Inter- and Intra-Population Variation of Foliage Calcium and Magnesium in Two Chinese Pine Species
Abstract
:1. Introduction
2. Results
2.1. Difference in Ca and Mg Concentrations among Populations of Two Pines
2.2. Correlation between Calcium and Magnesium
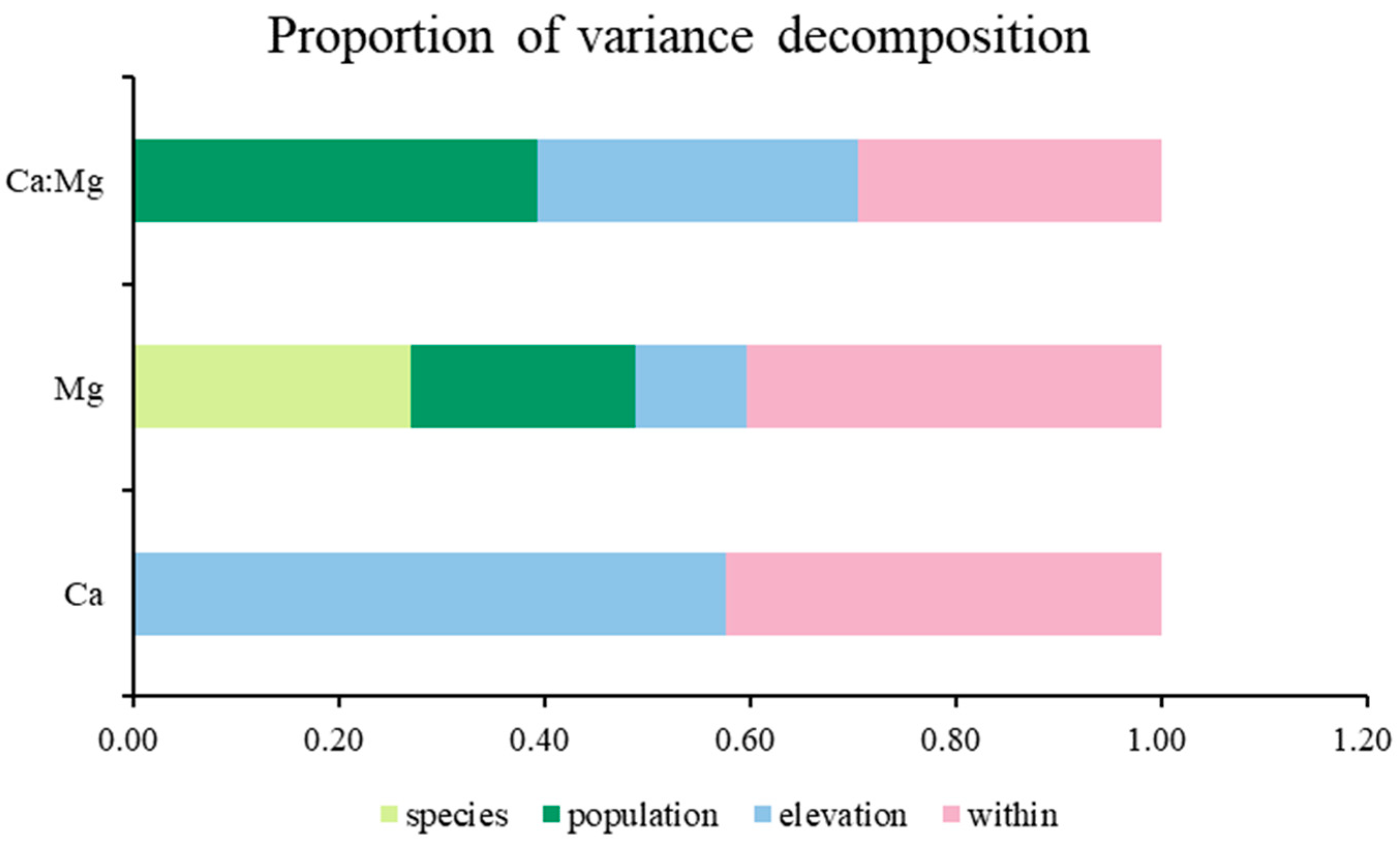
2.3. Source of Variation
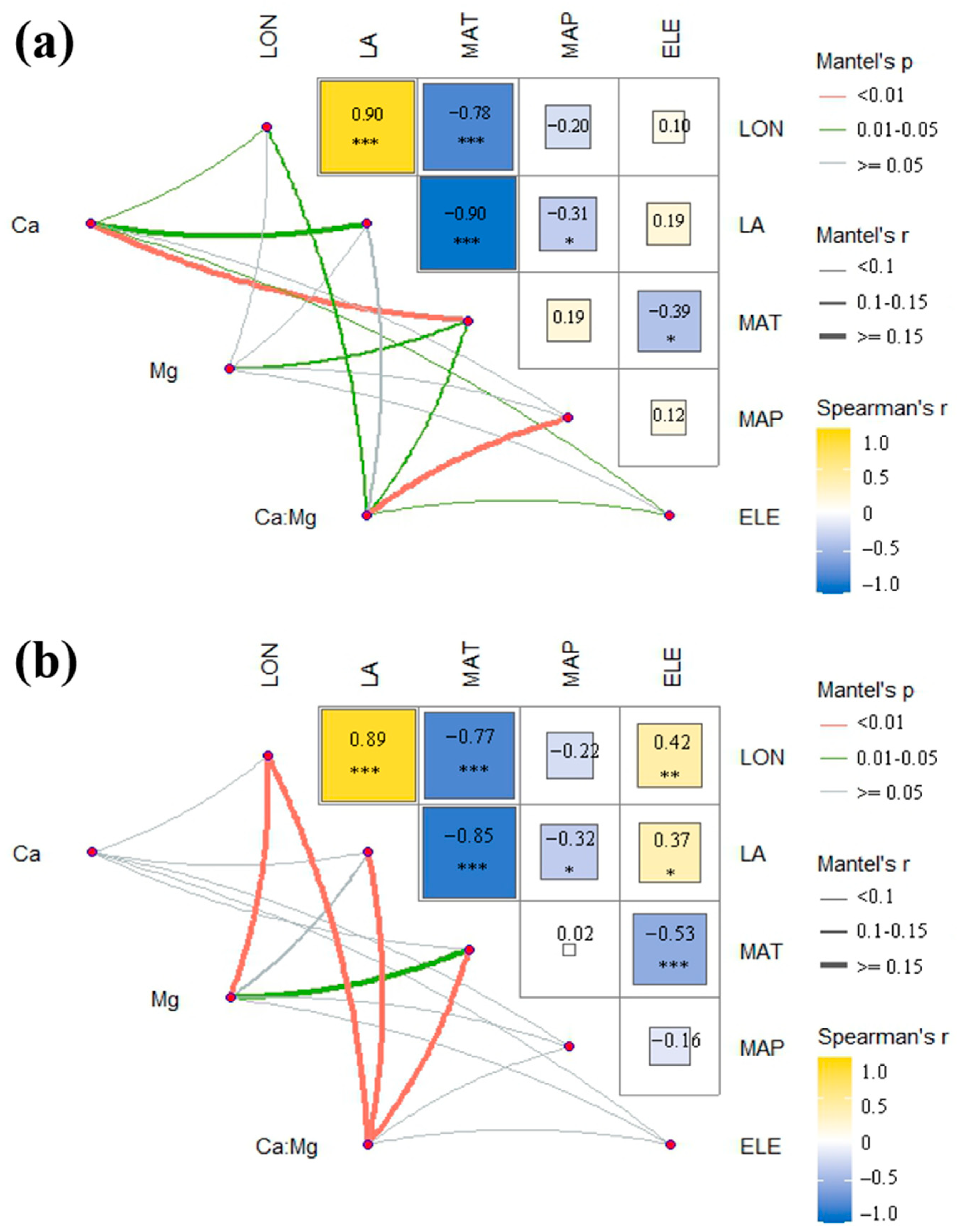
2.4. Correlation with Environmental Factors
3. Discussion
3.1. Concentration of Calcium and Magnesium
3.2. Correlation between Calcium and Magnesium
3.3. Genetic and Environmental Variation of Calcium and Magnesium
4. Materials and Methods
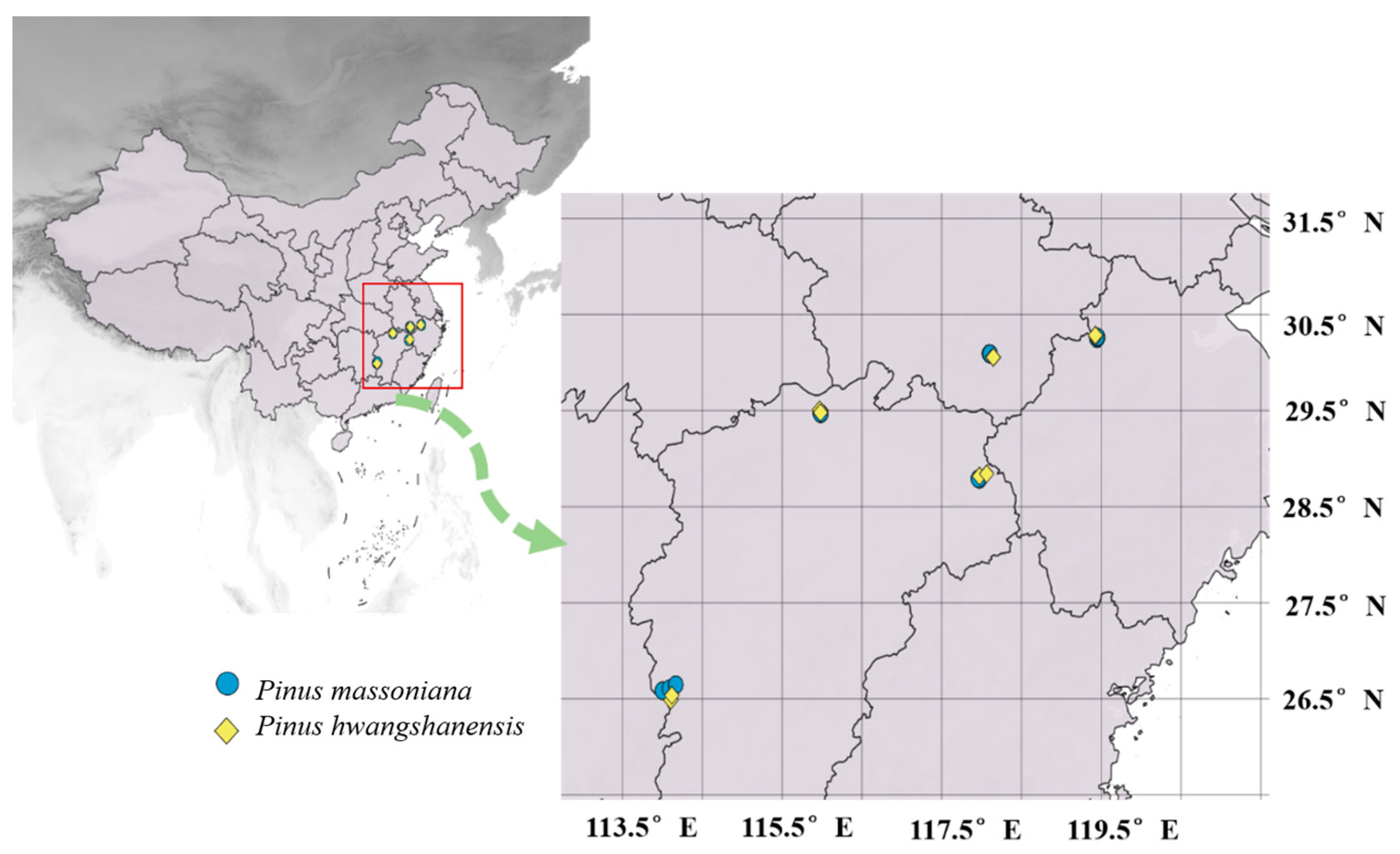
4.1. Plant Sampling
4.2. Chemical Analyses
4.3. Environmental Variables
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium-Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; de Kroon, H. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef]
- Edwards, E.J.; Chatelet, D.S.; Sack, L.; Donoghue, M.J. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. J. Ecol. 2014, 102, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Agren, G.I.; Weih, M. Plant stoichiometry at different scales: Element concentration patterns reflect environment more than genotype. New Phytol. 2012, 194, 944–952. [Google Scholar] [CrossRef]
- Nakajima, N.; Morikawa, H.; Igarashi, S.; Senda, M. Differential effect of calcium and magnesium on mechanical properties of pea stem cell walls. Plant Cell Physiol. 1981, 22, 1305–1315. [Google Scholar]
- Mladkova, P.; Mladek, J.; Hejduk, S.; Hejcman, M.; Pakeman, R.J. Calcium plus magnesium indicates digestibility: The significance of the second major axis of plant chemical variation for ecological processes. Ecol. Lett. 2018, 21, 885–895. [Google Scholar] [CrossRef]
- Podgorska, A.; Burian, M.; Gieczewska, K.; Ostaszewska-Bugajska, M.; Zebrowski, J.; Solecka, D.; Szal, B. Altered Cell Wall Plasticity Can Restrict Plant Growth under Ammonium Nutrition. Front. Plant Sci. 2017, 8, 1344. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, W.S. Preharvest Spraying of CaCl2 Alleviates the Scape Bending of Gerbera ‘Harmony’ Flowers by Strengthening the Pectin Crosslinks through Ca2+ Bonds. Horticulturae 2022, 8, 523. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.F.; Li, L.G.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Wang, J.Q.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T. New Aspects of Magnesium Function: A Key Regulator in Nucleosome Self-Assembly, Chromatin Folding and Phase Separation. Int. J. Mol. Sci. 2019, 20, 4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishfaq, M.; Wang, Y.Q.; Yan, M.W.; Wang, Z.; Wu, L.Q.; Li, C.J.; Li, X.X. Physiological Essence of Magnesium in Plants and Its Widespread Deficiency in the Farming System of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Gerendas, J.; Fuhrs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.L.; Nazim, H.; Liang, Z.S.; Yang, D.F. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z.Y. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Tian, X.Y.; He, D.D.; Bai, S.; Zeng, W.Z.; Wang, Z.; Wang, M.; Wu, L.Q.; Chen, Z.C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Fromm, J. Wood formation of trees in relation to potassium and calcium nutrition. Tree Physiol. 2010, 30, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen, B.; Behling, S.J.; Lawrence, G.B.; Sullivan, J.H. Photosynthetic and Growth Response of Sugar Maple (Acer saccharum Marsh.) Mature Trees and Seedlings to Calcium, Magnesium, and Nitrogen Additions in the Catskill Mountains, NY, USA. PLoS ONE 2015, 10, e0136148. [Google Scholar] [CrossRef] [PubMed]
- Lautner, S.; Ehlting, B.; Windeisen, E.; Rennenberg, H.; Matyssek, R.; Fromm, J. Calcium nutrition has a significant influence on wood formation in poplar. New Phytol. 2007, 173, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Baukloh, C.; Fromm, J. The effect of calcium starvation on assimilate partitioning and mineral distribution of the phloem. J. Exp. Bot. 1993, 44, 1703–1707. [Google Scholar] [CrossRef]
- Westermark, U. Calcium promoted phenolic coupling by superoxide radical—A possible lignification reaction in wood. Wood Sci. Technol. 1982, 16, 71–78. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Allen, H.L.; McKeand, S.E.; Dougherty, P.M. Stem sinuosity in loblolly pine with nitrogen and calcium additions. Forest Ecol. Manag. 2012, 265, 55–61. [Google Scholar] [CrossRef]
- Alves, E.E.N.; Rodriguez, D.R.O.; de Azevedo Rocha, P.; Vergütz, L.; Junior, L.S.; Hesterberg, D.; Pessenda, L.C.R.; Tomazello-Filho, M.; da Costa, L.M. Synchrotron-based X-ray microscopy for assessing elements distribution and speciation in mangrove tree-rings. Results Chem. 2021, 3, 100121. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; Hevia, A.; Sanchez-Salguero, R.; Santini, L.; de Carvalho, H.W.P.; Roig, F.A.; Tomazello, M. Exploring wood anatomy, density and chemistry profiles to understand the tree-ring formation in Amazonian tree species. Dendrochronologia 2022, 71, 125915. [Google Scholar] [CrossRef]
- Dwivedi, P.; Sucre, E.; Turnblom, E.C.; Harrison, R.B. Investigating Relationships between Nutrient Concentrations, Stem Sinuosity, and Tree Improvement in Douglas-Fir Stands in Western Washington. Forests 2019, 10, 541. [Google Scholar] [CrossRef] [Green Version]
- Hevia, A.; Sanchez-Salguero, R.; Camarero, J.J.; Querejeta, J.I.; Sanguesa-Barreda, G.; Gazol, A. Long-term nutrient imbalances linked to drought-triggered forest dieback. Sci. Total Environ. 2019, 690, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.; Wimmer, R. Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Sun, O.; Payn, T. Magnesium nutrition and photosynthesis in Pinus radiata: Clonal variation and influence of potassium. Tree Physiol. 1999, 19, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Sun, O.J.; Gielen, G.J.H.P.; Sands, R.; Smith, C.T.; Thorn, A.J. Growth, Mg nutrition and photosynthetic activity in Pinus radiata: Evidence that NaCl addition counteracts the impact of low Mg supply. Trees 2001, 15, 335–340. [Google Scholar] [CrossRef]
- Laing, W.; Greer, D.; Sun, O.; Beets, P.; Lowe, A.; Payn, T. Physiological impacts of Mg deficiency in Pinus radiata: Growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Ende, H.; Zöttl, H. Effects of magnesium fertilizer on the vitality and nutrition of a European beech (Fagus sylvatica L.) stand in the Southern Black Forest of West Germany. Water Air Soil Pollut. 1990, 54, 561–566. [Google Scholar] [CrossRef]
- Xue, J.M.; Clinton, P.W.; Leckie, A.C.; Graham, J.D. Magnesium fertilizer, weed control and clonal effects on wood stiffness of juvenile Pinus radiata at two contrasting sites. Forest Ecol. Manag. 2013, 306, 128–134. [Google Scholar] [CrossRef]
- Ovington, J. The calcium and magnesium contents of tree species crown in close stands. New Phytol. 1959, 58, 164–175. [Google Scholar] [CrossRef]
- Tausz, M.; Trummer, W.; Wonisch, A.; Goessler, W.; Grill, D.; Jimenez, M.S.; Morales, D. A survey of foliar mineral nutrient concentrations of Pinus canariensis at field plots in Tenerife. Forest Ecol. Manag. 2004, 189, 49–55. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Nutrient return via litterfall in two contrasting Pinus sylvestris forests in the Pyrenees under different thinning intensities. Forest Ecol. Manag. 2008, 256, 1840–1852. [Google Scholar] [CrossRef]
- Carlson, C.A.; Fox, T.R.; Allen, H.L.; Albaugh, T.J.; Rubilar, R.A.; Stape, J.L. Growth Responses of Loblolly Pine in the Southeast United States to Midrotation Applications of Nitrogen, Phosphorus, Potassium, and Micronutrients. Forest Sci. 2014, 60, 157–169. [Google Scholar] [CrossRef]
- Michopoulos, P.; Farmaki, L.; Thomaidis, I. Foliar status and factors affecting foliar and soil chemistry in a natural aleppo pine forest. J. Plant Nutr. 2017, 40, 1443–1452. [Google Scholar] [CrossRef]
- Pongrac, P.; Baltrenaite, E.; Vavpetic, P.; Kelemen, M.; Kladnik, A.; Budic, B.; Vogel-Mikus, K.; Regvar, M.; Baltrenas, P.; Pelicon, P. Tissue-specific element profiles in Scots pine (Pinus sylvestris L.) needles. Trees 2019, 33, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Gernandt, D.S.; Lopez, G.G.; Garcia, S.O.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.E. Sylva Sinica; China Forestry Publishing House: Beijing, China, 1983; Volume 1. [Google Scholar]
- Zhao, S.; Wang, H. Division of breeding areas and improvement strategies for Pinus massoniana. J. Sichuan For. Sci. Technol. 1991, 12, 42–45. [Google Scholar]
- Tong, Z.; Fan, Y. Dividing the distribution area of Pinus taiwanensis based on climatic and ecologic factors. J. Cent.-South For. Colloge 1993, 13, 81–87. [Google Scholar]
- Wang, L.; Zhuo, Z. On the positional problem of the Pinus taiwanensis forest in the vegetation altitude belts of Mt. Lushan. Acta Phytoecol. Et Geobot. Sin. 1989, 13, 28–35. [Google Scholar]
- Li, M.; He, Z.; Jiang, L.; Gu, X.; Jin, M.; Chen, B.; Liu, J. Distribution pattern and driving factors of species diversity and phylogenetic diversity along altitudinal gradient on the south slope of Daiyun Mountain. Acta Ecol. Sin. 2020, 41, 1148–1157. [Google Scholar]
- Ye, J.; Yan, L.; Chen, M.; Li, Y.; Zheng, X.; Zhang, K.; Zhong, L.; Tang, N. Study on nutrient distribution in fast-growing, high-resistance and polysarcous Tugong pine in the Southeast of Guangxi. J. Shanxi Agric. Sci. 2016, 44, 364–368. [Google Scholar]
- Ma, Z.; Gao, S.; Yang, W.; Wu, F. Seasonal release characteristics of Ca, Mg and Mn of foliar litter of six tree species in subtropical evergreen broadleaved forest. Chin. J. Appl. Ecol. 2015, 26, 2913–2920. [Google Scholar]
- Grubb, P.J.; Turner, I.M.; Burslem, D.P. Mineral nutrient status of coastal hill dipterocarp forest and adinandra belukar in Singapore: Analysis of soil, leaves and litter. J. Trop. Ecol. 1994, 10, 559–577. [Google Scholar] [CrossRef]
- Angel, H.Z.; Priest, J.S.; Stovall, J.P.; Oswald, B.P.; Weng, Y.H.; Williams, H.M. Individual tree and stand-level carbon and nutrient contents across one rotation of loblolly pine plantations on a reclaimed surface mine. New Forest 2019, 50, 733–753. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Rey, M.X.; Couto-Vazquez, A.; Gonzalez-Prieto, S.J. Age and thinning effects on elemental composition of Pinus pinaster and Pinus radiata needles. Iforest 2021, 14, 299–306. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.K. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol. Biochem. 2002, 34, 37–42. [Google Scholar] [CrossRef]
- Jimenez, M.N.; Navarro, F.B. Monthly foliar-nutrient pattern in a semiarid Aleppo pine plantation five years after thinning. Forest Ecol. Manag. 2015, 343, 63–72. [Google Scholar] [CrossRef]
- Yalcin, I.E.; Ozyigit, I.I.; Dogan, I.; Demir, G.; Yarci, C. Determining Element Accumulations in Turkish Red Pine Used as a Bioindicator for Estimating of Existing Pollution on Both Sides of Bosphorus in Istanbul. Fresen Environ. Bull. 2020, 29, 4963–4972. [Google Scholar]
- Lopez-Serrano, F.R.; de las Heras, J.; Gonazalez-Ochoa, A.I.; Garcia-Morote, F.A. Effects of silvicultural treatments and seasonal patterns on foliar nutrients in young post-fire Pinus halepensis forest stands. Forest Ecol. Manag. 2005, 210, 321–336. [Google Scholar] [CrossRef]
- Segura, C.; Fernandez-Ondono, E.; Jimenez, M.N.; Navarro, F.B. Thinning affects the needlefall nutrient return to soil in a semiarid Aleppo pine afforestation while the nutrient dynamics remain unchanged. Forest Ecol. Manag. 2017, 405, 257–270. [Google Scholar] [CrossRef]
- Zhu, M.; Ye, G.; You, S.; You, L.; Bai, Y.; Gao, W. Caloric values and correlations of nutrient elements in three forestation species of a coastal sandy land. J. Zhejiang A F Univ. 2012, 29, 829–834. [Google Scholar]
- Chen, W.; Kou, L.; Jiang, L.; Gao, W.; Yang, H.; Wang, H.; Li, S. Short-term responses of foliar multi-element stoichiometry and nutrient resorption of slash pine to N addition in subtropical China. Chin. J. Appl. Ecol. 2017, 28, 1094–1102. [Google Scholar]
- Sun, Z.-G.; Chen, Q.; Li, X.-Z.; Lu, X.-W.; Chen, X.-Z.; Su, J.-W.; Liu, Y.-C.; Liu, Y.-G.; Huai, K.-Y. The Effect of Zinc, Boron and Magnesium on the Growth of Young Pinus kesiya var. langbianensis Forest. J. West China For. Sci. 2019, 48, 143–147. [Google Scholar]
- Sun, Y.; Wang, J.; Zhang, H.; Wang, J.; Wu, T.; Zhang, P. Variation in needle stoichiometric characters of Pinus thunbergii along a coastal-inlandgradient. Chin. J. Ecol. 2019, 38, 1662–1668. [Google Scholar]
- Tong, Z.; He, L.; Ou, Y.; Wang, Y. Nutrient Accumulation and Distribution Characteristics of Pinus yunnanensis Plantation in Central Yunnan Plateau. Guangxi For. Sci. 2019, 48, 46–50. [Google Scholar]
- Albaugh, J.M.; Blevins, L.; Allen, H.L.; Albaugh, T.J.; Fox, T.R.; Stape, J.L.; Rubilar, R.A. Characterization of Foliar Macro- and Micronutrient Concentrations and Ratios in Loblolly Pine Plantations in the Southeastern United States. South J. Appl. For. 2010, 34, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Bassaco, M.V.M.; Motta, A.C.V.; Maeda, S.; Prior, S.A.; Marques, R.; Magri, E.; Bognola, I.A.; Gomes, J.B.V. Influence of industrial forest residue applications on Pinus taeda: Soil, litter, growth, nutrition, and wood quality characteristics. New Forest 2023, 54, 83–106. [Google Scholar] [CrossRef]
- Huang, D.L.; Gong, X.M.; Liu, Y.G.; Zeng, G.M.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.F.; Xu, P.A.; Cheng, M.; et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef]
- Wen, J.H.; Tao, H.M.; Du, B.M.; Hui, D.F.; Sun, N.X.; Umair, M.; Liu, C.J. Plantations modified leaf elemental stoichiometry compared to the native shrub community in karst areas, Southwest of China. Trees 2021, 35, 987–999. [Google Scholar] [CrossRef]
- Rios, J.J.; Lochlainn, S.O.; Devonshire, J.; Graham, N.S.; Hammond, J.P.; King, G.J.; White, P.J.; Kurup, S.; Broadley, M.R. Distribution of calcium (Ca) and magnesium (Mg) in the leaves of Brassica rapa under varying exogenous Ca and Mg supply. Ann. Bot. 2012, 109, 1081–1089. [Google Scholar] [CrossRef]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Patino, S.; Baker, T.R.; Nardoto, G.B.; Martinelli, L.A.; Quesada, C.A.; Paiva, R.; Schwarz, M.; Horna, V.; Mercado, L.M.; et al. Basin-wide variations in foliar properties of Amazonian forest: Phylogeny, soils and climate. Biogeosciences 2009, 6, 2677–2708. [Google Scholar] [CrossRef] [Green Version]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Woodward, F.I.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- Brown, G.; Luu, I.; O’Sullivan, G. Trace Metal Concentrations in Pine Needles at Varying Elevation in Proximity to Roadways in an Urban Environment. J. Environ. Prot. 2017, 8, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Soethe, N.; Lehmann, J.; Engels, C. Nutrient availability at different altitudes in a tropical montane forest in Ecuador. J. Trop. Ecol. 2008, 24, 397–406. [Google Scholar] [CrossRef]
- Macek, P.; Klimes, L.; Adamec, L.; Dolezal, J.; Chlumska, Z.; de Bello, F.; Dvorskry, M.; Rehakova, K. Plant Nutrient Content Does Not Simply Increase with Elevation under the Extreme Environmental Conditions of Ladakh, NW Himalaya. Arct. Antarct. Alp. Res. 2012, 44, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef] [PubMed]
| Ca | P. massoniana | P. hwangshanensis | ||||||||
| Population | H | J | L | S | T | H | J | L | S | T |
| Mean | 4.00 ab | 4.01 b | 2.59 b | 5.62 a* | 3.39 b | 3.78 a | 2.85 a | 3.11 a | 2.97 a | 3.42 a |
| Median | 3.53 | 4.27 | 2.62 | 5.48 | 3.13 | 3.31 | 2.95 | 3.28 | 3.08 | 3.40 |
| Variance | 3.96 | 5.06 | 0.28 | 0.65 | 1.38 | 1.77 | 0.42 | 0.38 | 0.91 | 0.76 |
| SD | 1.99 | 2.25 | 0.53 | 0.81 | 1.18 | 1.33 | 0.65 | 0.61 | 0.95 | 0.87 |
| Minimum | 1.74 | 1.10 | 1.49 | 4.73 | 1.77 | 2.18 | 1.57 | 1.91 | 1.88 | 2.46 |
| Maximum | 8.38 | 8.22 | 3.53 | 7.08 | 5.09 | 5.94 | 3.62 | 3.81 | 4.26 | 5.19 |
| CV | 49.77% | 56.08% | 20.60% | 14.38% | 34.72% | 35.18% | 22.71% | 19.74% | 32.05% | 25.41% |
| Mg | P. massoniana | P. hwangshanensis | ||||||||
| Population | H | J | L | S | T | H | J | L | S | T |
| Mean | 0.92 c* | 1.41 a* | 1.07 bc | 1.13 abc | 1.32 ab* | 0.67 c | 1.09 a | 1.00 ab | 0.87 abc | 0.84 bc |
| Median | 0.92 | 1.37 | 0.93 | 1.02 | 1.38 | 0.68 | 1.13 | 0.92 | 0.79 | 0.89 |
| Variance | 0.05 | 0.14 | 0.07 | 0.07 | 0.05 | 0.02 | 0.02 | 0.09 | 0.05 | 0.05 |
| SD | 0.23 | 0.38 | 0.27 | 0.27 | 0.21 | 0.13 | 0.15 | 0.31 | 0.23 | 0.22 |
| Minimum | 0.60 | 0.92 | 0.73 | 0.90 | 0.98 | 0.45 | 0.83 | 0.72 | 0.69 | 0.44 |
| Maximum | 1.28 | 2.23 | 1.48 | 1.48 | 1.53 | 0.82 | 1.32 | 1.77 | 1.27 | 1.12 |
| CV | 24.62% | 26.85% | 25.33% | 23.74% | 16.20% | 19.19% | 13.38% | 30.89% | 26.13% | 26.09% |
| Ca:Mg | P. massoniana | P. hwangshanensis | ||||||||
| Population | H | J | L | S | T | H | J | L | S | T |
| Mean | 4.33 a | 2.75 b | 2.47 b* | 5.20 a* | 2.56 b* | 5.65 a | 2.60 c | 3.23 c | 3.45 bc | 4.22 b |
| Median | 3.52 | 2.99 | 2.39 | 4.89 | 2.27 | 5.27 | 2.74 | 3.16 | 3.34 | 4.22 |
| Variance | 3.85 | 1.59 | 0.20 | 2.11 | 0.67 | 2.84 | 0.17 | 0.53 | 0.88 | 1.17 |
| SD | 1.96 | 1.26 | 0.44 | 1.45 | 0.82 | 1.69 | 0.41 | 0.73 | 0.94 | 1.08 |
| Minimum | 2.92 | 1.10 | 1.86 | 3.87 | 1.68 | 3.79 | 1.89 | 2.15 | 2.30 | 3.00 |
| Maximum | 9.11 | 4.83 | 3.08 | 7.77 | 4.28 | 8.73 | 3.05 | 4.33 | 4.48 | 5.94 |
| CV | 45.34% | 45.79% | 17.99% | 27.96% | 32.05% | 29.85% | 15.71% | 22.55% | 27.22% | 25.62% |
| Population | S | L | J | H | T |
|---|---|---|---|---|---|
| P. hwangshanensis | 0.664 | 0.512 | 0.792 * | 0.524 | 0.576 |
| P. massoniana | 0.012 | 0.713 * | 0.680 * | 0.419 | 0.504 |
| Species | Countries and Regions | Ca Concentration (mg·g−1) | Mg Concentration (mg·g−1) |
|---|---|---|---|
| P. elliottii | China, Fujian | 2.0 | 0.8 [60] |
| China, Jiangxi | 3.8 | 0.9 [61] | |
| P. kesiya var. langbianensis | China, Yunnan | 1.9 | 1.4 [62] |
| P. massoniana | China, Sichuan | 6.8 | 1.3 [51] |
| P. thunbergii | China, Shandong | 4.5 | 1.6 [63] |
| P. yunnanensis | China, Yunnan | 3.2 | 1.4 [64] |
| P. brutia | Turkey, Istanbul | 2.3~4.6 | 0.7~0.8 [57] |
| P. canariensis | Spain, Tenerife | 9.1 | 1.5 [39] |
| P. halepensis | Spain, Yeste/Calasparra | 7.0/7.5 | 2.7/2.0 [58] |
| Greece, Parnitha | 7.9 | 1.5 [42] | |
| Spain, Granada | 2.4 | 0.2 [59] | |
| Spain, Granada | 12.3 | 2.5 [56] | |
| P. pinaster | Spain, Galicia | 1.1 | 1.6 [54] |
| P. radiata | Spain, Galicia | 3.6 | 0.9 [54] |
| P. sylvestris | Finland, Suonenjoki | 4.3 | 0.5 [55] |
| Lithuania, Neris | 4.9 | 1.2 [43] | |
| P. taeda | USA, 11 Southern States | 1.8 | 1.0 [65] |
| USA, Texas | 2.8~4.0 | 1.8~2.3 [53] | |
| USA, 8 southeastern States | 1.7 | 1.0 [41] | |
| Brazil, Southern region | 1.8 | 0.5 [66] |
| Population | Abbreviation | Longitude (°E) | Latitude (°N) | Site (plot) and Elevation (m) | |
|---|---|---|---|---|---|
| P. massoniana | P. hwangshanensis | ||||
| Mt. Sanqingshan, Jiangxi | S | 118°00′~118°06′ | 28°52′~28°57′ | 300, 400 | 1062, 1500 |
| Mt. Lushan, Jiangxi | L | 115°50′~116°10′ | 29°28′~29°45′ | 300, 400, 500 | 900, 1000, 1100 |
| Mt. Jinggangshan, Jiangxi | J | 113°39′~114°23′ | 26°27′~26°40′ | 300, 500, 600 | 900, 1100, 1300 |
| Mt. Huangshan, Anhui | H | 116°49′~118°59′ | 29°13′~31°05′ | 350, 450, 550 | 900, 1100, 1200 |
| Mt. Tianmushan, Zhejiang | T | 118°36′~120°06′ | 29°52′~30°55′ | 340, 480, 580 | 1200, 1400, 1500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Yang, Y.; Fan, M.; Huang, K.; Wang, L.; Lv, T.; Yi, X.; Chen, L.; Fang, Y. Inter- and Intra-Population Variation of Foliage Calcium and Magnesium in Two Chinese Pine Species. Plants 2023, 12, 562. https://doi.org/10.3390/plants12030562
Hu M, Yang Y, Fan M, Huang K, Wang L, Lv T, Yi X, Chen L, Fang Y. Inter- and Intra-Population Variation of Foliage Calcium and Magnesium in Two Chinese Pine Species. Plants. 2023; 12(3):562. https://doi.org/10.3390/plants12030562
Chicago/Turabian StyleHu, Meng, Yuan Yang, Mingyang Fan, Kexin Huang, Lu Wang, Ting Lv, Xiangui Yi, Lin Chen, and Yanming Fang. 2023. "Inter- and Intra-Population Variation of Foliage Calcium and Magnesium in Two Chinese Pine Species" Plants 12, no. 3: 562. https://doi.org/10.3390/plants12030562





