Duckweeds for Phytoremediation of Polluted Water
Abstract
1. Introduction
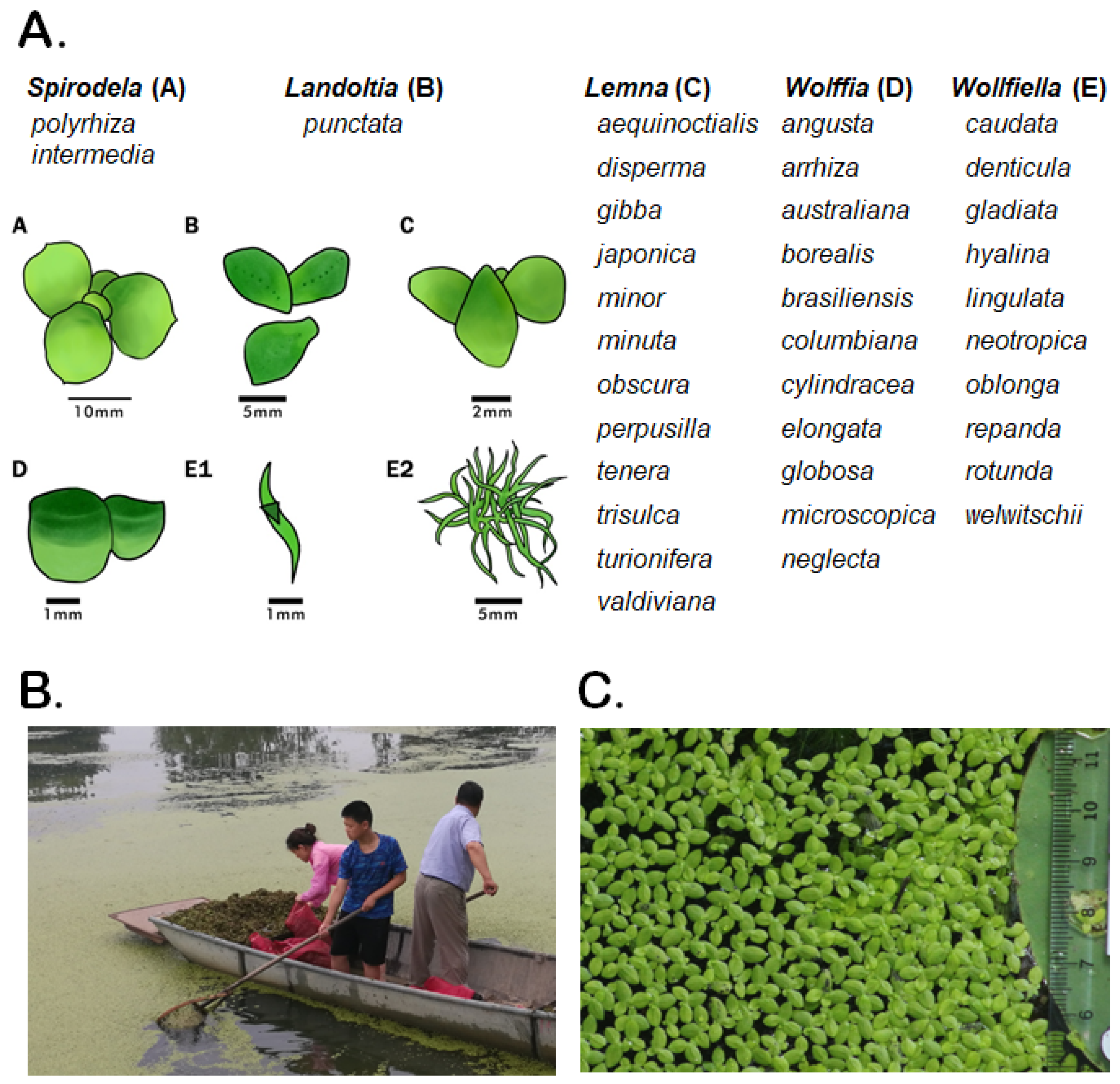
2. Duckweeds (Lemnaceae): Tiny Aquatic Plants with Unique Properties
3. Duckweeds for Remediating Water Contaminated with Nitrogen and Phosphorus
4. Duckweeds for Remediating Water Contaminated with Organic Compounds
4.1. Organic Agrochemicals
4.2. Pharmaceuticals and Personal Care Products (PPCPs)
4.2.1. Antibiotics
4.2.2. Analgesics and Anti-Inflammatory Drugs
4.3. Other Industrial Organic Compounds
5. Duckweeds for Remediating Water Contaminated with Heavy Metals and Metalloids
5.1. Heavy Metals
5.2. Metalloids: Boron and Arsenic
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, J.; Gupta, A.; Chandra, H. Managing Water Quality with Aquatic Macrophytes. Revis. Environ. Sci. Technol. 2008, 7, 255–266. [Google Scholar] [CrossRef]
- Priya, A.; Avishek, K.; Pathak, G. Assessing the Potentials of Lemna minor in the Treatment of Domestic Wastewater at Pilot Scale. Environ. Monit. Assess. 2012, 184, 4301–4307. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- González-González, R.B.; Flores-Contreras, E.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Bio-Removal of Emerging Pollutants by Advanced Bioremediation Techniques. Environ. Res. 2022, 214, 113936. [Google Scholar] [CrossRef]
- Dhote, S.; Dixit, S. Water Quality Improvement through Macrophytes—A Review. Environ. Monit Assess. 2009, 152, 149–153. [Google Scholar] [CrossRef]
- Newete, S.W.; Byrne, M.J. The Capacity of Aquatic Macrophytes for Phytoremediation and Their Disposal with Specific Reference to Water Hyacinth. Environ. Sci. Pollut. Res. 2016, 23, 10630–10643. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of Common Duckweed (Lemna minor) in Phytoremediation of Chemicals in the Environment: State and Future Perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Becker, J.A.; Stefanakis, A.I. Pharmaceuticals and personal care products as emerging water contaminants. In Pharmaceutical Sciences: Breakthroughs in Research and Practice; Information Resources Management Association, Ed.; IGI Global: Hershey, PA, USA, 2017; pp. 1457–1475. ISBN 978-1-5225-1762-7. [Google Scholar] [CrossRef]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural Molecular Mechanisms of Plant Hyperaccumulation and Hypertolerance towards Heavy Metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy Metal and Metalloid Toxicity in Horticultural Plants: Tolerance Mechanism and Remediation Strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Landolt, E. Biosystematic Investigations in the Family of Duckweeds, Lemnaceae: The Family of Lemnaceae, a Monographic Study. Vol. 1: Morphology, Karyology, Ecology, Geographic Distribution, Systematic Position, Nomenclature, Descriptions; Eidgenössischen Technischen Hochschule, Stiftung Rübel, Geobotanisches Institut: Zürich, Switzerland, 1986. [Google Scholar]
- Tippery, N.P.; Les, D.H. Tiny Plants with Enormous Potential: Phylogeny and Evolution of Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–38. ISBN 978-3-030-11044-4. [Google Scholar]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in Vitro Growth Rates of Duckweeds (Lemnaceae)—The Most Rapidly Growing Higher Plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Edelman, M.; Appenroth, K.-J.; Sree, K.S.; Oyama, T. Ethnobotanical History: Duckweeds in Different Civilizations. Plants 2022, 11, 2124. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970, 45, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Rail, S.; Roth, A.; Hemleben, V. Evidence for Uptake of Plamid DNA into Intact Plants (Lemna perpusilla) Proved by an E. Coli Transformation Assay. Z. Nat. C 1980, 35, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Hoffman-Falk, H.; Marder, J.B.; Edelman, M. Regulation of Protein Metabolism: Coupling of Photosynthetic Electron Transport to in Vivo Degradation of the Rapidly Metabolized 32-Kilodalton Protein of the Chloroplast Membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 1380–1384. [Google Scholar] [CrossRef]
- Ben-Tal, Y.; Cleland, C.F. Uptake and Metabolism of [14C] Salicylic Acid in Lemna gibba G3. Plant Physiol. 1982, 70, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ingemarsson, B. Nitrogen Utilization in Lemna: I. Relations between Net Nitrate Flux, Nitrate Reduction, and in Vitro Activity and Stability of Nitrate Reductase. Plant Physiol. 1987, 85, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J.; Sree, K.S.; Fakhoorian, T.; Lam, E. Resurgence of Duckweed Research and Applications: Report from the 3rd International Duckweed Conference. Plant Mol. Biol. 2015, 89, 647–654. [Google Scholar] [CrossRef]
- An, D.; Li, C.; Zhou, Y.; Wu, Y.; Wang, W. Genomes and Transcriptomes of Duckweeds. Front. Chem. 2018, 6, 230. [Google Scholar] [CrossRef]
- Baek, G.; Saeed, M.; Choi, H.-K. Duckweeds: Their Utilization, Metabolites and Cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef]
- Yang, G.-L.; Feng, D.; Liu, Y.-T.; Lv, S.-M.; Zheng, M.-M.; Tan, A.-J. Research Progress of a Potential Bioreactor: Duckweed. Biomolecules 2021, 11, 93. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Ziegler, P.; Sree, K.S. Accumulation of Starch in Duckweeds (Lemnaceae), Potential Energy Plants. Physiol. Mol. Biol. Plants 2021, 27, 2621–2633. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Borisjuk, N.; Lam, E. Telling Duckweed Apart: Genotyping Technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013, 19, 1–10. [Google Scholar]
- Borisjuk, N.; Chu, P.; Gutierrez, R.; Zhang, H.; Acosta, K.; Friesen, N.; Sree, K.S.; Garcia, C.; Appenroth, K.J.; Lam, E. Assessment, Validation and Deployment Strategy of a Two-Barcode Protocol for Facile Genotyping of Duckweed Species. Plant Biol. 2015, 17, 42–49. [Google Scholar] [CrossRef]
- Braglia, L.; Lauria, M.; Appenroth, K.J.; Bog, M.; Breviario, D.; Grasso, A.; Gavazzi, F.; Morello, L. Duckweed Species Genotyping and Interspecific Hybrid Discovery by Tubulin-Based Polymorphism Fingerprinting. Front. Plant Sci. 2021, 12, 625670. [Google Scholar] [CrossRef]
- Les, D.H.; Crawford, D.J. Landoltia (Lemnaceae), a new genus of duckweeds. Novon 1999, 9, 530–533. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.-J.; Sree, K.S. Duckweed (Lemnaceae): Its Molecular Taxonomy. Front. Sustain. Food Syst. 2019, 3, 117. [Google Scholar] [CrossRef]
- Zhou, Y.; Borisjuk, N. Small Aquatic Duckweed Plants with Big Potential for the Production of Valuable Biomass and Wastewater Remediation. Int. J. Environ. Sci. Nat. Resour. 2019, 16, 555942. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Yuan, X.Y.; Wang, J.R.; Bao, T.F.; Shi, W.M. Screening duckweed (Lemnaceae) species for efficient removal of waterbody nitrogen in the Tai Lake region and preliminary study on nitrogen removal mechanism. Soils 2010, 42, 390–397. (In Chinese) [Google Scholar]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Bao, S.; Fu, T.; He, Z.; Wang, F.; Zhao, H. Potential of Duckweed in the Conversion of Wastewater Nutrients to Valuable Biomass: A Pilot-Scale Comparison with Water Hyacinth. Bioresour. Technol. 2014, 163, 82–91. [Google Scholar] [CrossRef]
- Kishchenko, O.; Stepanenko, A.; Straub, T.; Zhou, Y.; Neuhäuser, B.; Borisjuk, N. Ammonium Uptake, Mediated by Ammonium Transporters, Mitigates Manganese Toxicity in Duckweed, Spirodela polyrhiza. Plants 2023, 12, 208. [Google Scholar] [CrossRef]
- Walsh, É.; Coughlan, N.E.; O’Brien, S.; Jansen, M.A.K.; Kuehnhold, H. Density Dependence Influences the Efficacy of Wastewater Remediation by Lemna minor. Plants 2021, 10, 1366. [Google Scholar] [CrossRef]
- Walsh, É.; Kuehnhold, H.; O’Brien, S.; Coughlan, N.E.; Jansen, M.A.K. Light Intensity Alters the Phytoremediation Potential of Lemna minor. Environ. Sci. Pollut. Res. 2021, 28, 16394–16407. [Google Scholar] [CrossRef]
- Walsh, É.; Paolacci, S.; Burnell, G.; Jansen, M.A.K. The Importance of the Calcium-to-Magnesium Ratio for Phytoremediation of Dairy Industry Wastewater Using the Aquatic Plant Lemna minor L. Int. J. Phytoremediation 2020, 22, 694–702. [Google Scholar] [CrossRef]
- Guo, L.; Jin, Y.; Xiao, Y.; Tan, L.; Tian, X.; Ding, Y.; He, K.; Du, A.; Li, J.; Yi, Z.; et al. Energy-Efficient and Environmentally Friendly Production of Starch-Rich Duckweed Biomass Using Nitrogen-Limited Cultivation. J. Clean. Produc. 2020, 251, 119726. [Google Scholar] [CrossRef]
- Lam, E.; Appenroth, K.J.; Michael, T.; Mori, K.; Fakhoorian, T. Duckweed in Bloom: The 2nd International Conference on Duckweed Research and Applications Heralds the Return of a Plant Model for Plant Biology. Plant Mol. Biol. 2014, 84, 737–742. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a Model Plant System in the Genomics and Post-Genomics Era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläßer, C.; Nussbaumer, T.; Luo, M.C.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.A.; Shanklin, J.; et al. The Spirodela polyrhiza Genome Reveals Insights into Its Neotenous Reduction Fast Growth and Aquatic Lifestyle. Nat. Commun. 2014, 5, 3311. [Google Scholar] [CrossRef]
- Michael, T.P.; Bryant, D.; Gutierrez, R.; Borisjuk, N.; Chu, P.; Zhang, H.; Xia, J.; Zhou, J.; Peng, H.; El Baidouri, M.; et al. Comprehensive Definition of Genome Features in Spirodela polyrhiza by High-Depth Physical Mapping and Short-Read DNA Sequencing Strategies. Plant J. 2017, 89, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.T.N.; Fiebig, A.; Novák, P.; Macas, J.; Cao, H.X.; Stepanenko, A.; Chen, G.; Borisjuk, N.; Scholz, U.; Schubert, I. Chromosome-Scale Genome Assembly for the Duckweed Spirodela intermedia, Integrating Cytogenetic Maps, PacBio and Oxford Nanopore Libraries. Sci. Rep. 2020, 10, 19230. [Google Scholar] [CrossRef]
- Van Hoeck, A.; Horemans, N.; Monsieurs, P.; Cao, H.X.; Vandenhove, H.; Blust, R. The First Draft Genome of the Aquatic Model Plant Lemna minor Opens the Route for Future Stress Physiology Research and Biotechnological Applications. Biotechnol. Biofuels 2015, 8, 188. [Google Scholar] [CrossRef]
- Abramson, B.W.; Novotny, M.; Hartwick, N.T.; Colt, K.; Aevermann, B.D.; Scheuermann, R.H.; Michael, T.P. The genome and preliminary single-nuclei transcriptome of Lemna minuta reveals mechanisms of invasiveness. Plant Physiol. 2022, 188, 879–897. [Google Scholar] [CrossRef]
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J.; et al. Genome and time-of-day transcriptome of Wolffia australiana link morphological minimization with gene loss and less growth control. Genome Res. 2020, 31, 225–238. [Google Scholar] [CrossRef]
- Ernst, E. Status of the Lemna gibba 7742a and Lemna minor 8627 genomes. ISCDRA 2016, 3, 9–10. [Google Scholar]
- Sree, K.S.; Appenroth, K.-J. Worldwide Genetic Resources of Duckweed: Stock Collections. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 39–46. ISBN 978-3-030-11044-4. [Google Scholar]
- Lam, E.; Appenroth, K.J.; Ma, Y.; Shoham, T.; Sree, K.S. Registration of Duckweed Clones/Strains-Future Approach. Duckweed Forum 2020, 8, 35–37. [Google Scholar]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How Green Is My River? A New Paradigm of Eutrophication in Rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). World Fertilizer Trends and Outlook to 2022. 2019. Available online: https://www.fao.org/3/ca6746en/CA6746EN.pdf?eloutlink=imf2fao (accessed on 20 October 2022).
- Sylvester-Bradley, R.; Kindred, D.R. Analysing Nitrogen Responses of Cereals to Prioritize Routes to the Improvement of Nitrogen Use Efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef]
- He, F.; Wu, Z. Application of Aquatic Plants in Sewage Treatment and Water Quality Improvement. Chin. Bull. Bot. 2003, 20, 641–647. [Google Scholar]
- Cao, L.; Wang, W. Wastewater Management in Freshwater Pond Aquaculture in China. In Sustainability in Food and Water: An Asian Perspective; Sumi, A., Fukushi, K., Honda, R., Hassan, K.M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 181–190. [Google Scholar]
- Zimmo, O.R.; van der Steen, N.P.; Gijzen, H.J. Nitrogen Mass Balance across Pilot-Scale Algae and Duckweed-Based Wastewater Stabilisation Ponds. Water Res. 2004, 38, 913–920. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.J. Growing Duckweed for Biofuel Production: A Review. Plant Biol. J. 2015, 17, 16–23. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Peterson, A.; Zha, X.; Cheng, J.; Li, S.; Cui, D.; Zhu, H.; Kishchenko, O.; Borisjuk, N. Biodiversity of Duckweeds in Eastern China and Their Potential for Bioremediation of Municipal and Industrial Wastewater. J. Geosci. Environ. Protect. 2018, 6, 108–116. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Yu, L.; Zhu, M.; Xu, H.; Zhao, J.; Ma, Y.; Zhou, G. Comparative Analysis of Duckweed Cultivation with Sewage Water and SH Media for Production of Fuel Ethanol. PLoS ONE 2014, 9, e115023. [Google Scholar] [CrossRef]
- Cheng, J.J.; Stomp, A.-M. Growing Duckweed to Recover Nutrients from Wastewaters and for Production of Fuel Ethanol and Animal Feed. Clean Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Tavares, F.A.; Belli Filho, P. High Nutrient Removal Rate from Swine Wastes and Protein Biomass Production by Full-Scale Duckweed Ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Y.-P.; Zhang, T.-T.; Zhao, Y.; Shen, Y.; Huang, L.; Gao, X.; Guo, J.-S. The Logistic Growth of Duckweed (Lemna minor) and Kinetics of Ammonium Uptake. Environ. Technol. 2014, 35, 562–567. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Babourina, O.; Rengel, Z.; Yang, X.E.; Pu, P.M. Ammonium and Nitrate Uptake by the Floating Plant Landoltia punctata. Ann. Bot. 2007, 99, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Fang, Y.; Jin, Y.; Yi, Z.; Li, J.; Du, A.; He, K.; Huang, Y.; Zhao, H. Ammonium Detoxification Mechanism of Ammonium-Tolerant Duckweed (Landoltia punctata) Revealed by Carbon and Nitrogen Metabolism under Ammonium Stress. Environ. Pollut. 2021, 277, 116834. [Google Scholar] [CrossRef]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The Dynamics of NO3− and NH4+ Uptake in Duckweed Are Coordinated with the Expression of Major Nitrogen Assimilation Genes. Plants 2022, 11, 11. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Lu, C.; Shen, Z.; Xu, F.; Chen, Y. Characterization of Pseudomonas Mendocina LR Capable of Removing Nitrogen from Various Nitrogen-Contaminated Water Samples When Cultivated with Cyperus alternifolius L. J. Biosci. Bioeng. 2012, 114, 182–187. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen Transformations in Modern Agriculture and the Role of Biological Nitrification Inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, K.; Geisen, S.; Yang, W.; Yan, Y.; Gu, D.; Liu, N.; Borisjuk, N.; Luo, Y.; Friman, V.-P. The Effect of Microbial Inoculant Origin on the Rhizosphere Bacterial Community Composition and Plant Growth-Promotion. Plant Soil 2020, 452, 105–117. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Bachand, P.A.M.; Horne, A.J. Denitrification in Constructed Free-Water Surface Wetlands: II. Effects of Vegetation and Temperature. Ecol. Eng. 1999, 14, 17–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Y.; Zhang, H.; Shi, W. Aerobic Denitrifying Characteristics of Duckweed Rhizosphere Bacterium RWX31. Afr. J. Microbiol. Res. 2013, 7, 211–219. [Google Scholar]
- Ashok, V.; Hait, S. Remediation of Nitrate-Contaminated Water by Solid-Phase Denitrification Process—A Review. Environ. Sci. Pollut. Res. 2015, 22, 8075–8093. [Google Scholar] [CrossRef]
- Borisjuk, N.; Peterson, A.A.; Lv, J.; Qu, G.; Luo, Q.; Shi, L.; Chen, G.; Kishchenko, O.; Zhou, Y.; Shi, J. Structural and Biochemical Properties of Duckweed Surface Cuticle. Front. Chem. 2018, 6, 317. [Google Scholar] [CrossRef]
- Lu, Y.; Kronzucker, H.J.; Shi, W. Stigmasterol Root Exudation Arising from Pseudomonas Inoculation of the Duckweed Rhizosphere Enhances Nitrogen Removal from Polluted Waters. Environ. Pollut 2021, 287, 117587. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Nakai, S.; Hosomi, M.; Zhang, H.; Kronzucker, H.J.; Shi, W. Stimulation of Nitrogen Removal in the Rhizosphere of Aquatic Duckweed by Root Exudate Components. Planta 2014, 239, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.C.; Koch, R. Influence of Exposure Concentration and Duration on Effects and Recovery of Lemna minor Exposed to the Herbicide Norflurazon. Arch. Environ. Contam. Toxicol. 2013, 64, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Žurga, P.; Brusić, I.; Horvatić, J.; Moslavac, M. Growth Inhibition and Recovery Patterns of Common Duckweed Lemna minor L. after Repeated Exposure to Isoproturon. Ecotoxicology 2020, 29, 1538–1551. [Google Scholar] [CrossRef]
- Burns, M.; Hanson, M.L.; Prosser, R.S.; Crossan, A.N.; Kennedy, I.R. Growth Recovery of Lemna gibba and Lemna minor Following a 7-Day Exposure to the Herbicide Diuron. Bull. Environ. Contam. Toxicol. 2015, 95, 150–156. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Oxidative Stress in Duckweed (Lemna minor L.) Induced by Glyphosate: Is the Mitochondrial Electron Transport Chain a Target of This Herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dosnon-Olette, R.; Couderchet, M.; Oturan, M.A.; Oturan, N.; Eullaffroy, P. Potential Use of Lemna minor for the Phytoremediation of Isoproturon and Glyphosate. Int. J. Phytoremediation 2011, 13, 601–612. [Google Scholar] [CrossRef]
- Mitsou, K.; Koulianou, A.; Lambropoulou, D.; Pappas, P.; Albanis, T.; Lekka, M. Growth Rate Effects, Responses of Antioxidant Enzymes and Metabolic Fate of the Herbicide Propanil in the Aquatic Plant Lemna minor. Chemosphere 2006, 62, 275–284. [Google Scholar] [CrossRef]
- Prasertsup, P.; Ariyakanon, N. Removal of Chlorpyrifos by Water Lettuce (Pistia Stratiotes L.) and Duckweed (Lemna minor L.). Int. J. Phytoremediation 2011, 13, 383–395. [Google Scholar] [CrossRef]
- Olette, R.; Couderchet, M.; Biagianti, S.; Eullaffroy, P. Toxicity and Removal of Pesticides by Selected Aquatic Plants. Chemosphere 2008, 70, 1414–1421. [Google Scholar] [CrossRef]
- Dosnon-Olette, R.; Couderchet, M.; El Arfaoui, A.; Sayen, S.; Eullaffroy, P. Influence of Initial Pesticide Concentrations and Plant Population Density on Dimethomorph Toxicity and Removal by Two Duckweed Species. Sci. Total Environ. 2010, 408, 2254–2259. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The Treatment of Duckweed with a Plant Biostimulant or a Safener Improves the Plant Capacity to Clean Water Polluted by Terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef]
- Tront, J.M.; Saunders, F.M. Sequestration of a Fluorinated Analog of 2,4-Dichlorophenol and Metabolic Products by L. minor as Evidenced by 19F NMR. Environ. Pollut. 2007, 145, 708–714. [Google Scholar] [CrossRef]
- Yılmaz, Ö.; Taş, B. Feasibility and Assessment of the Phytoremediation Potential of Green Microalga and Duckweed for Zeta-Cypermethrin Insecticide Removal. Desalination Water Treat. 2021, 209, 131–143. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Nassiri Koopaei, N.; Abdollahi, M. Health Risks Associated with the Pharmaceuticals in Wastewater. DARU J. Pharm. Sci. 2017, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.d.J.S.; Kulzer, J.; Pujol de Lima, P.d.R.; Barbosa, S.C.; Primel, E.G. Updated Knowledge, Partitioning and Ecological Risk of Pharmaceuticals and Personal Care Products in Global Aquatic Environments. Environ. Sci. Process. Impacts 2022, 24, 1982–2008. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of Pharmaceutical Active Compounds in Wastewater by Constructed Wetlands: Performance and Mechanisms. J. Environ. Manag. 2023, 325, 116478. [Google Scholar] [CrossRef]
- Cleuvers, M. Initial Risk Assessment for Three β-Blockers Found in the Aquatic Environment. Chemosphere 2005, 59, 199–205. [Google Scholar] [CrossRef]
- Kaza, M.; Nalecz-Jawecki, G.; Sawicki, J. The Toxicity of Selected Pharmaceuticals to the Aquatic Plant Lemn. Minor. Fresenius Environ. Bull. 2007, 16, 524–531. [Google Scholar]
- Reinhold, D.; Vishwanathan, S.; Park, J.J.; Oh, D.; Michael Saunders, F. Assessment of Plant-Driven Removal of Emerging Organic Pollutants by Duckweed. Chemosphere 2010, 80, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Amy-Sagers, C.; Reinhardt, K.; Larson, D.M. Ecotoxicological Assessments Show Sucralose and Fluoxetine Affect the Aquatic Plant, Lemna minor. Aquat. Toxicol. 2017, 185, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Cascone, A.; Forni, C.; Migliore, L. Flumequine Uptake and the Aquatic Duckweed, Lemna minor L. Water Air Soil Pollut. 2004, 156, 241–249. [Google Scholar] [CrossRef]
- Gomes, M.P.; Gonçalves, C.A.; de Brito, J.C.M.; Souza, A.M.; da Silva Cruz, F.V.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin Induces Oxidative Stress in Duckweed (Lemna minor L.): Implications for Energy Metabolism and Antibiotic-Uptake Ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of Amoxicillin to the Duckweed Spirodela polyrhiza: Growth, Oxidative Stress, Biochemical Traits and Antibiotic Degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity and Degradation of Antibiotic Ofloxacin in Duckweed (Spirodela polyrhiza) System. Ecotoxicol. Environ. Saf. 2019, 179, 88–95. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Gatidou, G.; Damalas, D.; Thomaidis, N.S.; Stasinakis, A.S. Fate of Antimicrobials in Duckweed Lemna minor Wastewater Treatment Systems. J. Hazard. Mater. 2017, 330, 116–126. [Google Scholar] [CrossRef]
- Matamoros, V.; Nguyen, L.X.; Arias, C.A.; Salvadó, V.; Brix, H. Evaluation of Aquatic Plants for Removing Polar Microcontaminants: A Microcosm Experiment. Chemosphere 2012, 88, 1257–1264. [Google Scholar] [CrossRef]
- Pietrini, F.; Di Baccio, D.; Aceña, J.; Pérez, S.; Barceló, D.; Zacchini, M. Ibuprofen Exposure in Lemna gibba L.: Evaluation of Growth and Phytotoxic Indicators, Detection of Ibuprofen and Identification of Its Metabolites in Plant and in the Medium. J. Hazard. Mater. 2015, 300, 189–193. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. Removal of Selected Emerging PPCP Compounds Using Greater Duckweed (Spirodela polyrhiza) Based Lab-Scale Free Water Constructed Wetland. Water Res. 2017, 126, 252–261. [Google Scholar] [CrossRef]
- Di Baccio, D.; Pietrini, F.; Bertolotto, P.; Pérez, S.; Barcelò, D.; Zacchini, M.; Donati, E. Response of Lemna gibba L. to High and Environmentally Relevant Concentrations of Ibuprofen: Removal, Metabolism and Morpho-Physiological Traits for Biomonitoring of Emerging Contaminants. Sci. Total Environ. 2017, 584–585, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Torbati, S. Feasibility and Assessment of the Phytoremediation Potential of Duckweed for Triarylmethane Dye Degradation with the Emphasis on Some Physiologicalresponses and Effect of Operational Parameters. Turk. J. Biol. 2015, 39, 438–446. [Google Scholar] [CrossRef]
- Can-Terzi, B.; Goren, A.Y.; Okten, H.E.; Sofuoglu, S.C. Biosorption of Methylene Blue from Water by Live Lemna minor. Environ. Technol. Innov. 2021, 22, 101432. [Google Scholar] [CrossRef]
- Torbati, S. Toxicological Risks of Acid Bordeaux B on Duckweed and the Plant Potential for Effective Remediation of Dye-Polluted Waters. Environ. Sci. Pollut. Res. 2019, 26, 27699–27711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, L. Removal of eight perfluoroalkyl acids from aqueous solutions by aeration and duckweed. Sci. Total Environ. 2020, 724, 138357. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Nwachukwu, E.O.; Sikoki, F.D. Assessing and Modelling the Efficacy of Lemna Paucicostata for the Phytoremediation of Petroleum Hydrocarbons in Crude Oil-Contaminated Wetlands. Sci. Rep. 2020, 10, 8489. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. Clean Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Uysal, Y.; Taner, F. Bioremoval of Cadmium by Lemna minor in Different Aquatic Conditions. Clean Soil Air Water 2010, 38, 370–377. [Google Scholar] [CrossRef]
- Sekomo, C.B.; Rousseau, D.P.L.; Saleh, S.A.; Lens, P.N.L. Heavy Metal Removal in Duckweed and Algae Ponds as a Polishing Step for Textile Wastewater Treatment. Ecol. Eng. 2012, 44, 102–110. [Google Scholar] [CrossRef]
- Parra, L.-M.M.; Torres, G.; Arenas, A.D.; Sánchez, E.; Rodríguez, K. Phytoremediation of Low Levels of Heavy Metals Using Duckweed (Lemna minor). In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 451–463.minor). In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 451–463. [Google Scholar]
- Abdallah, M.A.M. Phytoremediation of Heavy Metals from Aqueous Solutions by Two Aquatic Macrophytes, Ceratophyllum Demersum and Lemna gibba L. Environ. Technol. 2012, 33, 1609–1614. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Cadmium Removal by Lemna minor and Spirodela polyrhiza. Int. J. Phytoremediation 2014, 16, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Vieira, M.N.; Marques, J.E.; Pereira, R. Bioremediation of an Iron-Rich Mine Effluent by Lemna minor. Int. J. Phytoremediation 2014, 16, 1228–1240. [Google Scholar] [CrossRef]
- Miranda, A.F.; Muradov, N.; Gujar, A.; Stevenson, T.; Nugegoda, D.; Ball, A.S.; Mouradov, A. Application of Aquatic Plants for the Treatment of Selenium-Rich Mining Wastewater and Production of Renewable Fuels and Petrochemicals. J. Sust. Bioenergy Syst. 2014, 04, 97–112. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Lead and Cadmium Removal from Water Using Duckweed—Lemna gibba L.: Impact of PH and Initial Metal Load. Alex. Eng. J. 2015, 54, 1297–1304. [Google Scholar] [CrossRef]
- Bokhari, S.H.; Ahmad, I.; Mahmood-Ul-Hassan, M.; Mohammad, A. Phytoremediation Potential of Lemna minor L. for Heavy Metals. Int. J. Phytoremediation 2016, 18, 25–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, T.; Kishchenko, O. Potential of Lemnoideae Species for Phytoremediation of Fresh Water with Elevated Manganese Concentration. Innov. Biosyst. Bioeng. 2019, 3, 232–238. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.S.; Appenroth, K.-J. Duckweeds for Water Remediation and Toxicity Testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Md Din, M.F.; Dahalan, F.A.; Kamyab, H. Comprehensive Review on Phytotechnology: Heavy Metals Removal by Diverse Aquatic Plants Species from Wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, Y.; Li, X.; Yang, C.; Han, Z.; Zeng, G.; Lu, L.; He, S. Effect of Zinc Ions on Nutrient Removal and Growth of Lemna Aequinoctialis from Anaerobically Digested Swine Wastewater. Bioresour. Technol. 2018, 249, 457–463. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Liu, C.; Kang, X.; Chen, L.; Liang, X.; Jin, L. Duckweed Diversity Decreases Heavy Metal Toxicity by Altering the Metabolic Function of Associated Microbial Communities. Chemosphere 2018, 203, 76–82. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Duan, D.; Li, H.; Lei, T.; Wang, M.; Zhao, H.; Zhao, Y. The Influence of Duckweed Species Diversity on Ecophysiological Tolerance to Copper Exposure. Aquat. Toxicol. 2015, 164, 92–98. [Google Scholar] [CrossRef]
- Stout, L.M.; Dodova, E.N.; Tyson, J.F.; Nüsslein, K. Phytoprotective Influence of Bacteria on Growth and Cadmium Accumulation in the Aquatic Plant Lemna minor. Water Res. 2010, 44, 4970–4979. [Google Scholar] [CrossRef]
- Chen, L.C.; Fang, Y.; Jin, Y.L.; Chen, Q.; Zhao, Y.G.; Xiao, Y.; Zhao, H. Biosorption of Pb2+ by dried powder of duckweed (Lemna aequinoctialis). Chin. J. Appl. Environ. Biol. 2013, 19, 1046–1052. (In Chinese) [Google Scholar] [CrossRef]
- Nie, X.Q.; Dong, F.Q.; Liu, N.; Zhang, D.; Liu, M.X.; Yang, J.; Zhang, W. Biosorption and biomineralization of uranium (VI) from aqueous solutions by Landoltia puntata. Spectrosc. Spect. Anal. 2015, 35, 2613–2619. (In Chinese) [Google Scholar]
- Li, Y.; Yang, C.; Zhong, Y.; Tang, J. Adsorption properties of the dry powers of two duckweed species for Cd2+. Jiangsu Agric. Sci. 2017, 45, 248–254. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in Plants: Deficiency and Toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Kaur, R.; Kumar, A.; Bhardwaj, R. Biogeochemical Cycling, Tolerance Mechanism and Phytoremediation Strategies of Boron in Plants: A Critical Review. Chemosphere 2022, 300, 134505. [Google Scholar] [CrossRef]
- Davis, S.M.; Drake, K.D.; Maier, K.J. Toxicity of Boron to the Duckweed, Spirodella polyrrhiza. Chemosphere 2002, 48, 615–620. [Google Scholar] [CrossRef]
- Del-Campo Marín, C.M.; Oron, G. Boron Removal by the Duckweed Lemna Gibba: A Potential Method for the Remediation of Boron-Polluted Waters. Water Res. 2007, 41, 4579–4584. [Google Scholar] [CrossRef]
- Gür, N.; Türker, O.C.; Böcük, H. Toxicity Assessment of Boron (B) by Lemna minor L. and Lemna gibba L. and Their Possible Use as Model Plants for Ecological Risk Assessment of Aquatic Ecosystems with Boron Pollution. Chemosphere 2016, 157, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Türker, O.C.; Baran, T. A Combination Method Based on Chitosan Adsorp-tion and Duckweed (Lemna gibba L.) Phytoremediation for Boron (B) Removal from Drinking Water. Int. J. Phytoremediation 2018, 20, 175–183. [Google Scholar] [CrossRef]
- Türker, O.C.; Yakar, A.; Gür, N. Bioaccumulation and Toxicity Assessment of Irrigation Water Contaminated with Boron (B) Using Duckweed (Lemna gibba L.) in a Batch Reactor System. J. Hazard. Mater. 2017, 324, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Türker, O.C. Simultaneous Boron (B) Removal and Electricity Generation from Domestic Wastewater Using Duckweed-Based Wastewater Treatment Reactors Coupled with Microbial Fuel Cell. J. Environ. Manag. 2018, 228, 20–31. [Google Scholar] [CrossRef]
- Türker, O.C.; Yakar, A.; Türe, C.; Saz, Ç. Boron (B) Removal and Bioelec-tricity Captured from Irrigation Water Using Engineered Duckweed-Microbial Fuel Cell: Effect of Plant Species and Vegetation Structure. Environ. Sci. Pollut. Res. Int. 2019, 26, 31522–31536. [Google Scholar] [CrossRef]
- Liu, C.; Gu, W.; Dai, Z.; Li, J.; Jiang, H.; Zhang, Q. Boron Accumulation by Lemna minor L. under Salt Stress. Sci. Rep. 2018, 8, 8954. [Google Scholar] [CrossRef]
- Uruc Parlak, K. Effects of Boron and NaCl on Antioxidant Defence Mechanisms in Duckweeds (Spirodela polyrhiza L.). Pak. J. Biol. Sci. 2021, 24, 989–996. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic Arsenic: Toxicity, Speciation, Transfor-mations, and Remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Aquatic Arsenic: Phytoremediation Using Floating Macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Kumar, P.; Ohri, P.; Bhardwaj, R.; Alam, P.; Ah-mad, P. Arsenic as Hazardous Pollutant: Perspectives on Engineering Remediation Tools. Sci. Total Environ. 2022, 838, 155870. [Google Scholar] [CrossRef]
- Mkandawire, M.; Taubert, B.; Dudel, E.G. Capacity of Lemna gibba L. (Duckweed) for Uranium and Arsenic Phytoremediation in Mine Tailing Waters. Int. J. Phytoremediation 2004, 6, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, M.; Dudel, E.G. Accumulation of Arsenic in Lemna gibba L. (Duckweed) in Tailing Waters of Two Abandoned Uranium Mining Sites in Saxony, Germany. Sci. Total Environ. 2005, 336, 81–89. [Google Scholar] [CrossRef]
- Charlier, H.A.J.; Albertson, C.; Thornock, C.; Warner, L.; Hurst, T.; Ellis, R. Comparison of the Effects of Arsenic (V), Cadmium (II), and Mercury (II) Single Metal and Mixed Metal Exposure in Radish, Raphanus Sativus, Fescue Grass, Festuca Ovina, and Duckweed, Lemna minor. Bull. Environ. Contam. Toxicol. 2005, 75, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, S.; Guédez, M.; Lué-Merú, M.P.; Nelson, G.; Alvaro, A.; Jesús, A.C.; Gyula, Z. Arsenic Removal from Waters by Bioremediation with the Aquatic Plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour. Technol. 2008, 99, 8436–8440. [Google Scholar] [CrossRef]
- Duman, F.; Ozturk, F.; Aydin, Z. Biological Responses of Duckweed (Lemna minor L.) Exposed to the Inorganic Arsenic Species As(III) and As(V): Ef-fects of Concentration and Duration of Exposure. Ecotoxicology 2010, 19, 983–993. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Pratas, J.; Prasad, M.N.V. Accumulation of Arsenic by Aquatic Plants in Large-Scale Field Conditions: Opportunities for Phytoremediation and Bioindication. Sci. Total Environ. 2012, 433, 390–397. [Google Scholar] [CrossRef]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic Up-take by Lemna minor in Hydroponic System. Int. J. Phytoremediation 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Yang, G.-L.; Yang, M.-X.; Lv, S.-M.; Tan, A.-J. The Effect of Chelating Agents on Iron Plaques and Arsenic Accumulation in Duckweed (Lemna minor). J. Hazard. Mater. 2021, 419, 126410. [Google Scholar] [CrossRef]
- De Souza, T.D.; Borges, A.C.; de Matos, A.T.; Veloso, R.W.; Braga, A.F. Kinetics of Arsenic Absorption by the Species Eichhornia crassipes and Lemna Valdiviana under Optimized Conditions. Chemosphere 2018, 209, 866–874. [Google Scholar] [CrossRef]
- De Souza, T.D.; Borges, A.C.; Braga, A.F.; Veloso, R.W.; Teixeira de Matos, A. Phytoremediation of Arsenic-Contaminated Water by Lemna Valdiviana: An Optimization Study. Chemosphere 2019, 234, 402–408. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T.; Okumura, C.; Rahman, M.M. Arsenic Accumulation in Duckweed (Spirodela polyrhiza L.): A Good Option for Phytoremediation. Chemosphere 2007, 69, 493–499. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Ueda, K.; Maki, T.; Rahman, M.M. Influence of EDTA and Chemical Species on Arsenic Accumulation in Spirodela polyrhiza L. (Duckweed). Ecotoxicol. Environ. Saf. 2008, 70, 311–318. [Google Scholar] [CrossRef]
- Seth, C.S.; Chaturvedi, P.K.; Misra, V. Toxic Effect of Arsenate and Cadmium Alone and in Combination on Giant Duckweed (Spirodela polyrrhiza L.) in Response to Its Accumulation. Environ. Toxicol. 2007, 22, 539–549. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Liu, Y.; Chen, B. Arsenic Uptake, Accumulation and Phytofiltration by Duckweed (Spirodela polyrhiza L.). J. Environ. Sci. 2011, 23, 601–606. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, F.-J.; Huang, Q.; Williams, P.N.; Sun, G.-X.; Zhu, Y.-G. Arsenic Uptake and Speciation in the Rootless Duckweed Wolffia globosa. New Phytol. 2009, 182, 421–428. [Google Scholar] [CrossRef]
- Zhang, X.; Uroic, M.K.; Xie, W.-Y.; Zhu, Y.-G.; Chen, B.-D.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Phytochelatins Play a Key Role in Arsenic Accumulation and Tolerance in the Aquatic Macrophyte Wolffia globosa. Environ. Pollut. 2012, 165, 18–24. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Su, J.-Q.; Zhu, Y.-G. Arsenite Oxidation by the Phyllosphere Bacterial Community Associated with Wolffia Australiana. Environ. Sci. Technol. 2014, 48, 9668–9674. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Fang, Y.; Xu, Y.L.; Tan, L.; Li, Q.; Liu, Y.; Lai, F.; Jin, Y.L.; Du, A.P.; He, K.Z.; et al. Frond transformation system mediated by Agrobacterium tumefaciens for Lemna minor. Plant Mol. Biol. 2018, 98, 319–331. [Google Scholar] [CrossRef]
- Heenatigala, P.P.M.; Yang, J.; Bishopp, A.; Sun, Z.; Li, G.; Kumar, S.; Hu, S.; Wu, Z.; Lin, W.; Yao, L.; et al. Development of Efficient Protocols for Stable and Transient Gene Transformation for Wolffia globosa Using Agrobacterium. Front. Chem. 2018, 6, 227. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xu, S.; Tang, X.; Zhao, J.; Yu, C.; He, G.; Xu, H.; Wang, S.; Tang, Y.; et al. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing in Lemna aequinoctialis. Plant Biotechnol. J. 2019, 17, 2143–2152. [Google Scholar] [CrossRef]
- Peterson, A.; Kishchenko, O.; Zhou, Y.; Vasylenko, M.; Giritch, A.; Sun, J.; Borisjuk, N.; Kuchuk, M. Robust Agrobacterium-Mediated Transient Expression in Two Duckweed Species (Lemnaceae) Directed by Non-Replicating, Replicating, and Cell-to-Cell Spreading Vectors. Front. Bioeng. Biotechnol. 2021, 9, 761073. [Google Scholar] [CrossRef] [PubMed]
- Megateli, S.; Semsari, S.; Couderchet, M. Toxicity and removal of heavy metals (cadmium, copper, and zinc) by Lemna gibba. Ecotoxicol. Environ. Saf. 2009, 72, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.D.; Akbulut, H. Effect of circulation on wastewater treatment by Lemna gibba and Lemna minor (Floating Aquatic Macrophytes). Int. J. Phytoremediation 2011, 13, 970–984. [Google Scholar] [CrossRef]
- Ekta, C.; Praveen, S. Chromium and cadmium removal from wastewater using duckweed-Lemna gibba L. and ultrastructural deformation due to metal toxicity. Int. J. Phytoremediation 2019, 21, 279–286. [Google Scholar]
- Khellaf, N.; Zerdaoui, M. Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicol. (Lond. Engl.) 2010, 19, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Romero-Hernández, J.A.; Amaya-Chávez, A.; Balderas-Hernández, P.; Roa-Morales, G.; González-Rivas, N. Balderas-Plata, M.A. Tolerance and hyperaccumulation of a mixture of heavy metals (Cu, Pb, Hg, and Zn) by four aquatic macrophytes. Int. J. Phytoremediation 2017, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Conte, B.; Cobianchi, R.C.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, accumulation, and removal of heavy metals by three aquatic macrophytes, Int. J. Phytoremediat. 2012, 14, 374–387. [Google Scholar] [CrossRef]
- Kaur, L.; Gadgil, K.; Sharma, S. Role of pH in the accumulation of lead and nickel by common duckweed (Lemna minor). Int. J. Bioassays 2012, 12, 191–195. [Google Scholar]
- Azeez, N.M.; Sabbar, A.A. Efficiency of duckweed (Lemna minor L.) in phytotreatment of wastewater pollutants from Basrah oil refinery. J. Appl. Phytotechnology Environ. Sanit. 2012, 1, 163–172. [Google Scholar]
- Daud, M.K.; Ali, S.; Abbas, Z.; Zaheer, I.E.; Riaz, M.A.; Malik, A.; Hussain, A.; Rizwan, M.; Rehman, M.Z.; Zhu, S.J. Potential of duckweed (Lemna minor) for the phytoremediation of landfill leachate. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Jafari, N.; Akhavan, M. Effect of pH and heavy metal concentration on phytoaccumulation of zinc by three duckweed species. Am.–Eurasian J. Agric. Environ. Sci. 2011, 10, 34–41. [Google Scholar]
- Kumar, M.V.; Tripathi, B.D. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef]
- Al-Balawna, Z.A. Remove heavy metals (Cd and Pb) from irrigation water in Jordan valley by using duckweeds (Spirodela polyrhiza). Acta Sci. Agric. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Loveson, A.; Sivalingam, R.; Syamkumar, R. Aquatic macrophyte Spirodela polyrrhiza as a phytoremediation tool in polluted wetland water from Eloor, Ernakulam district, Kerala. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 51–58. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. https://doi.org/10.3390/plants12030589
Zhou Y, Stepanenko A, Kishchenko O, Xu J, Borisjuk N. Duckweeds for Phytoremediation of Polluted Water. Plants. 2023; 12(3):589. https://doi.org/10.3390/plants12030589
Chicago/Turabian StyleZhou, Yuzhen, Anton Stepanenko, Olena Kishchenko, Jianming Xu, and Nikolai Borisjuk. 2023. "Duckweeds for Phytoremediation of Polluted Water" Plants 12, no. 3: 589. https://doi.org/10.3390/plants12030589
APA StyleZhou, Y., Stepanenko, A., Kishchenko, O., Xu, J., & Borisjuk, N. (2023). Duckweeds for Phytoremediation of Polluted Water. Plants, 12(3), 589. https://doi.org/10.3390/plants12030589







