Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Fennel Seeds
2.2. F. vulgare Sprouting and Preparation of Aqueous and Ethanolic Extracts
2.3. Total Phenolic Content (TPC), Antioxidant Activity (AOA), Total Flavonoids (TF), and Total Flavonols (TFL) in Raw FS and during Sprouting
2.4. Phenolics Quantification in FS and FSS Using HPLC-DAD
2.5. Quantification of Volatile Components by GC–MS
2.6. Animals and Experimental Design
2.6.1. Determination of Kidney Function
2.6.2. Oxidative Stress Biomarkers
2.6.3. Histopathological Studies
2.7. Statistical Analysis
3. Results
3.1. Bioactive Compounds and Antioxidant Activity of FS and FSS
3.2. Quantification of Phenolic Compounds in FS and FSS by HPLC-DAD
3.3. Determination of Volatile Component Concentrations in FS and FSS Using GC–MS
3.4. The Weight Gain, the Relative Weight of Organs, and Hypoglycemic Efficiency
3.5. The Hypolipidemic Efficiency
3.6. The Kidney’s Functions
3.7. Antioxidant Biomarkers
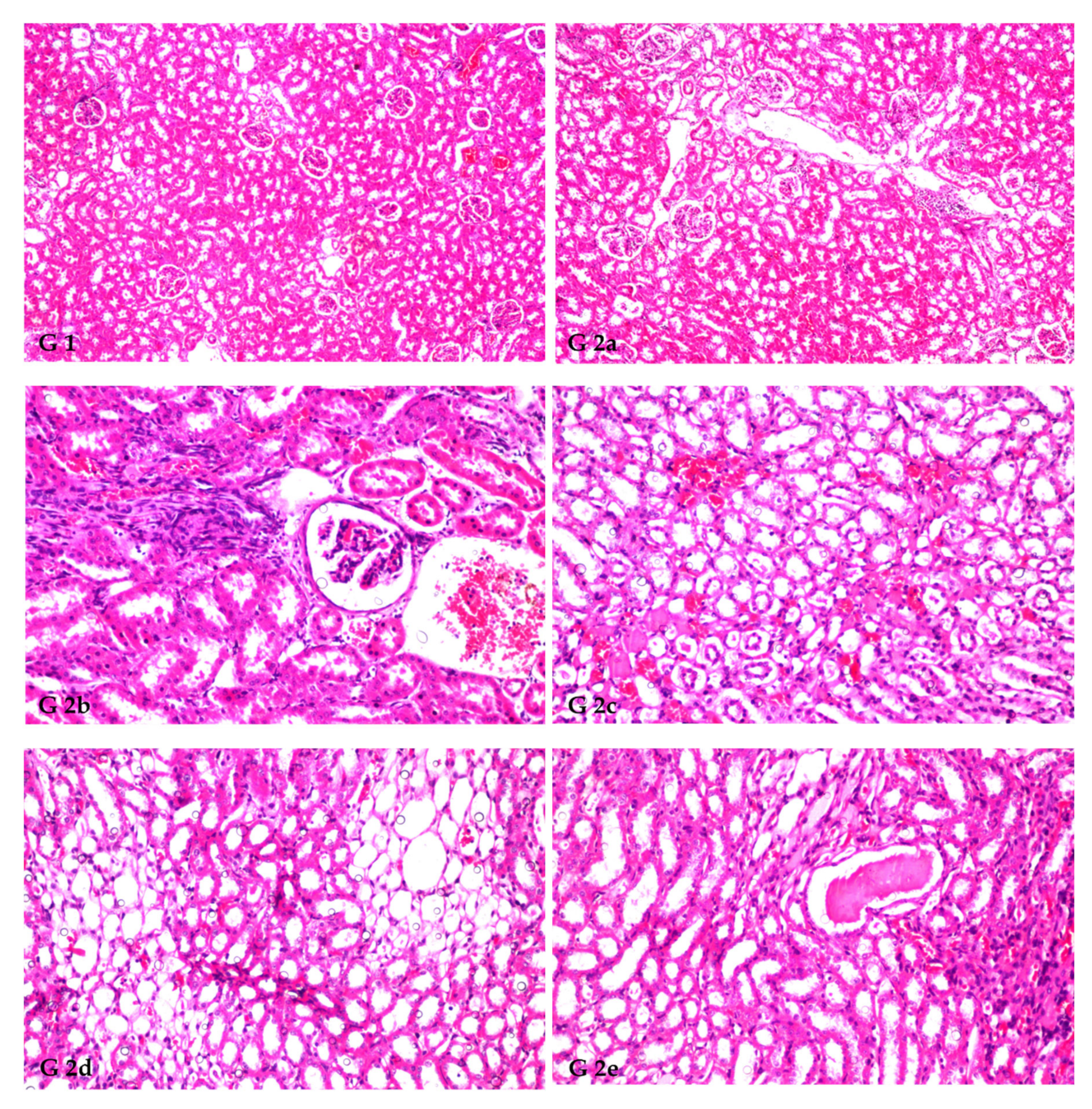
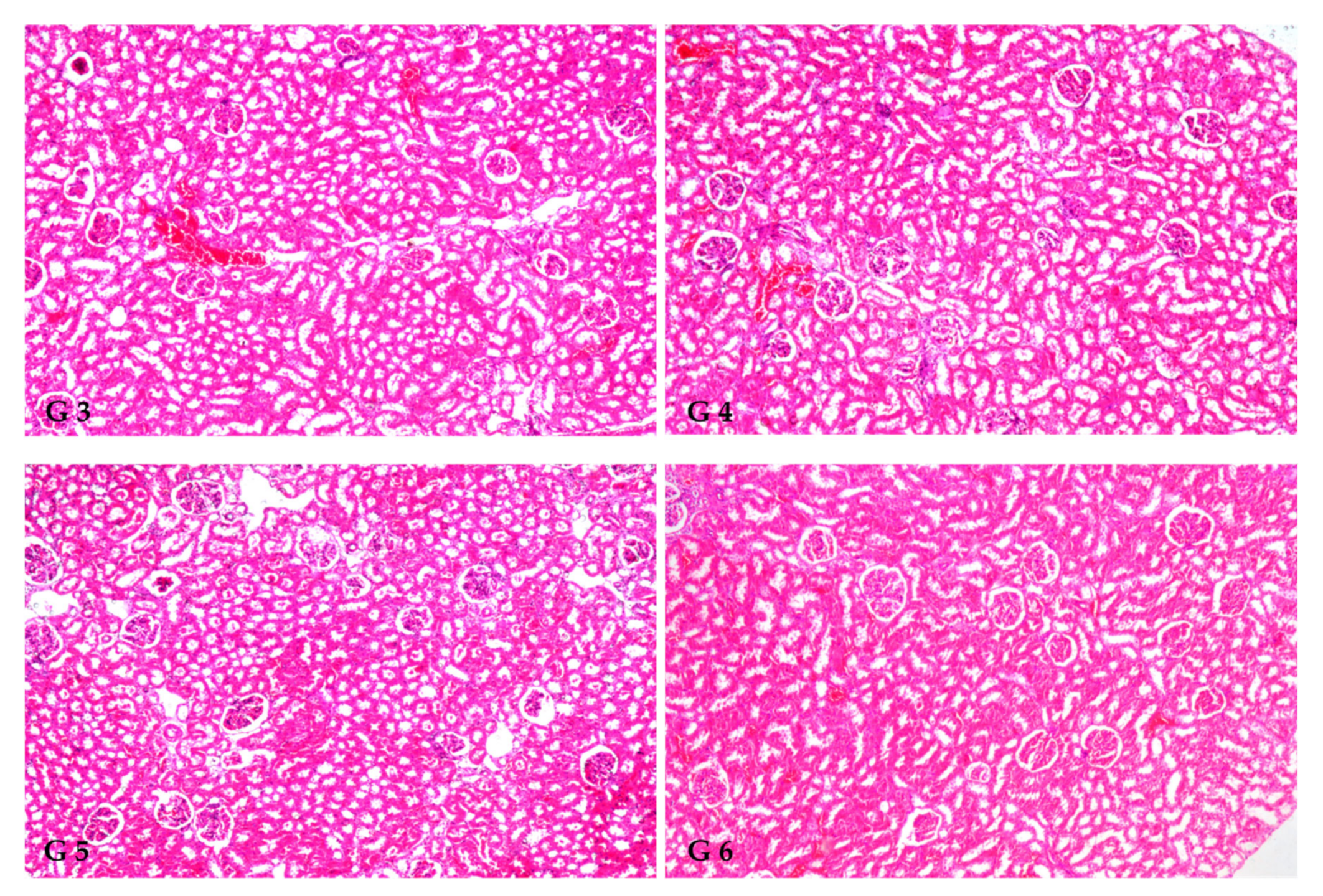
3.8. Renal Histoarchitecture
4. Discussion
5. Conclusions
Institutional Review Board Statement
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Pallardó, F.V.; Knecht, E.; Sanz, P. Increased Oxidative Stress and Impaired Antioxidant Response in Lafora Disease. Mol. Neurobiol. 2015, 51, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and Oxidative Stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Díaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Comparedt To Healthy Controls Before and after Antidepressant Treatment: Results from A Meta-Analysis. J. Clin. Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Statistics, K.D. Chronic Kidney Disease in the United States. 2021. Available online: https://www.cdc.gov/kidneydisease/pdf/Chronic-Kidney-Disease-in-the-US-2021-h.pdf (accessed on 12 October 2022).
- Chen, Z.; Shrestha, R.; Yang, X.; Wu, X.; Jia, J.; Chiba, H.; Hui, S.-P. Oxidative Stress and Lipid Dysregulation in Lipid Droplets: A Connection to Chronic Kidney Disease Revealed in Human Kidney Cells. Antioxidants 2022, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Guebre-Egziabher, F.; Alix, P.M.; Koppe, L.; Pelletier, C.C.; Kalbacher, E.; Fouque, D.; Soulage, C.O. Ectopic Lipid Accumulation: A Potential Cause for Metabolic Disturbances and A Contributor to the Alteration of Kidney Function. Biochimie 2013, 95, 1971–1979. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Fuda, H.; Tsukui, T.; Wu, X.; Shen, N.; Saito, N.; Chiba, H.; Hui, S.-P. Oxidative Stress Linked Organ Lipid Hydroperoxidation and Dysregulation in Mouse Model of Nonalcoholic Steatohepatitis: Revealed by Lipidomic Profiling of Liver and Kidney. Antioxidants 2021, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.H.; Abdou, H.S.; Azeem, A.S.A.E.; Abdel-Rahim, E.A. The Antioxidative Effects of Some Medicinal Plants as Hypoglycemic Agents on Chromosomal Aberration and Abnormal Nucleic Acids Metabolism Produced by Diabetes Stress in Male Adult Albino Rats. J. Diabetes Mellit. 2011, 1, 6–14. [Google Scholar] [CrossRef]
- Suslick, K.S. Kirk-Othmer Encyclopedia of Chemical Technology; J. Wiley & Sons: New York, NY, USA, 1998; pp. 517–541. [Google Scholar]
- Ozturk, F.; Ucar, M.; Ozturk, I.C.; Vardi, N.; Batcioglu, K. Carbon Tetrachloride-Induced Nephrotoxicity and Protective Effect of Betaine in Sprague-Dawley Rats. Urology 2003, 62, 353–356. [Google Scholar] [CrossRef]
- Slater, T.F. Free Radical Mechanisms in Tissue Injury. In Cell Function and Disease; Plenum Press: New York, NY, USA, 1988; pp. 209–218. [Google Scholar]
- Khan, M.R.; Rizvi, W.; Khan, G.N.; Khan, R.A.; Shaheen, S. Carbon Tetrachloride-Induced Nephrotoxicity in Rats: Protective Role of Digera Muricata. J. Ethnopharmacol. 2009, 122, 91–99. [Google Scholar] [CrossRef]
- Afsar, T.; Khan, M.R.; Razak, S.; Ullah, S.; Mirza, B. Antipyretic, Anti-inflammatory and Analgesic Activity of Acacia hydaspica R. Parker and its Phytochemical Analysis. BMC Complement Altern. Med. Rev. 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef] [PubMed]
- Al Zhrani, M.M.; Althwab, S.A.; Aljutaily, T.; Alfheeaid, H.A.; Ashoush, I.S.; Barakat, H. Protective Effect of Moringa-Based Beverages Against Hyperlipidemia and Hyperglycemia in Type 2 Diabetes-Induced Rats. Food Res. 2021, 5, 279–289. [Google Scholar] [CrossRef]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. [Google Scholar] [CrossRef]
- Shojaiefar, S.; Sabzalian, M.R.; Mirlohi, A.; Mirjalili, M.H. Seed Yield Stability with Modified Essential Oil Content and Composition in Self-Compatible Progenies of Bitter Fennel (Foeniculum Vulgare Mill.). Ind. Crops Prod. 2022, 182, 114821. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Yang, I.J.; Lee, D.U.; Shin, H.M. Anti-Inflammatory and Antioxidant effects of Coumarins Isolated From Foeniculum Vulgare in Lipopolysaccharide-Stimulated Macrophages and 12-O-Tetradecanoylphorbol-13-Acetate-Stimulated Mice. Immunopharmacol. Immunotoxicol. 2015, 37, 308–317. [Google Scholar] [CrossRef]
- Gerhke, S.A.; Shibli, J.A.; Salles, M.B. Potential of the Use of An Antioxidant Compound to Promote Peripheral Nerve Regeneration After Injury. Neural. Regen. Res. 2015, 10, 1673–5374. [Google Scholar]
- Odeh, A.; Allaf, A.W. Determination of Polyphenol Component Fractions and Integral Antioxidant Capacity of Syrian Aniseed and Fennel Seed Extracts Using Gc–Ms, Hplc Analysis, and Photochemiluminescence Assay. Chem. Pap. 2017, 71, 1731–1737. [Google Scholar] [CrossRef]
- Alam, P.; Abdel-Kader, M.S.; Alqarni, M.H.; Zaatout, H.H.; Ahamad, S.R.; Shakeel, F. Chemical Composition of Fennel Seed Extract and Determination of Fenchone in Commercial Formulations by Gc–Ms Method. J. Food Sci.Technol. 2019, 56, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.; Ali Shah, S.A.; Ahmed, T.; Zahid, S. Neuroprotective Effects of Foeniculum Vulgare Seeds Extract on Lead-Induced Neurotoxicity in Mice Brain. Drug Chem. Toxicol. 2018, 41, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Parle, M. Cholinergic Basis of Memory-Strengthening Effect of Foeniculum Vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H. Foeniculum vulgare Mill (Umbelliferae) Attenuates Stress and Improves Memory in Wister Rats. Trop. J. Pharm. Res. 2013, 12, 553–558. [Google Scholar] [CrossRef]
- Imran, A.; Xiao, L.; Ahmad, W.; Anwar, H.; Rasul, A.; Imran, M.; Aziz, N.; Razzaq, A.; Arshad, M.U.; Shabbir, A.; et al. Foeniculum Vulgare (Fennel) Promotes Functional Recovery and Ameliorates Oxidative Stress Following A Lesion to the Sciatic Nerve in Mouse Model. J. Food Biochem. 2019, 43, e12983. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Tamta, G.; Nand, V.; Singh, J.P. Nutritional Profiling, Antibacterial Potential, and Cluster Analysis in Foeniculum Vulgare Seeds Against Human Pathogenic Bacteria. J. Food Process. Preserv. 2022, 46, e16763. [Google Scholar] [CrossRef]
- Kalleli, F.; Bettaieb Rebey, I.; Wannes, W.A.; Boughalleb, F.; Hammami, M.; Saidani Tounsi, M.; M’Hamdi, M. Chemical Composition and Antioxidant Potential of Essential Oil and Methanol Extract from Tunisian and French Fennel (Foeniculum vulgare Mill.) Seeds. J. Food Biochem. 2019, 43, e12935. [Google Scholar] [CrossRef]
- Burkhardt, A.; Sintim, H.Y.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Schlegel, V. Method for Attaining Fennel (Foeniculum vulgare Mill.) Seed Oil Fractions with Different Composition and Antioxidant Capacity. J. Appl. Res. Med. Aromat. Plants 2015, 2, 87–91. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Bouhlali, E.d.T.; Derouich, M.; El-Rhaffari, L. Essential Oil and Chemical Composition of Wild and Cultivated Fennel (Foeniculum vulgare Mill.): A Comparative Study. S. Afr. J. Bot 2020, 135, 93–100. [Google Scholar] [CrossRef]
- Smoum, R.; Haj, C.; Hirsch, S.; Nemirovski, A.; Yekhtin, Z.; Bogoslavsky, B.; Bakshi, G.K.; Chourasia, M.; Gallily, R.; Tam, J.; et al. Fenchone Derivatives as a Novel Class of CB2 Selective Ligands: Design, Synthesis, X-ray Structure and Therapeutic Potential. Molecules 2022, 27, 1382. [Google Scholar] [CrossRef]
- Dobrikov, G.M.; Valcheva, V.; Nikolova, Y.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Enantiopure Antituberculosis Candidates Synthesized from (-)-fenchone. Eur. J. Med. Chem. 2014, 77, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; An, J.; Seol, G.H.; Han, S.H.; Yee, J.; Min, S.S. Trans-Anethole Alleviates Trimethyltin Chloride-Induced Impairments in Long-Term Potentiation. Pharmaceutics 2022, 14, 1422. [Google Scholar] [CrossRef] [PubMed]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Gwinner, W.; Gröne, H.J. Role of Reactive Oxygen Secies in Glomerulonephritis. Nephrol. Dial. Transplant 2000, 15, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Finkel, T. From Sulfenylation to Sulfhydration: What a Thiolate Needs to Tolerate. Sci. Signal. 2012, 5, pe10. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Thiol Chemistry and Specificity in Redox Signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Hashem Abdel-Razik, A.R.; Abdel Aziz, R.L. Rescue Effects of Aqueous Seed Extracts of Foeniculum vulgare and Carum Carvi Against Cadmium-Induced Hepatic, Renal and Gonadal Damage in Female Albino Rats. Asian Pac. J. Trop. Med. 2017, 10, 1123–1133. [Google Scholar] [CrossRef]
- Sadrefozalayi, S.; Farokhi, F. Effect of the Aqueous Extract of Foeniculum vulgare (fennel) on the Kidney in Experimental PCOS Female Rats. Avicenna. J. Phytomed. 2014, 4, 110–117. [Google Scholar]
- Hernández-Saavedra, D.; Pérez-Ramírez, I.F.; Ramos-Gómez, M.; Mendoza-Díaz, S.; Loarca-Piña, G.; Reynoso-Camacho, R. Phytochemical Characterization and Effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis Infusions on Obesity-Associated Cardiovascular Risk. Med. Chem. Res. 2016, 25, 163–172. [Google Scholar] [CrossRef]
- Yawadio Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant Activity of Various Extracts and Fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Barakat, H.; Rohn, S. Effect of Different Cooking Methods on Bioactive Compounds in Vegetarian, Broccoli-Based Bars. J. Funct. Foods 2014, 11, 407–416. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics Extracted from Potato, Sugar Beet, and Sesame Processing By-Products. Int. J. Food Prop. 2012, 16, 1148–1168. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76a rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Almundarij, T.I. Phenolic Compounds and Hepatoprotective Potential of Anastatica hierochuntica Ethanolic and Aqueous Extracts Against CCl4-Induced Hepatotoxicity in Rats. J. Tradit. Chin. Med. 2020, 40, 947. [Google Scholar]
- Moradabadi, L.; Montasser Kouhsari, S.; Fehresti Sani, M. Hypoglycemic Effects of Three Medicinal Plants in Experimental Diabetes: Inhibition of Rat Intestinal α-glucosidase and Enhanced Pancreatic Insulin and Cardiac Glut-4 mRNAs Expression. Iran J. Pharm. Res. 2013, 12, 387–397. [Google Scholar] [PubMed]
- Nwagha, U.; Ikekpeazu, E.; Ejezie, F.; Neboh, E.; Maduka, I. Atherogenic Index of Plasma as Useful Predictor of Cardiovascular Risk Among Postmenopausal Women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar]
- Beutler, E. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase In Vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Banchroft, J.D.; Stevans, A.; Turnes, D.R. Theory and Practice of Histological Techniques, 4th ed.; Elsevier Health Sciences: London, UK, 1996. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Eva, Y.; Annisa, A.; Andrafikar. Effectiveness of Jicama Probiotic Yoghurt (Pachyrhizus Erosus) on Blood Glucose in Diabetic Mice. KnE. Life Sci 2019, 4, 250–261. [Google Scholar] [CrossRef]
- Hasanein, P.; Felehgari, Z.; Emamjomeh, A. Preventive Effects of Salvia officinalis L. Against Learning and Memory Deficit Induced by Diabetes in Rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Kowalczyk, D.; Złotek, U. Impact of Germination Time and Type of Illumination on the Antioxidant Compounds and Antioxidant Capacity of Lens culinaris Sprouts. Sci. Hortic. 2012, 140, 87–95. [Google Scholar] [CrossRef]
- Vadivel, V.; Biesalski, H.K. Effect of Certain Indigenous Processing Methods on The Bioactive Compounds of Ten Different Wild Type Legume Grains. J. Food Sci. Technol. 2012, 49, 673–684. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts During their Growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of Caffeic Acid in Peanut Sprouts and Evaluation of Its In Vitro Effectiveness Against Oxidative Stress-Induced Erythrocyte Hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea Fennel (Crithmum Maritimum L.): Phytochemical Profile, Antioxidative, Cholinesterase Inhibitory and Vasodilatory Activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and Antimicrobial Activities of essential Oil and Extracts of Fennel (Foeniculum Vulgare Mill.) Seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Zaki, M.F. Antioxidant Activities of Phenolics, Flavonoids and Vitamin C in two Cultivars of Fennel (Foeniculum Vulgare Mill.) in Responses to Organic and Bio-Organic Fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, T.; Sun, Y.; Zhang, M.; Tian, J.; Chen, H.; Shen, Z.; Khan Abro, H.; Su, N.; Cui, J. Protective Effect of Selenium-Enriched Red Radish Sprouts on Carbon Tetrachloride-Induced Liver Injury in Mice. J. Food Sci. 2019, 84, 3027–3036. [Google Scholar] [CrossRef]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of Germination on Legume Phenolic Compounds and their Antioxidant Activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum Vulgare L.) and Chamomile (Matricaria Chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Fennel (Foeniculum Vulgare Mill. Subsp. Piperitum) Florets, A Traditional Culinary Spice in Italy: Evaluation of Phenolics and Volatiles in Local Populations, and Comparison with the Composition of Other Plant Parts. J. Sci. Food Agric. 2017, 97, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.k.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and Anticarcinogenic Effects of Methanolic Extract and Volatile Oil of Fennel Seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Allaithy, S.A. Chemical Compound of Cumin and Fennel Seed Extracts Against Some Types of Pathogenic Bacteria. Iraq Med. J 2017, 1, 1–6. [Google Scholar]
- Suleiman, W.B.; Helal, E.E.-H. Chemical Constituents and Potential Pleiotropic Activities of Foeniculum Vulgare (Fennel) Ethanolic Extract; In Vitro Approach. Egypt. J. Chem. 2022, 65, 5–6. [Google Scholar] [CrossRef]
- Hong, S.J.; Yoon, S.; Jo, S.M.; Jeong, H.; Youn, M.Y.; Kim, Y.J.; Kim, J.K.; Shin, E.-C. Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients 2022, 14, 741. [Google Scholar] [CrossRef]
- Afiat, M.; Amini, E.; Ghazanfarpour, M.; Nouri, B.; Mousavi, M.S.; Babakhanian, M.; Rakhshandeh, H. The Effect of Short-term Treatment with Fennel on Lipid Profile in Postmenopausal Women: A Randomized Controlled Trial. J. Menopausal. Med. 2018, 24, 29–33. [Google Scholar] [CrossRef]
- Zakernezhad, F.; Barati, M.; Sanadgol, N.; Movahhedi, M.; Majd, A.; Golab, F. The Association Between Fennel Extract, Serum Lipid Profile, and Leptin Receptor Expression. Basic Clin. Neurosci. 2021, 12, 711–720. [Google Scholar] [CrossRef]
- Al-Amoudi, W.M. Protective Effects of Fennel Oil Extract Against Sodium Valproate-Induced Hepatorenal Damage in Albino Rats. Saudi J. Biol. Sci. 2017, 24, 915–924. [Google Scholar] [CrossRef]
- Adewole, S.; Salako, A.; Doherty, O.; Naicker, T. Effect of Melatonin on Carbon Tetrachloride-Induced Kidney Injury in Wistar Rats. Afr. J. Biomed. Res. 2007, 10, 153–164. [Google Scholar] [CrossRef]
- Stehouwer, C.A.; Zeldenrust, G.C.; den Ottolander, G.H.; Hackeng, W.H.L.; Donker, A.J.M.; Nauta, J.J.P. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. The Lancet 1992, 340, 319–323. [Google Scholar] [CrossRef]
- Asgharpour, M.; Alirezaei, A. Herbal Antioxidants in Dialysis Patients: A Review of Potential Mechanisms and Medical Implications. Ren. Fail. 2021, 43, 351–361. [Google Scholar] [CrossRef]
- Firuzi, O.; Khajehrezaei, S.; Ezzatzadegan, S.; Nejati, M.; Jahanshahi, K.-A.; Roozbeh, J. Effects of Silymarin on Biochemical and Oxidative Stress Markers in End-Stage Renal Disease Patients Undergoing Peritoneal Dialysis. Hemodial. Int. 2016, 20, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.-R. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering From Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-controlled Study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K.-s. SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxidative Med. Cell Longev. 2016, 2016, 7296092. [Google Scholar] [CrossRef] [PubMed]
- Erboga, M.; Aktas, C.; Erboga, Z.F.; Donmez, Y.B.; Gurel, A. Quercetin Ameliorates Methotrexate-Induced Renal Damage, Apoptosis and Oxidative Stress in Rats. Ren. Fail. 2015, 37, 1492–1497. [Google Scholar] [CrossRef]
- Ng, S.-C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of Lipid Oxidation Products in Quinoa (Chenopodium quinoa). Food Chem 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide Detoxification by Brain Cells. J. Neurosci. Res 2005, 79, 157–165. [Google Scholar] [CrossRef]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.-F.; Dai, M.-G. Antioxidant Properties of Proanthocyanidins Attenuate Carbon Tetrachloride (CCl4)–Induced stSeatosis and Liver Injury in Rats via CYP2E1 Regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef]
- Goswami, N.; Chatterjee, S. Assessment of Free Radical Scavenging Potential and Oxidative DNA Damage Preventive Activity of Trachyspermum ammi L. (Carom) and Foeniculum vulgare Mill. (Fennel) Seed Extracts. BioMed Res. Int. 2014, 2014, 582767. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-Induced Liver Injury. Biol. Pharm. Bull 2010, 33, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective Effect of Kaempferol Against Alcoholic Liver Injury in Mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-A.; Ke, B.-J.; Cheng, C.-S.; Wang, J.-J.; Wei, B.-L.; Lee, C.-L. Red Quinoa Bran Extracts Protects Against Carbon Tetrachloride-Induced Liver Injury and Fibrosis in Mice via Activation of Antioxidative Enzyme Systems and Blocking TGF-β1 Pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Samadi-Noshahr, Z.; Hadjzadeh, M.-A.-R.; Moradi-Marjaneh, R.; Khajavi-Rad, A. The Hepatoprotective Effects of Fennel Seeds Extract and trans-Anethole in Streptozotocin-induced Liver Injury in Rats. Food Sci. Nutr. 2021, 9, 1121–1131. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural Antioxidants in the Treatment and Prevention of Diabetic Nephropathy; A Potential Approach That Warrants Clinical Trials. Red. Rep. 2017, 22, 99–118. [Google Scholar] [CrossRef]
- Tan, S.M.; de Haan, J.B. Combating Oxidative Stress in Diabetic Complications with Nrf2 Activators: How Much Is Too Much? Red. Rep. 2014, 19, 107–117. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin Promotes Nrf2-ARE Anti-oxidative Pathway Through Activating Sirt1 to Resist AGEs-Induced Upregulation of Fibronetin and Transforming Growth Factor-β1 in Rat Glomerular Messangial Cells. Mol. Cell Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids Activate the Antioxidant Response Element Transcription System. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef]
- Yoh, K.; Hirayama, A.; Ishizaki, K.; Yamada, A.; Takeuchi, M.; Yamagishi, S.-i.; Morito, N.; Nakano, T.; Ojima, M.; Shimohata, H.; et al. Hyperglycemia Induces Oxidative and Nitrosative Stress and Increases Renal Functional Impairment in Nrf2-Deficient Mice. Genes Cells 2008, 13, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- de Haan, J.B. Nrf2 Activators as Attractive Therapeutics for Diabetic Nephropathy. Diabetes 2011, 60, 2683–2684. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Qian, Q.; Adaikalakoteswari, A.; Rabbani, N.; Babaei-Jadidi, R.; Thornalley, P.J. Activation of NF-E2–Related Factor-2 Reverses Biochemical Dysfunction of Endothelial Cells Induced by Hyperglycemia Linked to Vascular Disease. Diabetes 2008, 57, 2809–2817. [Google Scholar] [CrossRef]
- Nam, J.S.; Cho, M.H.; Lee, G.T.; Park, J.S.; Ahn, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; Ha, H.J.; Lee, H.C. The Activation of NF-κB and AP-1 in Peripheral Blood Mononuclear Cells Isolated from Patients with Diabetic Nephropathy. Diabetes Res. Clin. Prac. 2008, 81, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Keap1-Nrf2 Cellular Defense Pathway: Mechanisms of Regulation and Role in Protection Against Drug-Induced Toxicity. In Adverse Drug Reactions; Uetrecht, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 233–266. [Google Scholar]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The Protective Role of Nrf2 in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef]
- Peixoto, E.B.; Pessoa, B.S.; Biswas, S.K.; Lopes de Faria, J.B. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am. J. Nephrol. 2009, 29, 309–318. [Google Scholar] [CrossRef]
- Doğukan, A.; Akpolat, N.; Çeliker, H.; Ilhan, N.; Bahçecioğlu, H.; Günal, A.I. Protective Effect of Interferon-Alpha on Carbon Tetrachloride-Induced Nephrotoxicity. J. Nephrol. 2003, 16, 81–84. [Google Scholar]
- Khan, M.R.; Siddique, F. Antioxidant Effects of Citharexylum Spinosum in CCl4 Induced Nephrotoxicity in Rat. Exp. Toxicol. Pathol. 2012, 64, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Khan, M.R. Protective Effects of Monotheca buxifolia Fruit on Renal Toxicity Induced By CCl4 In Rats. BMC Complement. Altern. Med. 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Venkatanarayana, G.; Sudhakara, G.; Sivajyothi, P.; Indira, P. Protective Effects of Curcumin and Vitamin E On Carbon Tetrachloride-Induced Nephrotoxicity in Rats. EXCLI J. 2012, 11, 641. [Google Scholar]
- Ogeturk, M.; Kus, I.; Colakoglu, N.; Zararsiz, I.; Ilhan, N.; Sarsilmaz, M. Caffeic acid Phenethyl Ester Protects Kidneys Against Carbon Tetrachloride Toxicity in Rats. J. Ethnopharmacol. 2005, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
| Item | Sprouting Period (Day) | |||||
|---|---|---|---|---|---|---|
| Raw Seeds | 3 | 6 | 9 | 12 | 15 | |
| TPC (mg GAE g−1) | 70.42 ±3.28 a | 40.56 ±2.70 d | 51.10 ±3.79 c | 75.20 ±4.48 a | 62.04 ±2.12 b | 54.48 ±3.70 c |
| TF (mg QE g−1) | 4.83 ±0.45 a | 2.82 ±0.14 d | 4.29 ±0.21 b | 5.18 ±0.21 a | 3.86 ±0.25 c | 3.23 ±0.24 c |
| TFL (mg QE g−1) | 4.92 ±0.23 a | 3.22 ±0.23 c | 3.62 ±0.29 c | 4.54 ±0.38 ab | 4.39 ±0.29 b | 3.02 ±0.31 c |
| DPPH (µmol of TE g−1) | 9.36 ±0.07 b | 6.51 ±1.09 d | 9.91 ±1.29 b | 11.93 ±1.92 a | 8.02 ±0.06 c | 8.67 ±0.22 c |
| Item | No. | Compound | Phenolics (µg g−1) * | |
|---|---|---|---|---|
| Sprouting Period (Day) | ||||
| Raw Fennel Seed | 9-Day Sprouts | |||
| Phenolic acids | 1 | Pyrogallol | - | - |
| 2 | Quinol | - | - | |
| 3 | 3-Hydroxytyrosol | - | - | |
| 4 | Catechol | 9.26 ± 0.23 b | 72.26 ± 5.41 a | |
| 5 | p-Hydroxy benzoic acid | 32.25 ± 2.57 a | 32.60 ± 3.58 a | |
| 6 | Caffeic acid | 26.72 ± 5.98 b | 48.22 ± 7.25 a | |
| 7 | Chlorogenic acid | 6.79 ± 3.25 b | 71.25 ± 9.25 a | |
| 8 | Cinnamic acid | 9.38 ± 0.98 b | 18.83 ± 5.14 a | |
| 9 | Ellagic acid | 25.35 ± 4.98 b | 52.30 ± 8.24 a | |
| 10 | Vanillic acid | 587.40 ± 31.09 a | 129.08 ± 5.97 b | |
| 11 | Ferulic acid | 20.01 ± 2.49 b | 48.51 ± 4.75 a | |
| 12 | Gallic acid | - | - | |
| 13 | O-coumaric acid | 112.77 ± 5.68 a | 8.44 ± 1.28 b | |
| 14 | p-coumaric acid | 18.46 ± 2.97 a | 19.89 ± 2.58 a | |
| 15 | Benzoic acid | 30.38 ± 3.97 b | 110.35 ± 12.32 a | |
| 16 | Rosmarinic acid | 64.41 ± 6.17 b | 124.71 ± 15.28 a | |
| 17 | Syringic acid | 9.72 ± 0.98 b | 66.08 ± 10.28 a | |
| Flavonoids | 1 | Catechin | 123.46 ± 19.27 b | 151.46 ± 20.27 a |
| 2 | Kaempferol | 5913.55 ± 152.93 a | 2357.57 ± 251.71 b | |
| 3 | Myricetin | 236.93 ± 12.59 b | 166.94 ± 23.19 a | |
| 4 | Quercetin | 28.71 ± 1.25 b | 192.35 ± 18.97 a | |
| 5 | Rutin | 423.28 ± 20.02 b | 985.29 ± 12.35 a | |
| 6 | Resveratrol | 472.19 ± 25.98 | 402.24 ± 32.19 b | |
| 7 | Naringenin | - | - | |
| RT | MW | Compound | Concentration % | |
|---|---|---|---|---|
| Raw Fennel Seed | 9-Day Sprouts | |||
| 6.51 | 152 | α-Pinene | 0.44 | 1.08 |
| 9.19 | 136 | 1-methylidene-4-prop-1-en-2-ylcyclohexane | 7.17 | 3.32 |
| 10.94 | 152 | Fenchone | 11.18 | 7.42 |
| 14.30 | 148 | 1-methoxy-4-prop-2-enylbenzene | 23.65 | 22.89 |
| 15.89 | 136 | 4-methoxybenzaldehyde | 1.45 | 2.32 |
| 15.87 | 144 | Phenylhydrazine hydrochloride | 1.81 | - |
| 16.80 | 148 | 1-methoxy-4-[(E)-prop-1-enyl]benzene | 38.41 | 42.32 |
| 23.92 | 150 | 2-hydroxy-2-(4-methoxyphenyl)acetic acid | 1.65 | 3.34 |
| 25.00 | 222 | 4,5-dimethoxy-6-prop-2-enyl-1,3-benzodioxole | - | 4.23 |
| 26.10 | 180 | 2-Methoxyphenyl)methyl acetate | 1.80 | 0.51 |
| 26.84 | 165 | 1-(3-hydroxy-4-methoxyphenyl)ethane-1,2-diol | 1.86 | 0.52 |
| 34.20 | 296 | 7-Octadecenoic acid methyl ester | - | 0.89 |
| 35.70 | 282 | cis-13-Octadecenoic acid | 3.12 | 6.42 |
| 48.78 | 152 | 10-Nonadecanone | 1.57 | 0.53 |
| Groups | Weight Gain % | Organ Relative Weight (%) | FBG | ||
|---|---|---|---|---|---|
| Week 3 | Week 6 | Liver | Kidneys | ||
| G1 | 38.11 ±2.66 | 52.69 ±2.13 | 3.24 ±0.22 a | 0.79 ±0.06 a | 77.90 ±2.81a |
| G2 | 16.42 ±5.26 | 24.45 ±3.48 | 3.71 ±0.17 b | 0.84 ±0.03 a | 91.24 ±6.87 b |
| G3 | 25.33 ±5.37 | 35.45 ±6.19 | 3.24 ±0.04 a | 0.67 ±0.03 ab | 81.55 ±2.87 a |
| G4 | 35.50 ±4.88 | 62.17 ±4.58 | 2.91 ±0.24 a | 0.66 ±0.05 b | 79.99 ±6.69 a |
| G5 | 31.73 ±3.43 | 55.19 ±4.44 | 3.15 ±0.16 a | 0.78 ±0.02 a | 82.83 ±10.74 a |
| G6 | 40.51 ±5.26 | 66.27 ±5.21 | 2.96 ± 0.09 a | 0.74 ±0.02 a | 78.83 ±3.44 a |
| Groups | Lipid Profile Parameters | |||||
|---|---|---|---|---|---|---|
| TG | CHO | HDL-C | LDL-C | VLDL-C | AI | |
| G1 | 35.76 ±2.85 b | 69.40 ±5.20 b | 35.38 ±3.04 ab | 26.86 ±6.52 bcd | 7.15 ±0.57 c | 0.005 ±0.004 d |
| G2 | 53.59 ±5.53 a | 89.29 ±4.23 a | 23.33 ±2.56 c | 55.24 ±3.83 a | 10.72 ±1.10 a | 0.361 ±0.024 a |
| G3 | 47.71 ±4.30 ab | 72.33 ±4.53 b | 32.73 ±3.84 abc | 30.06 ±5.50 bc | 9.54 ±0.86 ab | 0.164 ±0.014 b |
| G4 | 41.71 ±4.08 ab | 60.33 ±4.35 b | 36.67 ±4.41 ab | 15.32 ±3.96 d | 8.34 ±0.82 abc | 0.056 ±0.011 c |
| G5 | 36.42 ±4.31 b | 74.81 ±6.15 b | 26.67 ±2.89 bc | 40.86 ±6.74 ab | 7.28 ±0.86 c | 0.135 ±0.012 b |
| G6 | 39.35 ±3.30 b | 67.31 ±3.37 b | 39.00 ±5.15 a | 20.44 ±4.61 cd | 7.87 ±0.66 bc | 0.004 ±0.006 d |
| Group | Kidney Function | |||||
|---|---|---|---|---|---|---|
| T. Protein (g dL−1) | Albumin (g dL−1) | Globulin (g dL−1) | Creatinine (mg dL−1) | Urea (mg dL−1) | BUN (mg dL−1) | |
| G1 | 8.05 ± 0.25 a | 3.27 ± 0.11 a | 4.78 ± 0.21 a | 1.13 ± 0.16 c | 48.79 ± 3.09 b | 22.93 ± 1.45 b |
| G2 | 6.26 ± 0.10 b | 2.60 ± 0.07 c | 3.67 ± 0.08 b | 2.00 ± 0.10 a | 60.42 ± 3.66 a | 28.40 ± 1.72 a |
| G3 | 7.36 ± 0.46 a | 2.86 ± 0.19 bc | 4.50 ± 0.47 a | 1.57 ± 0.06 b | 51.93 ± 5.27 ab | 24.41 ± 2.48 a |
| G4 | 7.85 ± 0.16 a | 3.33 ± 0.11 a | 4.52 ± 0.10 a | 1.26 ± 0.08 c | 44.36 ± 3.59 b | 20.85 ± 1.69 b |
| G5 | 7.28 ± 0.20 b | 3.12 ± 0.10 ab | 4.16 ± 0.17 a | 1.27 ± 0.02 c | 46.28 ± 1.56 b | 21.75 ± 0.74 b |
| G6 | 7.81 ± 0.68 a | 3.38 ± 0.13 a | 4.43 ± 0.75 a | 1.16 ± 0.07 c | 45.58 ± 1.44 b | 21.42 ± 0.68 b |
| Group | Antioxidant Biomarkers | |||
|---|---|---|---|---|
| GSH (µg dL−1) | MDA (nmol mL−1) | CAT (U L−1) | SOD (U L−1) | |
| G1 | 47.60 ± 4.89 a | 7.20 ± 0.43 cd | 46.69 ± 5.59 b | 73.32 ± 1.07 b |
| G2 | 36.63 ± 4.50 b | 12.51 ± 0.51 a | 23.83 ± 4.09 c | 46.75 ± 1.93 d |
| G3 | 43.56 ± 2.03 ab | 9.35 ± 0.64 b | 45.61 ± 5.71 b | 64.00 ± 0.92 c |
| G4 | 51.31 ± 2.66 a | 7.86 ± 0.36 cd | 51.13 ± 1.61 ab | 77.63 ± 1.58 a |
| G5 | 43.73 ± 4.43 ab | 8.57 ± 0.67 bc | 44.09 ± 6.60 b | 67.44 ± 1.23 c |
| G6 | 50.50 ± 1.58 a | 6.69 ± 0.35 d | 60.56 ± 4.26 a | 79.33 ± 1.58 a |
| Group | Antioxidant Biomarkers | |||
|---|---|---|---|---|
| GSH (µg g−1) | MDA (n mol g−1) | CAT (U g−1) | SOD (U L−1) | |
| G1 | 6.90 ± 0.54 a | 8.28 ± 0.47 c | 0.63 ± 0.06 bc | 0.96 ± 0.08 a |
| G2 | 5.31 ± 0.44 c | 14.38 ± 0.50 a | 0.32 ± 0.04 d | 0.58 ± 0.09 b |
| G3 | 6.36 ± 0.18 b | 10.75 ± 0.57 b | 0.61 ± 0.05 c | 0.80 ± 0.08 b |
| G4 | 7.49 ± 0.27 a | 9.03 ± 0.37 c | 0.69 ± 0.02 b | 0.97 ± 0.06 a |
| G5 | 6.34 ± 0.35 b | 9.85 ± 0.53 bc | 0.59 ± 0.05 c | 0.84 ± 0.08 b |
| G6 | 7.32 ± 0.12 a | 7.69 ± 0.40 d | 0.81 ± 0.04 a | 0.99 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Alkabeer, I.A.; Althwab, S.A.; Alfheeaid, H.A.; Alhomaid, R.M.; Almujaydil, M.S.; Almuziree, R.S.A.; Bushnaq, T.; Mohamed, A. Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats. Antioxidants 2023, 12, 325. https://doi.org/10.3390/antiox12020325
Barakat H, Alkabeer IA, Althwab SA, Alfheeaid HA, Alhomaid RM, Almujaydil MS, Almuziree RSA, Bushnaq T, Mohamed A. Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats. Antioxidants. 2023; 12(2):325. https://doi.org/10.3390/antiox12020325
Chicago/Turabian StyleBarakat, Hassan, Ibrahim Ali Alkabeer, Sami A. Althwab, Hani A. Alfheeaid, Raghad M. Alhomaid, Mona S. Almujaydil, Raya S. A. Almuziree, Taqwa Bushnaq, and Ahmed Mohamed. 2023. "Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats" Antioxidants 12, no. 2: 325. https://doi.org/10.3390/antiox12020325
APA StyleBarakat, H., Alkabeer, I. A., Althwab, S. A., Alfheeaid, H. A., Alhomaid, R. M., Almujaydil, M. S., Almuziree, R. S. A., Bushnaq, T., & Mohamed, A. (2023). Nephroprotective Effect of Fennel (Foeniculum vulgare) Seeds and Their Sprouts on CCl4-Induced Nephrotoxicity and Oxidative Stress in Rats. Antioxidants, 12(2), 325. https://doi.org/10.3390/antiox12020325








