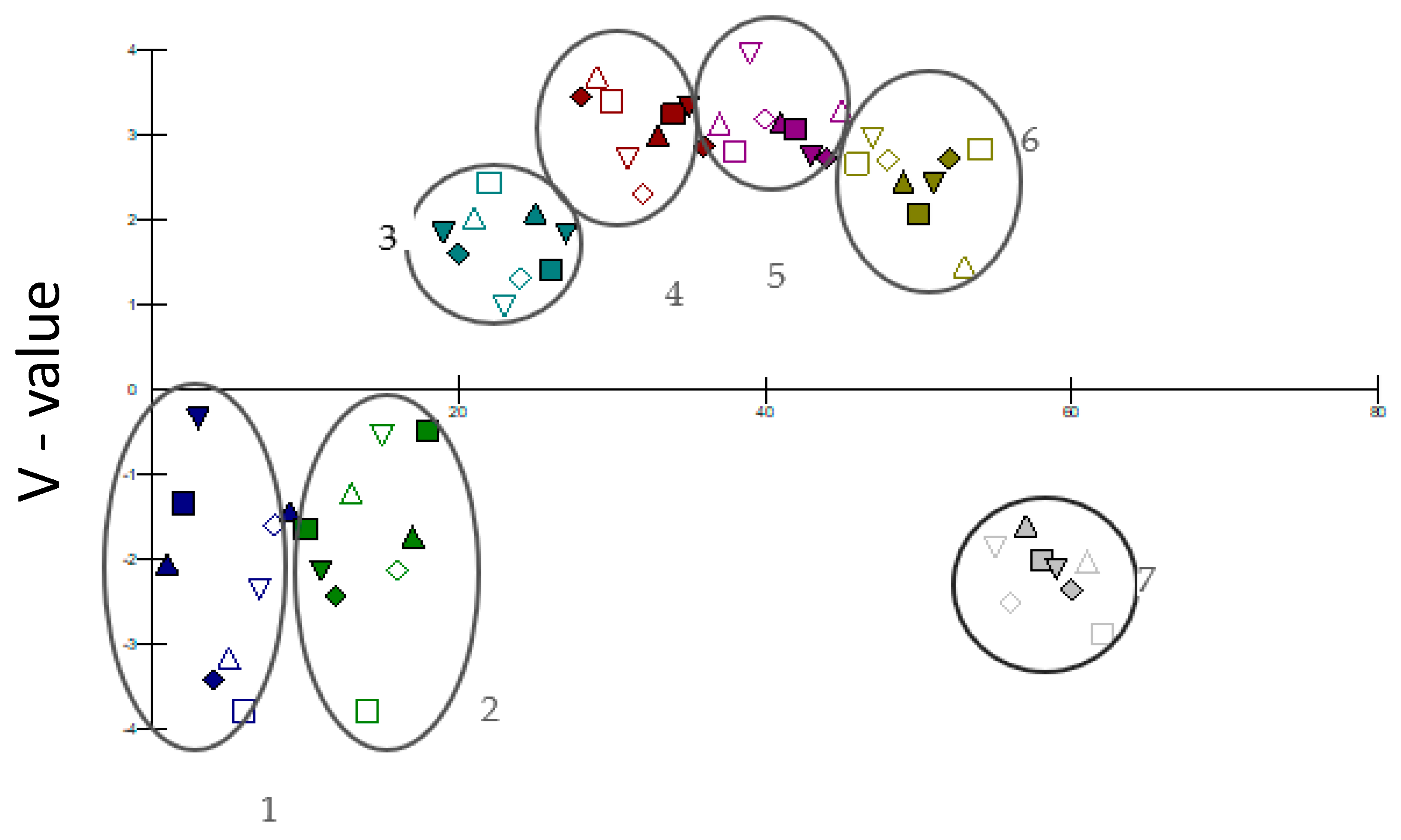

| Name | Dominant and Subdominant Plants |
|---|---|
| H1 | P.halepensis, Pistacia lentiscus L., Juniperus phoenicea L., Anthylis hermaniae L. |
| H2 | P. halepensis Mill, Poaceae, Cistus sp., Papilionaceae, Brassicaceae |
| H3 | P. pinea L., Pa. Lentiscus, Juniperus oxycedrus subsp. macrocarpa (Sm.) Neilr., A. hermaniae |
| H4 | P. pinea, Poaceae, Pa. lentiscus, Quercus coccifera L., Asparagus aphyllus L., Cistus sp. |
| H5 | Typha angustifolia L., Plantago coronopus L., T. hampeana, J. acutus L., Elymus farctus (Viv.) Runemark ex Melderis |
| H6 | J. subulatus Forssk., Phragmites australis (Cav.) Trin. ex Steud. |
| H7 | T. hampeana, Cakile maritima Scop. |
| Metric | Observed Value | Z | P |
|---|---|---|---|
| T | 31.04 3 | −1.34 | Ns |
| NODF 1 | 62.8 | 1.05 | Ns |
| WNODF 2 | 28.14 | 0.38 | Ns |
| C-score 4 | 141.34 | 4.6 | 5 × 10−4 |
| CU 5 | 1,375,256 | 319.38 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrakis, P.V.; Koulelis, P.P.; Solomou, A.D.; Spanos, K.; Spanos, I.; Feest, A. Insect Diversity in the Coastal Pinewood and Marsh at Schinias, Marathon, Greece: Impact of Management Decisions on a Degraded Biotope. Forests 2023, 14, 392. https://doi.org/10.3390/f14020392
Petrakis PV, Koulelis PP, Solomou AD, Spanos K, Spanos I, Feest A. Insect Diversity in the Coastal Pinewood and Marsh at Schinias, Marathon, Greece: Impact of Management Decisions on a Degraded Biotope. Forests. 2023; 14(2):392. https://doi.org/10.3390/f14020392
Chicago/Turabian StylePetrakis, Panos V., Panagiotis P. Koulelis, Alexandra D. Solomou, Kostas Spanos, Ioannis Spanos, and Alan Feest. 2023. "Insect Diversity in the Coastal Pinewood and Marsh at Schinias, Marathon, Greece: Impact of Management Decisions on a Degraded Biotope" Forests 14, no. 2: 392. https://doi.org/10.3390/f14020392
APA StylePetrakis, P. V., Koulelis, P. P., Solomou, A. D., Spanos, K., Spanos, I., & Feest, A. (2023). Insect Diversity in the Coastal Pinewood and Marsh at Schinias, Marathon, Greece: Impact of Management Decisions on a Degraded Biotope. Forests, 14(2), 392. https://doi.org/10.3390/f14020392











