Heart Rate and Body Temperature Evolution in an Interval Program of Passive Heat Acclimation at High Temperatures (100 ± 2 °C) in a Sauna
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Approvals
2.2. Experimental Protocol
2.3. Exposure Sessions
2.4. Heart Rate and Temperature Assessment
2.5. Health Security Protocol
2.6. Body Composition
2.7. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shibasaki, M.; Wilson, T.E.; Crandall, C.G. Neural Control and Mechanisms of Eccrine Sweating during Heat Stress and Exercise. J. Appl. Physiol. 2006, 100, 1692–1701. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J. Human Thermoregulation and the Cardiovascular System. Exp. Physiol. 2012, 97, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wingo, J.E. Exercise Intensity Prescription during Heat Stress: A Brief Review. Scand. J. Med. Sci. Sports 2015, 25, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, A.E.; Clegg, M.E.; Walsh, M.L.; White, M.D. Adaptation of Exercise Ventilation during an Actively-Induced Hyperthermia Following Passive Heat Acclimation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R605–R614. [Google Scholar] [CrossRef] [PubMed]
- Travers, G.; Nichols, D.; Riding, N.; González-Alonso, J.; Périard, J.D. Heat Acclimation with Controlled Heart Rate: Influence of Hydration Status. Med. Sci. Sports Exerc. 2020, 52, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Eymann, T.M.; Francisco, M.A.; Howard, M.J.; Minson, C.T. Passive Heat Therapy Improves Cutaneous Microvascular Function in Sedentary Humans via Improved Nitric Oxide-Dependent Dilation. J. Appl. Physiol. 2016, 121, 716–723. [Google Scholar] [CrossRef]
- Lorenzo, S.; Halliwill, J.R.; Sawka, M.N.; Minson, C.T. Heat Acclimation Improves Exercise Performance. J. Appl. Physiol. 2010, 109, 1140–1147. [Google Scholar] [CrossRef]
- Maloyan, A.; Eli-Berchoer, L.; Semenza, G.L.; Gerstenblith, G.; Stern, M.D.; Horowitz, M. HIF-1α-Targeted Pathways Are Activated by Heat Acclimation and Contribute to Acclimation-Ischemic Cross-Tolerance in the Heart. Physiol. Genom. 2005, 23, 79–88. [Google Scholar] [CrossRef]
- Coudevylle, G.R.; Sinnapah, S.; Robin, N.; Collado, A.; Hue, O. Conventional and Alternative Strategies to Cope with the Subtropical Climate of Tokyo 2020: Impacts on Psychological Factors of Performance. Front. Psychol. 2019, 10, 01279. [Google Scholar] [CrossRef]
- Akerman, A.P.; Tipton, M.; Minson, C.T.; Cotter, J.D. Heat Stress and Dehydration in Adapting for Performance: Good, Bad, Both, or Neither? Temperature 2016, 3, 412–436. [Google Scholar] [CrossRef]
- Bartolomé, I.; Toro-Román, V.; Siquier-Coll, J.; Muñoz, D.; Robles-Gil, M.C.; Maynar-Mariño, M. Acute Effect of Exposure to Extreme Heat (100±3° C) on Lower Limb Maximal Resistance Strength. Int J. Environ. Res. Public Health 2022, 19, 10934. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Littmann, A.E.; Chang, S.H.; Wester, L.A.; Knipper, J.S.; Shields, R.K. Heat Stress and Cardiovascular, Hormonal, and Heat Shock Proteins in Humans. J. Athl. Train 2012, 47, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Wilson, M.G.; Periard, J.D. Passive Heat Acclimation Improves Skeletal Muscle Contractility in Humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 312, R101–R107. [Google Scholar] [CrossRef] [PubMed]
- Ketelhut, S.; Ketelhut, R.G. The Blood Pressure and Heart Rate during Sauna Bath Correspond to Cardiac Responses during Submaximal Dynamic Exercise. Complement. Ther. Med. 2019, 44, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Poerschke, D.; Wilhelm, M.; Trachsel, L.D.; Tschanz, H.; Matter, F.; Jauslin, D.; Saner, H.; Schmid, J.-P. Acute Effects of Finnish Sauna and Cold-Water Immersion on Haemodynamic Variables and Autonomic Nervous System Activity in Patients with Heart Failure. Eur. J. Prev. Cardiol. 2016, 23, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Glazachev, O.S.; Kofler, W.; Dudnik, E.N.; Zapara, M.A.; Samartseva, V.G. Effect of Adaptation to Passive Hyperthermia on Aerobic Performance and Cardio-Respiratory Endurance in Amateur Athletes. Hum. Physiol. 2020, 46, 66–73. [Google Scholar] [CrossRef]
- Zapara, M.A.; Dudnik, E.N.; Samartseva, V.G.; Kryzhanovskaya, S.Y.; Susta, D.; Glazachev, O.S. Passive Whole-Body Hyperthermia Increases Aerobic Capacity and Cardio-Respiratory Efficiency in Amateur Athletes. Health N. Hav. 2020, 12, 14–26. [Google Scholar] [CrossRef][Green Version]
- Siquier- Coll, J.; Bartolomé, I.; Pérez-Quintero, M.; Grijota, F.J.; Muñoz, D.; Maynar-Mariño, M. Effect of Heat Exposure and Physical Exercise until Exhaustion in Normothermic and Hyperthermic Conditions on Serum, Sweat and Urinary Concentrations of Magnesium and Phosphorus. J. Therm. Biol. 2019, 84, 176–184. [Google Scholar] [CrossRef]
- Périard, J.; Racinais, S. Heat Stress in Sport and Exercise; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 3319935143. [Google Scholar]
- Tansey, E.A.; Johnson, C.D. Recent Advances in Thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef]
- Gorman, A.J.; Proppe, D.W. Mechanisms Producing Tachycardia in Conscious Baboons during Environmental Heat Stress. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 441–446. [Google Scholar] [CrossRef]
- Crandall, C.G.; Wilson, T.E. Human Cardiovascular Responses to Passive Heat Stress. Compr. Physiol. 2015, 5, 17–43. [Google Scholar] [CrossRef]
- Gagnon, D.; Schlader, Z.J.; Crandall, C.G. Sympathetic Activity during Passive Heat Stress in Healthy Aged Humans. J. Physiol. 2015, 593, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Leppaluoto, J.; Tuominen, M.; Vaananen, A.; Karpakka, J.; Vuori, J. Some Cardiovascular and Metabolic Effects of Repeated Sauna Bathing. Acta Physiol. Scand. 1986, 128, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Scoon, G.S.M.; Hopkins, W.G.; Mayhew, S.; Cotter, J.D. Effect of Post-Exercise Sauna Bathing on the Endurance Performance of Competitive Male Runners. J. Sci. Med. Sport 2007, 10, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.K.; Zaccardi, F.; Lee, E.; Willeit, P.; Khan, H.; Laukkanen, J.A. Acute Effects of Sauna Bathing on Cardiovascular Function. J. Hum. Hypertens. 2018, 32, 129–138. [Google Scholar] [CrossRef]
- Andrade-Mayorga, O.; Mancilla, R.; Díaz, E.; Alvarez, C. Heart Rate During an Exercise Test and Acute High-Intensity Interval Training in Type 2 Diabetes. Int. J. Sports Med. 2020, 41, 365–372. [Google Scholar] [CrossRef]
- Periard, J.D.; Travers, G.J.S.; Racinais, S.; Sawka, M.N. Cardiovascular Adaptations Supporting Human Exercise-Heat Acclimation. Auton. Neurosci. Basic Clin. 2016, 196, 52–62. [Google Scholar] [CrossRef]
- Alexander-Shani, R.; Mreisat, A.; Smeir, E.; Gerstenblith, G.; Stern, M.D.; Horowitz, M. Long-Term HIF-1α Transcriptional Activation Is Essential for Heat-Acclimation-Mediated Cross Tolerance: Mitochondrial Target Genes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R753–R762. [Google Scholar] [CrossRef]
- Ganio, M.S.; Brothers, R.M.; Shibata, S.; Hastings, J.L.; Crandall, C.G. Effect of Passive Heat Stress on Arterial Stiffness. Exp. Physiol. 2011, 96, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Ohori, T.; Nozawa, T.; Ihori, H.; Shida, T.; Sobajima, M.; Matsuki, A.; Yasumura, S.; Inoue, H. Effect of Repeated Sauna Treatment on Exercise Tolerance and Endothelial Function in Patients With Chronic Heart Failure. Am. J. Cardiol. 2012, 109, 100–104. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, S.L.; Hassmén, P.; Zhou, S.; Stevens, C.J. Passive Heating: Reviewing Practical Heat Acclimation Strategies for Endurance Athletes. Front. Physiol. 2018, 9, 1851. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.N.; Cohen, M.M.; Mantri, N.; O’Malley, C.J.; Greaves, R.F. Infrared Sauna as Exercise-Mimetic? Physiological Responses to Infrared Sauna vs Exercise in Healthy Women: A Randomized Controlled Crossover Trial. Complement Ther. Med. 2022, 64, 102798. [Google Scholar] [CrossRef]
- Vuori, I. Sauna Bather’s Circulation. Ann. Clin. Res. 1988, 20, 249–256. [Google Scholar]
- Taylor, N.A.S. Human Heat Adaptation. Compr. Physiol. 2014, 4, 325–365. [Google Scholar] [CrossRef] [PubMed]
- Periard, J.D.; Racinais, S.; Sawka, M.N. Adaptations and Mechanisms of Human Heat Acclimation: Applications for Competitive Athletes and Sports. Scand. J. Med. Sci. Sports 2015, 25, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M. Do Cellular Heat Acclimation Responses Modulate Central Thermoregulatory Activity? Physiology 1998, 13, 218–225. [Google Scholar] [CrossRef]
- Horowitz, M.; Assadi, H. Heat Acclimation-Mediated Cross-Tolerance in Cardioprotection: Do HSP70 and HIF-1α Play a Role? Ann. N. Y. Acad. Sci. 2010, 1188, 199–206. [Google Scholar] [CrossRef]
- Gibson, O.R.; Dennis, A.; Parfitt, T.; Taylor, L.; Watt, P.W.; Maxwell, N.S. Extracellular Hsp72 Concentration Relates to a Minimum Endogenous Criteria during Acute Exercise-Heat Exposure. Cell Stress Chaperones 2014, 19, 389–400. [Google Scholar] [CrossRef]
- Sandström, M.E.; Siegler, J.C.; Lovell, R.J.; Madden, L.A.; McNaughton, L. The Effect of 15 Consecutive Days of Heat–Exercise Acclimation on Heat Shock Protein 70. Cell Stress Chaperones 2008, 13, 169–175. [Google Scholar] [CrossRef]
- Fujii, N.; Zhang, S.Y.; McNeely, B.D.; Nishiyasu, T.; Kenny, G.P. Heat Shock Protein 90 Contributes to Cutaneous Vasodilation through Activating Nitric Oxide Synthase in Young Male Adults Exercising in the Heat. J. Appl. Physiol. 2017, 123, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.H.; Green, D.J. HSP90: An Unappreciated Mediator of Cutaneous Vascular Adaptation? J. Appl. Physiol. 2018, 124, 521. [Google Scholar] [CrossRef] [PubMed]
| Parameters | EG (n = 15) | CG (n = 14) |
|---|---|---|
| Age (years) | 22.34 ± 1.88 | 21.75 ± 1.71 |
| Height (m) | 171.86 ± 6.12 | 174.41 ± 4.67 |
| Weight (Kg) | 69.56 ± 6.41 | 70.82 ± 5,51 |
| HRb (bpm) | 79.43 ± 20.09 | 68.91 ± 11.48 |
| Fat mass (kg) | 11.96 ± 3.29 | 11.15 ± 2.47 |
| Fat mass (%) | 17.00 ± 3.55 | 15.63 ± 2.43 |
| Fat-free mass (kg) | 57.6 ± 4.08 | 59.66 ± 3.66 |
| Fat-free mass (%) | 83.00 ± 3.55 | 84.37 ± 2.43 |
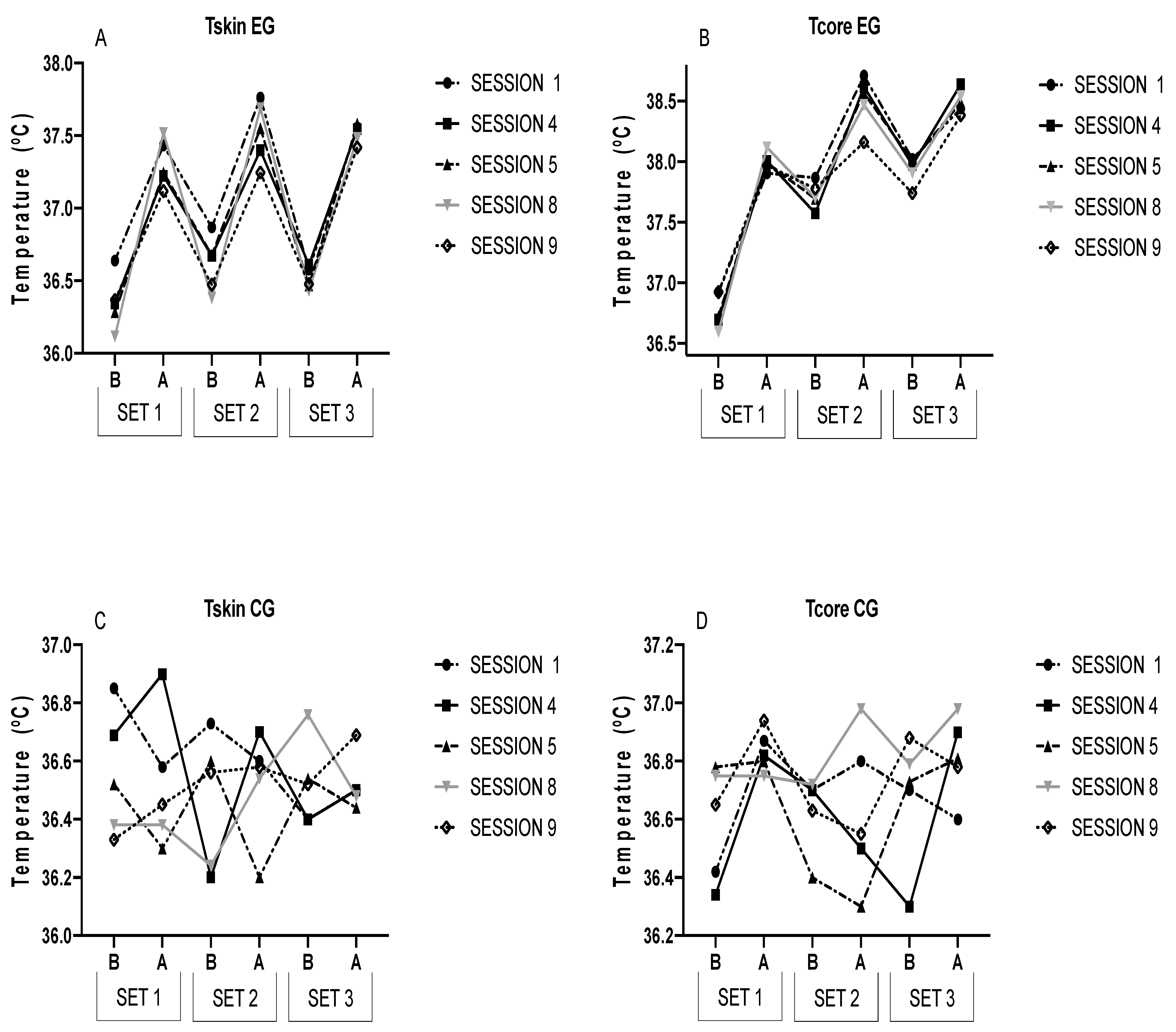
| EG | CG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tcore | p-Value | Tskin | p-Value | Tcore | p-Value | Tskin | p-Value | |||
| S1 | Set 1 | Before | 36.64 ± 0.63 | 0.001 | 36.92 ± 0.62 | 0.009 | 36.42 ± 0.56 | 0.085 | 36.85 ± 0.71 | 0.312 |
| After | 37.43 ± 0.84 | 37.91 ± 0.58 | 36.87 ± 0.37 | 36.58 ± 0.35 | ||||||
| Set 2 | Before | 36.87 ± 0.70 ** | 0.003 | 37.87 ± 0.64 * | 0.013 | 36.70 ± 0.31 | 0.128 | 36.73 ± 0.28 | 0.159 | |
| After | 37.76 ± 1.19 ** | 38.71 ± 0.78 | 36.80 ± 0.44 | 36.60 ± 0.47 | ||||||
| Set 3 | Before | 36.58 ± 0.65 ** | 0.074 | 38.02 ± 0.51 | 0.005 | 36.70 ± 0.37 | 0.327 | 36.40 ± 0.67 | 0.108 | |
| After | 37.56 ± 0.97 * | 38.44 ± 0.79 | 36.60 ± 0.41 | 36.50 ± 0.34 | ||||||
| S4 | Set 1 | Before | 36.34 ± 0.32 | 0.000 | 36.69 ± 0.68 | 0.000 | 36.34 ± 0.32 | 0.066 | 36.69 ± 0.68 | 0.096 |
| After | 37.22 ± 0.58 | 38.01 ± 0.82 | 36.82 ± 0.67 | 36.90 ± 0.61 | ||||||
| Set 2 | Before | 36.67 ± 0.53 ** | 0.001 | 37.57 ± 0.82 * | 0.003 | 36.70 ± 0.54 | 0.098 | 36.20 ± 0.76 | 0.059 | |
| After | 37.40 ± 0.73 ** | 38.62 ± 0.61 | 36.50 ± 0.38 | 36.70 ± 0.67 | ||||||
| Set 3 | Before | 36.61 ± 0.65 **† | 0.003 | 37.97 ± 0.61 * | 0.005 | 36.30 ± 0.49 | 0.254 | 36.40 ± 0.58 | 0.061 | |
| After | 37.54 ± 0.88 ** | 38.64 ± 0.79 * | 36.90 ± 0.31 | 36.50 ± 0.28 | ||||||
| S5 | Set 1 | Before | 36.28 ± 0.42 | 0.000 | 36.72 ± 0.57 ¥ | 0.000 | 36.78 ± 0.36 | 0.088 | 36.52 ± 0.28 | 0.073 |
| After | 37.24 ± 0.52 | 37.99 ± 0.64 | 36.80 ± 0.21 | 36.30 ± 0.62 | ||||||
| Set 2 | Before | 36.68 ± 0.51 ** | 0.002 | 37.69 ± 0.45 * | 0.006 | 36.40 ± 0.65 | 0.346 | 36.60 ± 0.45 | 0.455 | |
| After | 37.55 ± 0.75 * | 38.57 ± 0.89 | 36.30 ± 0.52 | 36.20 ± 0.74 | ||||||
| Set 3 | Before | 36.47 ± 0.71 **† | 0.022 | 38.01 ± 0.90 * | 0.000 | 36.73 ± 0.35 | 0.061 | 36.54 ± 0.53 | 0.316 | |
| After | 37.58 ± 0.87 ** | 38.49 ± 0.68 * | 36.81 ± 0.61 | 36.44 ± 0.51 | ||||||
| S8 | Set 1 | Before | 36.12 ± 0.35 ¥ | 0.000 | 36.59 ± 0.52 ¥¥# | 0.000 | 36.75 ± 0.49 | 0.086 | 36.38 ± 0.26 | 0.083 |
| After | 37.52 ± 0.49 | 38.12 ± 0.55 #ƒ | 36.75 ± 0.51 | 36.38 ± 0.39 | ||||||
| Set 2 | Before | 36.38 ± 0.51 ** | 0.001 | 37.70 ± 0.41 ¥ | 0.000 | 36.72 ± 0.48 | 0.102 | 36.24 ± 0.36 | 0.078 | |
| After | 37.69 ± 0.61 * | 38.47 ± 0.54 | 36.98 ± 0.48 | 36.54 ± 0.38 | ||||||
| Set 3 | Before | 36.43 ± 0.33 **†† | 0.008 | 37.91 ± 0.52 ** | 0.001 | 36.79 ± 0.62 | 0.074 | 36.76 ± 0.35 | 0.566 | |
| After | 37.48 ± 0.66 ** | 38.54 ± 0.83 | 36.98 ± 0.73 | 36.48 ± 0.31 | ||||||
| S9 | Set 1 | Before | 36.37 ± 0.72 § | 0.000 | 36.92 ± 0.40 ¥ | 0.004 | 36.65 ± 0.64 | 0.224 | 36.33 ± 0.31 | 0.117 |
| After | 37.12 ± 0.49 | 37.97 ± 0.52 | 36.94 ± 0.32 | 36.45 ± 0.39 | ||||||
| Set 2 | Before | 36.47 ± 0.42 **# | 0.047 | 37.78 ± 0.48 ¥ | 0.004 | 36.63 ± 0.51 | 0.089 | 36.56 ± 0.44 | 0.285 | |
| After | 37.24 ± 0.89 | 38.16 ± 0.84 | 36.55 ± 0.92 | 36.58 ± 0.41 | ||||||
| Set 3 | Before | 36.48 ± 0.55 **¥ | 0.002 | 37.74 ± 0.56 | 0.000 | 36.88 ± 0.43 | 0.234 | 36.52 ± 0.41 | 0.156 | |
| After | 37.42 ± 0.54 * | 38.38 ± 0.39 | 36.78 ± 0.38 | 36.69 ± 0.39 | ||||||
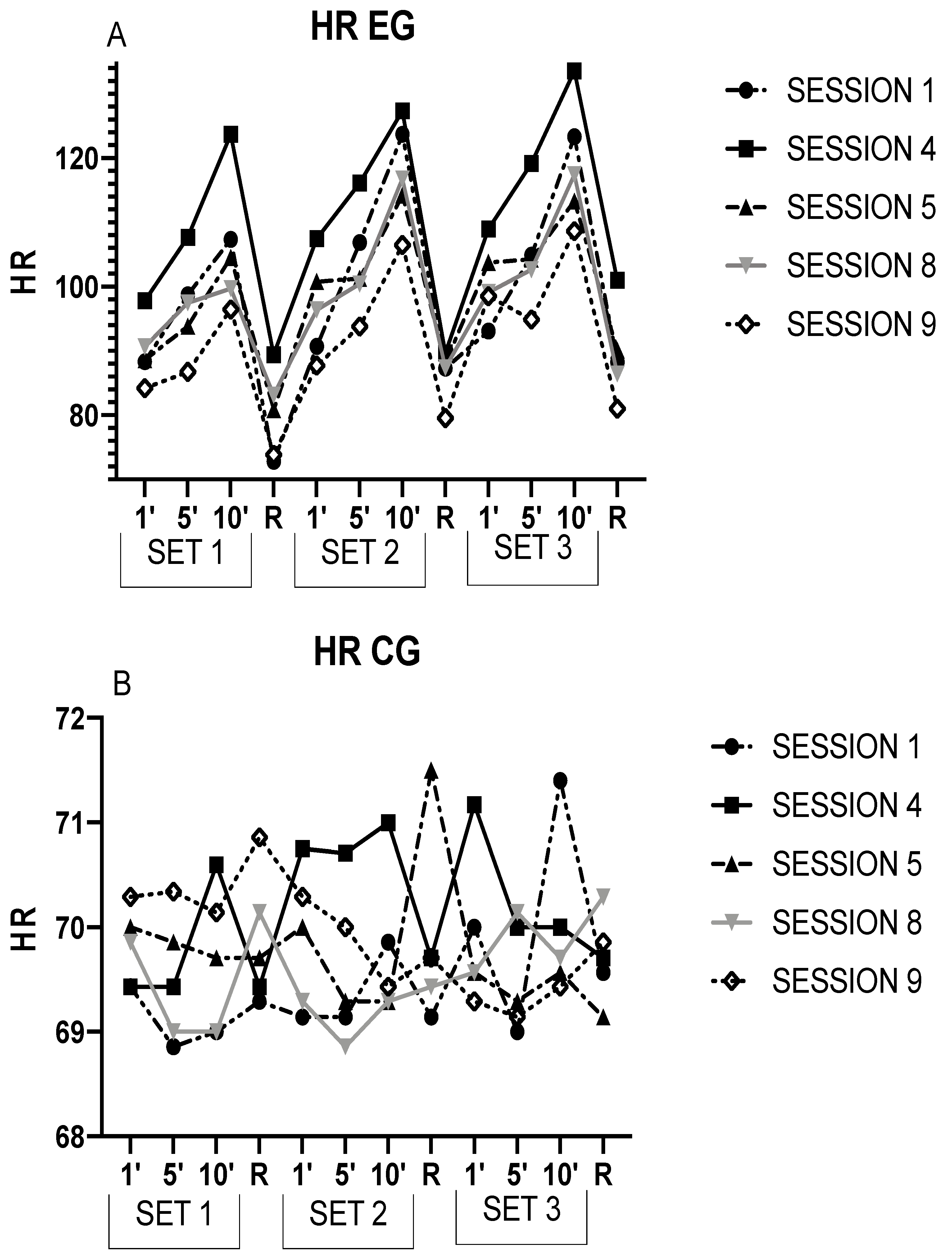
| EG | |||||||
| S1 | S4 | S5 | S8 | S9 | |||
| SET 1 | HR1 | 88.33 ± 19.67 | 97.79 ± 16.99 * | 88.58 ± 16.84 † | 90.69 ± 13.41 † | 84.2 ± 14.59 † | |
| HR5 | 98.71 ± 20.69 | 107.65 ± 20.72 * | 93.84 ± 19.35 | 97.47 ± 13.14 | 86.76 ± 14.48 *† | ||
| HR10 | 107.33 ± 26.45 | 123.68 ± 18.25 * | 104.53 ± 22.85 † | 99.71 ± 19.19 † | 96.48 ± 18.11 † | ||
| HRR1 | 72.86 ± 17.97 | 89.43 ± 24.41 * | 80.86 ± 19.20 † | 83.14 ± 15.25 | 73.86 ± 12.77 † | ||
| SET 2 | HR1 | 90.74 ± 22.11 | 107.44 ± 22.91 *ƒ | 100.8 ± 23.11 ƒ | 96.43 ± 19.57 † | 87.74 ± 15.61 † | |
| HR5 | 106.86 ± 14.17 | 116.12 ± 21.43 | 101.27 ± 17.56 † | 100.39 ± 16.67 †ƒ | 93.81 ± 11.34 † | ||
| HR10 | 123.68 ± 17.19 | 127.31 ± 18.97 | 114.25 ± 24.33 †ƒ | 116.80 ± 24.15 | 106.49 ± 19.08 *†ƒ | ||
| HRR2 | 87.29 ± 21.01 ƒ | 89.71 ± 16.19 | 88.14 ± 13.86 | 87.43 ± 14.46 | 79.57 ± 23.19 | ||
| SET 3 | HR1 | 93.09 ± 25.55 | 108.98 ± 21.37 | 103.8 ± 23.24 ƒ¥ | 99.12 ± 13.7 †ƒ | 98.54 ± 24.00 | |
| HR5 | 104.91 ± 14.27 | 119.16 ± 19.68 * | 104.27 ± 17.14 † | 102.65 ± 15.55 †ƒ | 94.87 ± 16.87 † | ||
| HR10 | 123.31 ± 14.17 | 133.54 ± 21.34 | 113.3 ± 18.4 † | 117.43 ± 20.89 †ƒ | 108.67 ± 23.09 †ƒ | ||
| HRR3 | 88.29 ± 15.56 ƒ | 101.00 ± 14.00 * | 90.14 ± 16.62 | 86.43 ± 13.14 † | 81.00 ± 23.90 † | ||
| CG | |||||||
| S1 | S4 | S5 | S8 | S9 | |||
| SET 1 | HR1 | 69.43 ± 10.91 | 69.43 ± 9.71 | 70.00 ± 10.33 | 69.86 ± 9.19 | 70.29 ± 9.23 | |
| HR5 | 68.86 ± 10.06 | 69.43 ± 8.94 | 69.86 ± 9.28 | 69.00 ± 10.54 | 70.34 ± 10.29 | ||
| HR10 | 69.00 ± 9.80 | 70.6 ± 10.45 | 69.71 ± 9.71 | 69.00 ± 8.98 | 70.14 ± 9.86 | ||
| HRR1 | 69.29 ± 10.19 | 69.43 ± 10.15 | 69.71 ± 8.62 | 70.14 ± 9.49 | 70.86 ± 9.89 | ||
| SET 2 | HR1 | 69.14 ± 9.62 | 70.75 ± 10.24 | 70.00 ± 10.21 | 69.29 ± 9.66 | 70.29 ± 9.71 | |
| HR5 | 69.14 ± 9.79 | 70.71 ± 10.01 | 69.29 ± 10.48 | 68.86 ± 9.30 | 70.00 ± 9.07 | ||
| HR10 | 69.86 ± 8.67 | 71.00 ± 8.83 | 69.29 ± 10.21 | 69.29 ± 9.64 | 69.43 ± 9.22 | ||
| HRR2 | 69.14 ± 9.17 | 69.71 ± 8.88 | 71.5 ± 7.31 | 69.43 ± 9.52 | 69.71 ± 9.27 | ||
| SET 3 | HR1 | 70.00 ± 11.1 | 71.17 ± 10.68 | 69.57 ± 9.73 | 69.57 ± 9.54 | 69.29 ± 9.74 | |
| HR5 | 69.00 ± 9.47 | 70.00 ± 8.16 | 69.29 ± 9.79 | 70.14 ± 8.88 | 69.14 ± 9.30 | ||
| HR10 | 71.40 ± 10.19 | 70.00 ± 7.98 | 69.57 ± 9.29 | 69.71 ± 9.43 | 69.43 ± 10.11 | ||
| HRR3 | 69.57 ± 10.05 | 69.71 ± 8.90 | 69.14 ± 9.82 | 70.29 ± 9.11 | 69.86 ± 9.41 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siquier-Coll, J.; Bartolomé, I.; Pérez-Quintero, M.; Toro-Román, V.; Grijota, F.J.; Maynar-Mariño, M. Heart Rate and Body Temperature Evolution in an Interval Program of Passive Heat Acclimation at High Temperatures (100 ± 2 °C) in a Sauna. Int. J. Environ. Res. Public Health 2023, 20, 2082. https://doi.org/10.3390/ijerph20032082
Siquier-Coll J, Bartolomé I, Pérez-Quintero M, Toro-Román V, Grijota FJ, Maynar-Mariño M. Heart Rate and Body Temperature Evolution in an Interval Program of Passive Heat Acclimation at High Temperatures (100 ± 2 °C) in a Sauna. International Journal of Environmental Research and Public Health. 2023; 20(3):2082. https://doi.org/10.3390/ijerph20032082
Chicago/Turabian StyleSiquier-Coll, Jesús, Ignacio Bartolomé, Mario Pérez-Quintero, Víctor Toro-Román, Francisco J. Grijota, and Marcos Maynar-Mariño. 2023. "Heart Rate and Body Temperature Evolution in an Interval Program of Passive Heat Acclimation at High Temperatures (100 ± 2 °C) in a Sauna" International Journal of Environmental Research and Public Health 20, no. 3: 2082. https://doi.org/10.3390/ijerph20032082
APA StyleSiquier-Coll, J., Bartolomé, I., Pérez-Quintero, M., Toro-Román, V., Grijota, F. J., & Maynar-Mariño, M. (2023). Heart Rate and Body Temperature Evolution in an Interval Program of Passive Heat Acclimation at High Temperatures (100 ± 2 °C) in a Sauna. International Journal of Environmental Research and Public Health, 20(3), 2082. https://doi.org/10.3390/ijerph20032082










