Abstract
In this article, we propose to explore the chemical interaction between Pseudosphinx tetrio L. and Allamanda cathartica L. using different analytical methods, including an innovative electrochemical approach (called electrochemical ecology) and multivariate analysis, and we investigate the potential antimicrobial effects (antibacterial and antifungal activities) of this interaction in order to gain a better understanding of their specific interaction. The analytical study presents a similar chemical profile between the leaves of healthy and herbivorous A. cathartica and the excretions of the caterpillars. The similar analytical profile of the leaves of A. cathartica and the excretions of P. tetrio, and the difference with the caterpillar bodies, suggests a selective excretion of compounds by the caterpillar. The measured antimicrobial activities support the physicochemical tests. The natural products found selectively in the excretions (rather than in the body) could explain the ability of P. tetrio to feed on this toxic Apocynaceae species.
1. Introduction
The tetrio sphinx, Pseudosphinx tetrio L. (Lepidoptera: Sphingidae), is a moth widely distributed in tropical and subtropical regions of the Americas and the Caribbean basin, where suitable host plants for the development of its larvae (large, conspicuous caterpillars) are present [1,2,3,4,5,6,7,8,9,10].
Adult females of P. tetrio [11] lay 50–100 eggs on the underside of the host plant’s leaves, from which they hatch in about three days [3]. After hatching, the gregarious larvae live in colonies for at least the first three developing stages, feeding initially on the upper surface of leaves and in later stages on all leaf tissues except leaf veins and midribs as they progress to the later instars. Adult larvae (fifth or sixth stages) feed singly or in small groups on entire leaves can reach a length of 63–69 mm [3,4]. The color of larvae is characteristic of aposematism (antipredation strategy: warning predators of bad taste or toxicity). According to an aposematic hypothesis, it becomes toxic to its predators when it ingests toxic phytocomponents from the milky sap of some plants [12]. The body is velvety black with yellow rings on each thoracic and abdominal segment, the head is dark orange-red, and the prothoracic shield, prominent legs and anal segment are orange-red with dark markings. The spine on the eighth abdominal segment is black and emerges from a raised, orange, button-like structure [4,13,14,15]. The aposematic larvae have few predators due to their venomous nature. However, the squirrel cuckoo, Piaya cayana Linn., of Belize, consumes P. tetrio by taking larvae, beating them against a branch until the poisonous gut contents are gone, and then swallowing the remains [16]. The larvae wrap themselves in a cocoon of silk or dead leaves and molt into new pupae about 7.0 cm long. The adult moths, colored in shades of gray, hatch from the pupae 53 days after egg laying and have a wingspan of 12.7–14.0 cm (eggs hatch in 3 days or more; average duration of the 5–6 larval stages is 23–4 days; the prepupal stage lasts about 4 days and the pupal stage about 22 days [3]). P. tetrio has the ability to eat up to twice its weight in food during a day, hence its name, “glutton caterpillar”. The larva of P. tetrio feeds preferentially on the leaves of the Apocynaceae family’s plants, such as Allamanda cathartica L. and Plumeria alba L., which are rich in toxic latex [17,18].
A. cathartica is commonly known as yellow allamanda, golden trumpet or “Liane à lait” [19]. This is a common name for the species. In the review paper by Ghosh and coworkers, it was described as a hermaphroditic, vine-like, woody shrub used primarily in landscaping, more spreading than tall, and with perennial leaves [20]. A. cathartica is a fast-growing species that is widely distributed worldwide, but mainly in tropical and subtropical regions, where the caterpillar P. tetrio is present [21,22]. A. cathartica is one of the fifteen species of the Apocynaceae family according to the “WFO Plant List” [23].
The plant is native to Brazil [24] and its various parts such as leaves, flowers, roots and stems are used in traditional medicine [25] for the treatment of jaundice [26], and have various pharmacological activities such as wound healing [26], antioxidant [27,28,29,30], antimicrobial [30,31,32], antifungal [33], antimalarial [34], anti-inflammatory [35]; anticancer [20,36,37] and gastrointestinal effects [38]. A dichloromethane (CH2Cl2) extract of the whole plant tested on two pathogenic dermatophytes, Trichophyton rubrum and Microsporum gypseum, showed moderate activity at a concentration of 50 μg/disk, but very strong activity at a concentration of 200 μg/disk, suggesting that A. cathartica may possess antidermatophyte constituents that could be useful in the treatment of ringworm [39,40].
In general, insects are not strictly restricted to the leaves and reproductive parts of living plants. Most studies conducted on strict herbivores [41] indicate that larval hosts of sphingids are concentrated on a few plant families whose foliage is particularly rich in alkaloids, milky sap, essential oils, irritant acids or other small toxic natural products (e.g., Rubiaceae, Euphorbiaceae, Apocynaceae, Vitaceae, Bignoniaceae, etc.).
However, all parts of A. cathartica are rich in secondary metabolites, which are exhaustively listed in the review by Petricevich and Abarca-Vargas [42], with plumieride, plumericin, and allamandin being the most characteristic. A total of 151 compounds were distributed as follows: 3 hydrocarbons in flowers; 7 alcohol compounds, 9 esters, 1 ether, 6 aldehydes, and 1 ketone in flowers, leaves, and stems extracts; 37 fatty acids and phospholipids; 43 volatile compounds mostly in flowers and leaves; 5 phenolic compounds and 6 flavonoids in the flowers and stems; 2 alkaloids in stems; 11 steroids and terpenes in leaves, stems and flowers; and 14 lactones in roots, stems, leaves, flowers and bark; 6 carbohydrates in leaves, stems, and nectar. Of the compounds identified, leaves contain: ursolic acid, β-amyrin; β-sitosterol, sesquiterpenes, plumericin, plumieride, long chain esters, flavonoids, polyphenols, allamandin, alkaloids, saponins and carbohydrates; stem and bark contain: ursolic acid, β-amyrin, β-sitosterol, triterpenoids, glucosides, alkaloids, flavonoids and polyphenols; flowers contain: quercetin, quercitrin, kaempferol, hesperetin, flavonoids, polyphenols and polysaccharides; and roots contain: lactones, allamandin, allamandicin, allamdin, plumieride iridoids, triterpenoids, alkaloids and several glucosides [36,38,43] (cited by [44]); [19,21,27,42]. Like A. cathartica, various parts of P. rubra are widely used in traditional medicine [45]. P. rubra contains many chemical constituents such as glycosides, phenolic compounds (phenolic acids and flavonoids), alkaloids, amino acids and terpenoids (iridoids) which give the flower antibacterial and antifungal activities. However, the main chemical constituents responsible for the pharmacological activities of P. rubra are: plumieride, fulvoplumierine, lupeol, rubrinol, stigmasterol, oleanolic acid, taraxasteryl acetate, rubranonoside, plumieride-p-E-coumarate, isoplumericin, rubrajalellol and plumericin [46,47,48,49]. These molecules form plant may have antimicrobial activities and effects and may be of interest for use against pathogenic microorganisms.
In nature, predatory insects are highly host-specific and exhibit a variety of traits. Direct associations between plants and insects are ubiquitous, and in the context of tropical chemical ecology, many different plant–herbivore interactions are highly specialized [50]. What attracts an egg-laying insect to one plant and prevents it from laying its eggs on another is often an aspect of plant chemistry that is recognized by the insect [51]. Nevertheless, plants can biosynthesize protective secondary metabolites (in the milky sap or via vascular tissue or in specialized tissues) in a self-defense process to reduce herbivory. In turn, herbivores may respond to these compounds and attempt to metabolize them, selectively excrete them, or use them (after ingestion into the body) for their own defense [52,53]. In 2022, McCoy and colleagues showed that for Eocene herbivorous insects that eat leaves with such defense mechanisms, a distinct burrowing or cutting behavior that disrupts the supply of protective compounds distal to the plant tissue allowed the insect to eat the leaf [54].
The coevolution of specialized plant–insect herbivore interactions has been the subject of decades of study [55,56,57]. Lepidoptera caterpillars, for example, often specialize on toxic plants and are able to either sequester [58,59,60], metabolize [61,62] or excrete chemical compounds from their hosts in an unmodified form [63]. Understanding how herbivores deal with toxic phytochemicals in their diet is necessary to understanding the evolution of the two most biodiverse groups of multicellular organisms: plants and insects.
In this article, we propose to explore the chemical interaction between P. tetrio and A. cathartica using different analytical methods, including an innovative electrochemical approach (called electrochemical ecology) and multivariate analysis, and we investigate the potential antimicrobial effects (antibacterial and antifungal activities) of this interaction, in order to gain a better understanding of their specificity.
2. Results and Discussion
2.1. Phytochemical Tests
Thin layer chromatography (TLC). First, a TLC study was conducted. The different results of this approach are presented in Table 1. In our first hypothesis (Hyp. 1), we propose that the caterpillars take up and store certain metabolites (for example, the metabolite with an Rf (Retention factor) of 0.16 on TLC 3 (orange color)). The second hypothesis proposed (Hyp. 2), also listed in Table 1, refers to the selective excretion of an unidentified molecule by the caterpillars (for example, the metabolite with an Rf of 0.95 on TLCs 2 and 3 (blue color)). Finally, the third hypothesis (Hyp. 3) refers to the response of the healthy plant to herbivory. For example, the metabolite with an Rf of 0.28 on TLC 1 (pink color), since this metabolite is present in healthy leaves and not in predated leaves. The opposite case is observed with the metabolite with an Rf value of 0.18 on TLC 4 (green color), which is present only in predated leaves and could therefore be considered a metabolic marker for herbivores.

Table 1.
Organic phases obtained with TLC results. (A) Healthy A. cathartica leaves, (B) herbivored A. cathartica leaves, (C) caterpillar bodies and (D) caterpillars’ feces. Solvent percentages are presented as highest polarity in solution (MeOH and EtOAc) *.
Nuclear magnetic resonance spectroscopy1H NMR. The 1H NMR data were obtained only from the organic extracts of the plants and caterpillars using DMSO-d6 as a solvent. Solvent signals from deuterated DMSO and residual water at 2.5 and 3.3 ppm, respectively, were removed (Figure 1). The analysis shows minor differences between the spectra of healthy and predated Allamanda leaves. This result is inconsistent with hypothesis 3 (in which a plant response to aggression was observed).
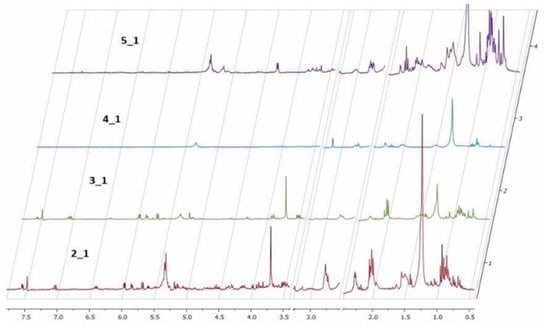
Figure 1.
1H NMR of organic extracts in DMSO-d6. (2_1) Healthy Allamanda leaves; (3_1) predated Allamanda leaves; (4_1) caterpillar bodies and (5_1) caterpillar feces.
The 1H NMR spectra of caterpillars’ bodies and feces show large differences between the samples, and the chemical profile of healthy Allamanda leaves and the caterpillar feces is very similar. This result confirms hypothesis 2 and shows the similarity between the chemical profile of A. cathartica leaves and caterpillar feces compared to the caterpillar body. These similarities and dissimilarities confirm our hypothesis (Hyp. 3) of the selective excretion of toxic compounds.
High performance liquid chromatography coupled with mass spectrometry (HPLC-MS). In this study, the samples containing the healthy and eaten leaves of A. cathartica, as well as the caterpillars and their feces, were analyzed using high-performance liquid chromatography techniques [19,64,65,66] (Figure 2). The similar profile observed in these spectra indicates the presence of the same main compounds in the healthy (A) and predated (B) A. cathartica leaves, with similar retention times (RT) of 20.00 min and 20.50 min, respectively. A second compound can be identified in these spectra with comparable retention times to hypothesis 3 (RTs: 25.52 min and 26.02 min). These results are in contrast to hypothesis 3 (as we can see in the TLCs and 1H NMR profiles). The analysis shows the presence of this compound in the caterpillar’s feces (D, RT: 25.73 min) but not in its body (C), which also confirms hypothesis 2 (in agreement with the 1H NMR and TLC results). Finally, the results show that the compounds found in P. tetrio’s bodies (C, RT: 20.43 min and 25.66 min) are significantly different from other compounds found in the feces (also confirming hypothesis 2).
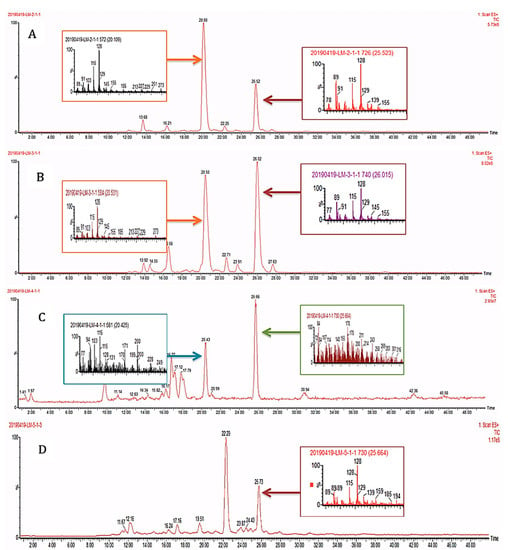
Figure 2.
HPLC-MS profiles of (A) healthy and (B) predated leaves of A. cathartica and (C) caterpillar bodies and (D) caterpillar feces.
Electrochemical investigation. Cyclic voltammetry (CV) studies have been used to investigate possible interactions between different compounds and were previously used to monitor plant defenses against external stressors [67,68,69,70]. Here, the predation of A. cathartica by P. tetrio has been monitored electrochemically using CV. We studied the electrochemical response of films (on the surface of a glassy carbon electrode) of organic and aqueous extracts of healthy and predated leaves of the plant and samples of bodies and feces of P. tetrio in 0.10 mol L−1 phosphate solution buffer at pH 7.0. The reported methodology is ultimately based on the voltammetry of microparticles (VMP) technique [71].
The behavior of films on glassy carbon electrodes of healthy and predated leaves of A. cathartica is shown in Figure 3. A series of chemically irreversible oxidation processes: A1, A2 and A3, were observed (0.28 V, 0.65 V and an undefined shoulder at 1.00 V, respectively). While the peaks A1 and A2 decrease with cycling until a fully passivated and stable state is reached at about the second cycle, an increase in the A3 peak was observed. Thus, the variations in the ratio of peaks A1 and A3 suggest differences in the nature of the redox species of A. cathartica and in leaves that are strengthened by them. These oxidation processes represent a profile observed in several plant species and are due to the oxidation of polyphenolic organic compounds. Indeed, the voltammogram of the predated leaves exhibits the same signals as healthy leaves, but peak A1 is depleted while the A3 signal is enhanced. Finally, a reduction process can be observed at about −1.0 V when the potential sweep is reversed to less positive potentials. Although these processes are slightly more negative in healthy leaves than in predated leaves, both are consistent with the typical behavior of the oxygen reduction reaction (ORR) in water. This result basically contradicts hypothesis 3 about the chemical response to the herbivory, but using this technique, we can identify slight differences between healthy and predated leaves, possibly related to polyphenols (reaction against oxidative stress) [72].
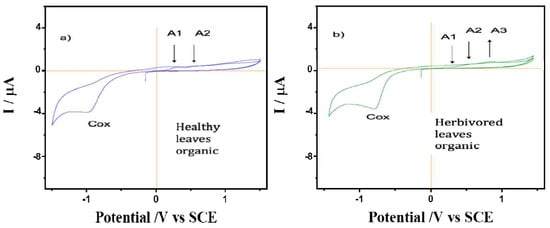
Figure 3.
Cyclic voltammograms of films from (a) healthy and (b) predated leaves of A. cathartica on glassy carbon electrode in contact with air-saturated 0.10 M phosphate buffer at pH 7.0. Scan rate 10 mV.s−1.
The CV of films on glassy carbon electrodes of P. tetrio’s bodies and its excrement after predation of leaves of A. cathartica are shown in Figure 4. CV shows no clear oxidation process in the initial anodic scan for the body of P. tetrio, but three oxidation processes for its excreta at 0.22, 0.45 and 0.78 V for A1, A2 and A3, respectively, which are similar to those found for the A. cathartica analysis. Again, a typical oxygen reduction reaction (ORR) in water was observed in the cathodic region in both analyses. In addition, a characteristic signal C1 appears in both samples, indicating the presence of oxidized metabolite of P. tetrio. This result confirms hypothesis 2, in agreement with the other analytical approaches.
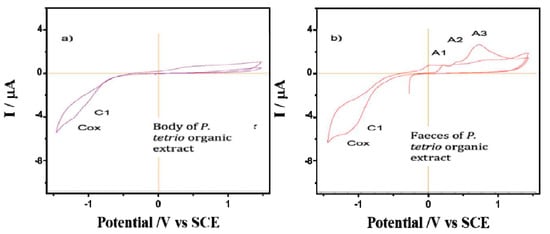
Figure 4.
Cyclic voltammograms of films from (a) P. tetrio’s bodies and (b) P. tetrio’s excrement after predation on leaves of A. cathartica on glassy carbon electrode in contact with air-saturated 0.10 M phosphate buffer at pH 7.0. Potential scan rate 10 mV.s−1.
A comparison was made between the CV of films from the water extracts of healthy and spoiled leaves of A. cathartica in contact with air-saturated 0.10 mol L−1 phosphate buffer at pH 7.0 (Figure 5). Here, signal A1 appears clearly recorded in the healthy leaves and is accompanied by a well-defined cathodic peak at—1.25 V (C2). Signal C2 disappears in the CV of the damaged leaves, while signal A1 decreases and signal A3 is clearly visible at 0.78 V. This result confirms hypothesis 3, which shows a reaction of leaves against herbivory; the disappearance of signal C2 and the appearance of signal A3 are a result of herbivory. This CV is reproduced to some extent reproduced in the aqueous extracts of P. tetrio and its excrement, as shown in Figure 6, thus suggesting the existence of common electroactive compounds.
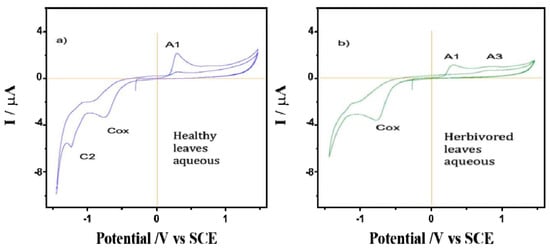
Figure 5.
Cyclic voltammograms of films from the water extracts of (a) healthy and (b) predated leaves of A. cathartica on glassy carbon electrode in contact with air-saturated 0.10 M phosphate buffer at pH 7.0. Potential scan rate 10 mV.s−1.
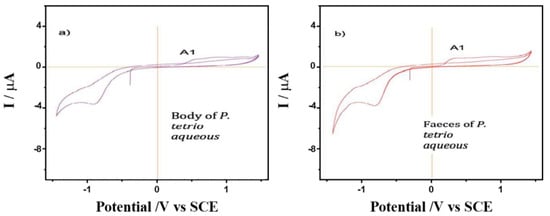
Figure 6.
Cyclic voltammograms of films from water extracts of (a) P. tetrio’s bodies and (b) its excrement after predation on leaves of A. cathartica on glassy carbon electrode in contact with air-saturated 0.10 M phosphate buffer at pH 7.0. Potential scan rate 10 mV.s−1.
Principal Component Analysis (PCA). A total of 24 chromatograms were acquired using HPLC-EI-MS, 12 from the aqueous fraction and 12 from the organic fraction (three biological replicates for each species). To identify differences in the metabolites or even in their concentration in the samples, the set of 24 samples was analyzed, obtained from XCMS and used for data analysis. Two spreadsheets were obtained after data processing of the HPLC-MS analysis, with 168 and 412 variables from the organic and aqueous fractions, respectively (retention time m/z). In the PCA analysis, two plots were generated, namely score and loading plots. The first one shows the sample groupings, whereas the second one indicates the contribution of each variable to these samples. The PCA analysis was focused on the detection of any inherent pattern within the data. As observed in Figure 7, samples of healthy and predated Allamanda leaves are closely related, with small differences from each other, when compared with the caterpillars’ bodies and feces. Further, when all samples are compared, the predated and not predated leaves from Allamanda are very similar to each other, and different from the other samples. In addition, the caterpillar body extracts present a great difference. In Figure 8 (loadings plot), the variables that influence the grouping observed are presented.
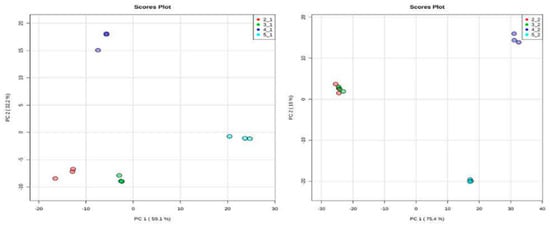
Figure 7.
Score plot of 12 samples of organic and aqueous extracts of all manipulation, obtained from 168 (left) and 412 (right) variables, respectively. (2_1and 2_2) red circle, healthy Allamanda leaves; (3_1and 3_2) green circle, predated Allamanda leaves; (4_1and 4_2) blue circle, caterpillar bodies and (5_1and 5_2) light blue circle, caterpillar feces.
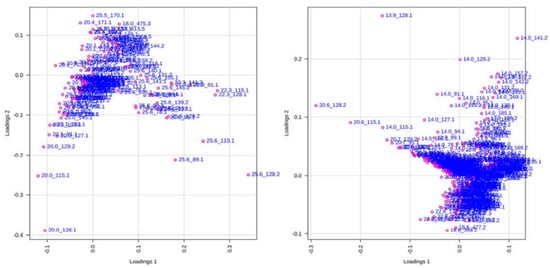
Figure 8.
Score plots of 12 samples of organic and aqueous extracts of all manipulation, obtained from 168 (left) and 412 (right) variables, respectively. Each dot represents one variable (retention time m/z).
2.2. Microbial Activities
The potential antimicrobial activities of P. tetrio/A. cathartica interaction are shown in Table 2. The yellow boxes show the effects of the consumption of the A. cathartica leaves by the caterpillar. The comparison between the healthy leaves (A) and the leaves eaten by the caterpillar (B) shows an increase or occurrence of inhibitory activity in favor of the leaves eaten by the caterpillar (B) on the microorganisms, regardless of the type of extraction (Aqueous (A); Organic (O)), AO versus BO for Escherichia coli and Candida albicans (diameter of inhibition: 7 mm versus 0 mm; 9,5 mm versus 7 mm); and AA versus BA for Escherichia coli and Candida albicans (diameter of inhibition: 7 mm versus 0 mm; 9 mm versus 8 mm)). The occurrence or enhancement of this bioactivity in the predated leaf could be explained by the induction of a chemical or biological defense reaction of the host plant against herbivory (proposed hypothesis 3).

Table 2.
Microbial activity results of aqueous and organic extracts, obtained from Allamanda cathartica (A. cathartica) leaves, caterpillar bodies and caterpillar feces on three bacterial (Escherichia coli; Staphylococcus aureus; Pseudomonas aeruginosa) and two fungal (Candida albicans; Aspergillus fumigatus) strains.
The green and blue boxes, respectively, represent the non-uptake and the uptake of bioactive compounds from the leaves into the bodies of the caterpillars. The samples obtained after extraction with organic solvents (O) show the same evolution of the inhibitory profile in three of the five microorganisms tested (Escherichia coli, Staphylococcus aureus and Aspergillus fumigatus). Our results suggest a selective excretion of bioactive compounds from ingested leaves into the feces without being retained or ingested in the caterpillar’s body (green boxes). Indeed, the feces of P. tetrio’s (DO) show inhibitory activity that is as strong or stronger than that of the eaten A. cathartica leaves (BO) [diameter of inhibition fecal matter (DO) versus eaten leaves (Bo): 11 mm versus 7 mm for Escherichia coli; 9.5 mm versus 8 mm for Staphylococcus aureus; and 10,5 mm versus 11 mm for Aspergillus fumigatus) whereas caterpillar body extracts (CO) showed no bio-inhibition (proposed hypothesis 2). The inhibitory effect of the organic and aqueous samples on Pseudomonas aeruginosa and Escherichia coli, respectively (blue boxes), shows an opposite profile. Indeed, the eaten leaves and the caterpillar body show a bio-inhibition that is not found in the caterpillar feces. This suggests that certain bioactive compounds in the eaten leaves, different from the previous ones, could be selectively taken up and stored specifically in the caterpillar body.
There are few data in the literature on the potential antimicrobial activity of predated leaves and the interaction between caterpillars and plants. Our samples showed higher antimicrobial activity than that reported by Islam et al. (2010) [31] for a concentrated methanol extract of healthy A. cathartica leaves fractionated into fractions soluble in petroleum ether, carbon tetrachloride, chloroform and water. The zones of inhibition observed by them have a diameter of less than 10 mm for E. coli, S. aureus, C. albicans and P. aeruginosa, at a tested concentration of 400 µg disk−1 against the 100 µg disk−1 in our case. At the same time, they showed higher antimicrobial activity with their methanolic and ethyl acetate extracts from healthy leaves [73]. Quercitrin was explicitly attributed antifungal and antibacterial activity [43].
Our qualitative results on biological activity may explain why P. tetrio can feed on this toxic Apocynaceae species. Indeed, the spectral (NMR) profiles of the eaten leaves and the caterpillar droppings are very similar, but very different from those of the caterpillar bodies. There is an association between the plant and caterpillar (specific herbivory). The plant produces organic chemical compounds for defense when the caterpillar attacks, and the caterpillar excretes or assimilates these molecules or derivatives by its own mechanism. The inhibition may be due to a synergistic effect of the different molecules contained in the extracts for all the microorganisms, hence the interest in using different analytical techniques. The genetic and cellular mechanisms by which metabolite diversity arises are becoming better understood, but the evolutionary explanations for the continued diversification of plant secondary metabolites have received less attention. Speed et al. (2019) [74] show a fundamental coevolutionary asymmetry between plants and their herbivores, which is that herbivores must resist all plant toxins, whereas plants must challenge and override only a single resistance trait.
Sequestration likely evolved as a protection from predators [75], [76] because caterpillars with chemical protection are less attractive to predators [77]. P. tetrio feeds on toxic species of the Apocynaceae and is aposematically colored, leading to the hypothesis that P. tetrio caterpillars may sequester compounds from their host. In many plant species, herbivory leads to the increased production of a secondary metabolite [78]. In particular, herbivores specialized in sequestration are thought to benefit from the induction of intermediate levels of chemical defenses, while plants eaten by such herbivores would benefit most from weak or strong induction. On the other hand, specialists that do not specialize in sequestration may not be deterred by induction unless it is high [55]. Caterpillars also have the ability to metabolize some organic chemical compounds and excrete others intact from the same host plant [63]. This is the case in Monarch butterflies, Danaus plexippus Linn. [58]. The study of Ramos et al., 2015 [79], compared the proteolytic system of the gut of P. tetrio and D. plexippus with the proteolytic system of the milky sap of their respective host plants (P. rubra and Calotropis procera Aiton). This revealed that the ability of the insect proteolytic systems (serine and cysteine peptidase inhibitors) to digest the milk sap proteins (in vivo) appears to be an important event favoring the caterpillars in overcoming plant chemical defenses.
3. Materials and Methods
3.1. Plant Material
Sample preparation. Healthy and eaten leaves of A. cathartica, as well as P. tetrio caterpillars were collected on the island of Guadeloupe (French West Indies), specifically in Le Gosier (16°13’00.5” N 61°31’09.9” W). The leaves were cleaned (with distilled water) and lyophilized. The caterpillars were kept in a cage for 24 h, to collect their feces, and starved for 48 h to use their corpse in the evaluation of biomolecule incorporation into the bodies of the caterpillars, excluding the digestive material. Finally, the dried leaves, the caterpillars’ bodies and feces were lyophilized and powdered. Afterwards, 50 g of each sample was extracted by maceration extraction for 48 h. Maceration was performed in a ternary mixture of dichloromethane/methanol/distilled water [80,81] (1:1:1 v/v, 200 mL). After the complete extraction, the solutions were filtered and extracted by liquid–liquid extraction and two phases were obtained for each manipulation (Table 3). Finally, these phases were dried by rotary evaporation.

Table 3.
Phases obtained for each manipulation after liquid–liquid extractions.
3.2. Phytochemical Tests
Analysis and quantification. Analyses were performed using several techniques such as Thin Layer Chromatography (TLC), Nuclear Magnetic Resonance Spectroscopy (1H-NMR), High Performance Liquid Chromatography coupled with Mass Spectrometry (HPLC-MS) and Cyclic Voltammetry (CV).
Thin layer chromatography (TLC). The thin layer chromatography of all the organic phases was carried out with a gradient of two different mixtures of elution solvents composed of CH2Cl2/MeOH or nhexane/AcOEt with a volume of 10 mL in different proportions (80/20, 50/50, 90/10, 95/5, 99/1, 50/50). Further, TLCs of all aqueous phases were made on cellulose TLC with a mixture of H2O/MeOH elution solvent in different proportions (50/50–100) as a mobile phase.
Nuclear magnetic resonance spectroscopy (1H-NMR). 15 mg of each extract was solubilized in 650 µL of DMSO and transferred to NMR tubes. 1D 1H nuclear magnetic resonance spectra were recorded with a BRUKER Avance 300 MHz spectrometer equipped with a BBO probe and automatic tube changer. Chemical shifts (δ) were expressed in ppm relative to tetramethylsilane (TMS) taken as the external reference, with internal calibration performed on the solvent signal. All spectra were processed using Topspin 2.1 software. The classical 1D proton with a 90° pulse width was performed. The spectra were acquired using 256 scans and 2 dummy scans of 32 K data points with a spectral width of 5411.255 Hz.
High performance liquid chromatography coupled with mass spectrometry (HPLC-MS). Triplicates of each extract were made and dissolved in 750 µL of methanol (LCMS grade) and 750 µL of ultra-pure water (ElgaPurelab Classic). The mixtures were sonicated in the ultrasonic bath then filtered on 13 mm and 0.45 µm PTFE filters and placed in amber vials. The analysis was performed on a Waters (Milford, MA, USA) Alliance e2695 liquid chromatographic system, equipped with a Waters 2996 photodiode array detector (PDA), coupled with a orthogonal quadrupole mass spectrometer (Micromass ZQ, Manchester, UK). The systems were controlled by MassLynx v.4.1 software (Micromass, Manchester, UK). The mass was equipped with an electrospray ionization ESI (Waters) source; the ionization was performed in positive mode. A double detection was carried out via mass spectrometry in ESI (range 71 to 1200 Da) and using a PDA diode array detector (UV detection between 210 and 400 nm). The analytical column was an XTERRA MS C18 column (2.1 x 100 mm, 3.5 µm) (Waters). The elution was performed with a mobile phase flow rate of 0.2 mL/min consisting of a mixture of ultra-pure water containing 0.1% formic acid (A) and methanol containing 0.1% formic acid (B). The program started at t = 0 min with a ratio (A: B) of 95:5, at t = 2 min (95:5), at t = 5 min (70:30), at t = 15 min (60:40), at t = 40 min (50:50), at t = 55 min (45:55), at t = 60 min (0:100), at t = 65 min (0:100), at t = 75 min (95:5) and at t = 80 min (95:5). The injection volume was 10 µl and detection was at 280 nm and at 320 nm. The ESI-MS parameters were as follows: desolvation gas (N2) flow rate: 650 L/h; cone gas flow rate: 40 L/h; drying gas temperature: 450 °C; source temperature: 120 °C; capillary voltage: 3 kV; cone voltage: 85 V; and RF lens voltage: 0.1 V.
Cyclic Voltammetry (CV). Cyclic voltammograms were recorded at 298 ± 1 K in a conventional three electrode cell using a platinum wire auxiliary electrode and an Ag/AgCl (3M NaCl) reference electrode. Measurements were carried out with CH I660 equipment using 0.10 M potassium phosphate buffer at pH 7.0 as a supporting electrolyte. The working electrode was prepared by evaporating 50 μL of an ethanol (EtOH) suspension of the extract of interest, ground leaves or insect samples, under air on a glassy carbon electrode (GCE, BAS MF 2012, geometrical area 0.071 cm2). In order to mimic the natural environment, no degasification of the electrolyte was performed.
Principal Component Analysis (PCA). The raw data from HPLC-MS were exported as netCDF files, using DataBridge software (Waters, USA), and pre-processed using XCMS Online [82,83] for feature detection, retention time correction and alignment of metabolites detected on HPLC-MS analysis. The dataset was created with 12 samples from each organic and aqueous extract fraction (3 samples from each manipulation (see Table 1)). Peak detection was performed using cent Wave peak detection (Δm/z = 10 ppm; minimum peak width, 5 s; maximum peak width, 20 s) and mzwid = 0.015, minfrac = 0.5, and bw = 5 were used for the retention time alignment. The processed data (csv file) were further exported to MetaboAnalyst 4.0 [84]. All data variables were scaled by the pareto method prior to PCA. PCA is an unsupervised method commonly used to identify patterns between multivariate samples [85,86] and was recently employed on the chemical variability of Allamanda cathartica extracts [87].
3.3. Antimicrobial Activities
To investigate the potential antimicrobial effects of the interaction between the caterpillar Pseudosphinx tetrio L. and one of its host plants, Allamanda cathartica, we focused our study on five pathogen microorganisms that are responsible of severe human disease and usually involved in hospital acquired illness: Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus for bacteria and Candida albicans and Aspergillus fumigatus for fungi. All strains were supplied by the ATCC (American Type Culture Collection).
The three bacteria were cultured on the non-selective medium Tryptic Soy Agar, whereas the two fungi were cultured on the selective medium Sabouraud, which is a selective medium for fungi and yeasts. These two media were provided by the manufacturer Grosseron, France, and prepared according to the instructions.
For each microorganism, starting cultures were prepared on Tryptic Soy Agar media for bacteria and Sabouraud media for fungi. From these starting cultures, microbial suspensions were created by picking two colonies from the starting cultures and adding them to 2 mL of sterile water. Finally, 200 µL of these microbial suspensions were used to inoculate the culture media used to test the antimicrobial activities.
Disc diffusion assay was used in order to test the antimicrobial activities of interaction between P. tetrio and A. cathartica. Sterile filter paper discs of Whatman no.1 (6 mm in diameter) were placed on the surface of the culture media with sterile forceps and gently pressed to ensure good contact with the surface. The different organic extracts were prepared as follows: 5 mg of each extract was mixed in 500 µL of Dimethyl sulfoxide (DMSO) and 10 µL of these mixtures were placed on antibiotic disks (100 μg/disk). The plates were incubated for 24 h to 48 h at 37 °C for bacteria and 30 °C for fungi. The zone of inhibition was calculated by measuring the diameter of the inhibition zone around the well (mm). All tests were conducted in duplicate.
4. Conclusions
In conclusion, we investigated the plant–herbivore interaction between the caterpillar Pseudosphinx tetrio and the flowering plant A. cathartica. In order to better understand this trophic relationship, several techniques were used, such as TLC, 1H NMR, HPLC-MS (analyzed using a 13 multivariate PCA) and an innovative approach using electrochemical methods (electrochemical ecology). The measured antimicrobial activities support the physicochemical tests. The results show a similar profile between the leaves of healthy and predated A. cathartica and the excretions of the caterpillars. The similar analytical profile between the leaves of A. cathartica and the excretions of P. tetrio, and the difference with the caterpillar bodies, suggests a selective excretion of compounds by the caterpillar (proposed hypothesis 2). These organic compounds found selectively in the excretions (rather than in the body) could explain the ability of P. tetrio to feed on this toxic Apocynaceae species.
Author Contributions
Conceptualization, G.C.-T. and M.S.; methodology, validation, formal analysis, investigation, L.M., M.M.L., M.M., M.V.C., D.P.V., M.V.P.-M., M.-N.S., L.P., A.D., Z.B., P.M. and G.C.-T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support of the “AgroEcoDiv” project from the European Union Fund (FEDER) and “Région Guadeloupe”. We would also like the Caribaea Initiative association for a two month study grant from Matignon. DPV would like to thank the OMICAS program: Optimización Multiescala In-silico de Cultivos Agrícolas Sostenibles (Infraestructura y validación en Arroz y Caña de Azúcar) Scientific Ecosystem belonging to the Colombia Científica Program, sponsored by The World Bank, The Ministry of Science, Technology and Innovation (MINCIENCIAS), ICETEX, the Colombian Ministry of Education and the Colombian Ministry of Commerce, Industry and Tourism, under GRANT ID: FP44842-217-2018, OMICAS Award ID: 792-61187.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks are due to Tara Joy Massad (Gorongosa National Park, Mozambique) for her attentive lecture and fruitful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cock, M.J.W. Hawk-moths (Sphingidae) of Trinidad, West Indies: An illustrated and annotated catalogue. Living World J. Trinidad Tobago Field Nat. Club 2018, 2018, 10–81. [Google Scholar]
- Ríos, S.; Drechsel, U. Nuevos registros de Pseudosphinx tetrio (Linnaeus 1771) en el Paraguay (Lepidoptera: Sphingidae). Parag. Biodivers. 2017, 4, 60–65. [Google Scholar]
- Santiago-Blay, J. Notes on Pseudosphinx tetrio (L.) (Sphingidae) in Puerto Rico. J. Lepid. Soc. 1985, 39, 208–214. [Google Scholar]
- Dunford, J.; Barbara, K. Tetrio Sphinx, Giant Gray Sphinx, Frangipani Hornworm, Pseudosphinx tetrio (Linnaeus) (Insecta: Lepidoptera: Sphingidae)1. EDIS 2019, 2005, 5. [Google Scholar] [CrossRef]
- Cock, M.J. Pseudosphinx tetrio (L.) (Lepidotera: Sphingidae) in Trinidad and Tobago. Living World J. Trinidad Tobago Field Nat. Club 2008, 2000, 49–52. [Google Scholar]
- Meurgey, F.; Ramage, T. Challenging the Wallacean shortfall: A total assessment of insect diversity on Guadeloupe (French West Indies), a checklist and bibliography. Insecta Mundi 2020, 786, 1–183. [Google Scholar]
- D’Aguilar, J. Catalogue raisonné des Insectes des Antilles françaises 1. Lépidoptèreq, Sphingidae. Annales Des Epiphyties 1950 1966, 17, 251–262. [Google Scholar]
- Chalumeau, F.; Delplanque, A. Catalogue commenté des Ctenuchidae (Lepidoptera) des Antilles françaises. Bull. De La Société Linnéenne De Lyon 1978, 47, 176–187. [Google Scholar] [CrossRef]
- Sautière, C. Insectes capturés aux abords et dans les limites de Parc national de la Guadeloupe. Cerambycidae, Scarabaeidae, Sphingidae. Bull. De L’entomologie Tourangelle 1999, 20, 13–34. [Google Scholar]
- Meurgey, F. Les Arthropodes Continentaux de Guadeloupe: Synthèse Bibliographique Pour un État des Lieux Des Connaissances; Ropport SHNLH pour le Parc national de Guadeloupe; 2011. [Google Scholar]
- MNHN & OFB. Fiche de Pseudosphinx tetrio (Linnaeus, 1771). Inventaire Natl. Du Patrim. Nat. INPN 2022, 2003. Available online: https://inpn.mnhn.fr/espece/cd_nom/641756 (accessed on 5 December 2022).
- Warrell, D.A. Researching nature’s venoms and poisons. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 860–866. [Google Scholar] [CrossRef] [PubMed]
- De Souza Leão Veiga, A.F.; de Melo, J.P.R.; Moreira, M.D. Aspectos Morfológicos da Forma Imatura de Pseudosphinx tetrio (LINNAEUS, 1771) (LEPIDOP-TERA: SPHINGIDAE). Jorn. De Ensino Pesqui. E Extensão-JEPEX 9. Recife 2009. [Google Scholar]
- Haxaire, J.; Rasplus, J.-Y. Contribution à la Connaissance des Sphingidae de Guyane Française. 1e Partie. Bull. De La Société Entomol. De Fr. 1986, 91, 275–285. [Google Scholar] [CrossRef]
- Haxaire, J.; Herbin, D. Les Lépidoptères Sphingidae de Bolivie. Ecologie et Systématique. 2ème Partie: Les Sous-Familles des Smerinthinae et Macroglossinae Pro Parte (1). Rev. Assoc. Rousillon. Entomol 2000, 9, 4–19. [Google Scholar]
- Meerman, J.C. The Annual Outbreak of Colorful Caterpillars of the Frangipani Hawkmoth (Pseudosphinx tetrio). Biol. Divers. Belize 2009. Available online: http://biological-diversity.info/pseudosphinx.htm (accessed on 8 December 2009).
- Sloan, S.A.; Zimmerman, J.K.; Sabat, A.M. Phenology of Plumeria alba and its Herbivores in a Tropical Dry Forest. Biotropica 2007, 39, 195–201. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Sudha, S.S. In-Vitro Antimicrobial Activity and In-Vivo Toxicity of Moringa Oleifera and Allamanda Cathartica against Multiple Drug Resistant Clinical Pathogens. Int. J. Pharma. Bio. Sci. 2013, 4, B768–B775. [Google Scholar]
- Khanam, M.N.; Anis, M.; Ahmad, S. Establishment of adventitious root cultures of Allamanda cathartica L. for the production of iridoid glycosides and its identification using HPTLC MS. Ind. Crops Prod. 2018, 125, 198–206. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.; Chan, E. Botany, uses, phytochemistry and pharmacology of selected Apocynaceae species: A review. Pharmcognosy Commun. 2013, 3, 2–11. [Google Scholar]
- Chandreyi, G.; Labani, H.; Sudip, K.N.; Sayantan, S.; Alokila, D.; Swagata, B.; Maitrayee, B.; Pranabesh, G.; Sirshendu Chatterjee Allamanda cathartica Linn. Apocynaceae: A mini review. Int. J. Herb. Med. 2019, 7, 29–33. [Google Scholar]
- Francis, J.K. Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions, Volume 1; U.S. Department of Agriculture, Forest Service, International Institute of Tropical Forestry: San Juan, PR, USA, 2004; p. IITF-GTR-26. [CrossRef]
- WFO. Allamanda L. Published on the Internet. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-4000001228 (accessed on 5 December 2022).
- Pessoa, C.; Costa-Lotufo, L.V.; Leyva, A.; de Moraes, M.E.A.; de Moraes, M.O. Anticancer potential of Northeast Brazilian plants. In Advances in Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 197–211. [Google Scholar] [CrossRef]
- Rahman, A.H.M.; Akter, M. Taxonomy and Traditional Medicinal Uses of Apocynaceae (Dogbane) Family of Rajshahi District, Bangladesh. Res. Rev. J. Bot. Sci. 2015, 4, 5–13. [Google Scholar]
- Nayak, S.; Nalabothu, P.; Sandiford, S.; Bhogadi, V.; Adogwa, A. Evaluation of wound healing activity of Allamanda cathartica. L. and Laurus nobilis. L. extracts on rats. BMC Complement. Altern Med. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Banerjee, S. Floral extracts of allamanda blanchetii and allamanda cathartica are comparatively higher resource of anti-oxidants and polysaccharides than leaf and stem extracts. Int. J. Curr. Pharm. Sci. 2018, 10, 36–39. [Google Scholar] [CrossRef]
- Hameed, A.; Nawaz, G.; Gulzar, T. Chemical composition, antioxidant activities and protein profiling of different parts of Allamanda cathartica. Nat. Prod. Res. 2014, 28, 2066–2071. [Google Scholar] [CrossRef]
- Sarker, R.; Tasnuva, S.; Farhana, I.; Sharmin, R.C. In vitro antioxidant, total phenolic, membrane stabilizing and antimicrobial activity of Allamanda cathartica L.: A medicinal plant of Bangladesh. J. Med. Plants Res. 2014, 8, 63–67. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Kolesnikov, E.; Mandal, A.R.; Kuznetsov, D. Allamanda cathartica flower’s aqueous extract-mediated green synthesis of silver nanoparticles with excellent antioxidant and antibacterial potential for biomedical application. MRS Commun. 2016, 6, 41–46. [Google Scholar] [CrossRef]
- Islam, M.R.; Ahamed, R.; Rahman, M.O.; Al-Amin, M.; Alam, K.D.; Lyzu, F. In Vitro Antimicrobial Activities of Four Medicinally Important Plants in Bangladesh. Eur. J. Sci. Res. 2010, 39, 199–206. [Google Scholar]
- Sharmin, M.; Banya, P.D.; Paul, L.; Chowdhury, F.F.K.; Afrin, S.; Acharjee, M.; Rahman, T.; Noor, R. Study of microbial proliferation and the in vitro antibacterial traits of commonly available flowers in Dhaka Metropolis. Asian Pac. J. Trop. Dis. 2015, 5, 91–97. [Google Scholar] [CrossRef]
- Souza, E.; Barcellos, V.D.A.; Sbaraini, N.; Reuwsaat, J.C.V.; Schneider, R.d.O.; da Silva, A.C.; Garcia, A.W.A.; von Poser, G.L.; Barbosa, E.G.; Lima, J.P.M.S.; et al. A Plumieridine-Rich Fraction From Allamanda polyantha Inhibits Chitinolytic Activity and Exhibits Antifungal Properties Against Cryptococcus neoformans. Front. Microbiol. 2020, 11, 2058. [Google Scholar] [CrossRef]
- Conrad, O.A.; Dike, I.P.; Agbara, U. In vivo antioxidant assessment of two antimalarial plants–Allamamda cathartica and Bixa orellana. Asian Pac. J. Trop. Biomed. 2013, 3, 388–394. [Google Scholar] [CrossRef]
- Boeing, T.; de Souza, P.; Bonomini, T.J.; Mariano, L.N.B.; Somensi, L.B.; Lucinda, R.M.; Malheiros, A.; da Silva, L.M.; Andrade, S.F.d. Antioxidant and anti-inflammatory effect of plumieride in dextran sulfate sodium-induced colitis in mice. Biomed. Pharmacother. 2018, 99, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Dessertine, A.L.; Blaylock, B.T.; Bryan, R.F. Isolation and structural elucidation of allamandin, and antileukemic iridoid lactone from allamanda cathartica. J. Org. Chem. 1974, 39, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Lim, Y.; Abdullah, N.; Nordin, F. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Phcog. Res. 2011, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Akah, P.A.; Offiah, V.N. Gastrointestinal Effects of Allamanda cathartica Leaf Extracts. Int. J. Pharmacogn. 1992, 30, 213–217. [Google Scholar] [CrossRef]
- Nithya, K.; Muthumary, J. Bioactive Metabolite Produced by Phomopsis sp., an Endophytic Fungus in Allamanda cathartica Linn. Recent Res. Sci. Technol. 2011, 3, 44–48. [Google Scholar]
- Nahar, A.; Ashrafuzzaman, S.; Islam, M.N.; Alam, M.S. Studies on antidermatophytic effect of Allamanda cathertica. Bangladesh J. Pharm. 2010, 5, 5–7. [Google Scholar] [CrossRef]
- Janzen, D.H. Patterns of Herbivory in a Tropical Deciduous Forest. Biotropica 1981, 13, 271–282. [Google Scholar] [CrossRef]
- Petricevich, V.; Abarca-Vargas, R. Allamanda cathartica: A Review of the Phytochemistry, Pharmacology, Toxicology, and Biotechnology. Molecules 2019, 24, 1238. [Google Scholar] [CrossRef]
- Hema, K.; Krishnaveni, R. Antibacterial and antifungal activities of Allamanda cathartica Linn. Int. J. Pharma Bio. Sci. 2014, 5, 588–593. [Google Scholar]
- Chaithra, A.B.; Satish, S.; Karunakara, H. Therapeutic uses of Allamanda Cathartica Linn. With a note on its pharmacological actions: A Review. Int. J. Pharma Chem. Res. 2016, 2, 227–232. [Google Scholar]
- Bihani, T. Plumeria rubra L.–A review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol. 2021, 264, 113291. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Hussain, M.; Syed, S.K.; Saadullah, M.; Alqahtani, A.M.; Alqahtani, T.; Aldahish, A.A.; Asiri, S.; Zeng, L.-H. Pharmacological Justification for the Medicinal Use of Plumeria rubra Linn. in Cardiovascular Disorders. Molecules 2021, 27, 251. [Google Scholar] [CrossRef] [PubMed]
- Shefali, A.; Kanchan, D.B.; Shailey, S.; Mamta, L.; Tanuja, U.S.; Deepak, K. A review on phytopharmacological activity of plumeria species. Int. J. Eng. Sci. Math. 2018, 7. Available online: http://www.ijma.us (accessed on 5 December 2022).
- Siti Mohamed Isa; Ablat, A.; Mohamad, J. The Antioxidant and Xanthine Oxidase Inhibitory Activity of Plumeria rubra Flowers. Molecules 2018, 23, 400. [Google Scholar] [CrossRef]
- Thakur, M.; Gupta, P.; Roy, A.; Chandrakar, S.; Sahu, J.; Gupta, U.P. Therapeutic potential of Plumeria rubra plant: A review. Adv. J. Bioact. Mol. 2020, 1, 40–47. [Google Scholar]
- Forister, M.L.; Novotny, V.; Panorska, A.K.; Baje, L.; Basset, Y.; Butterill, P.T.; Cizek, L.; Coley, P.D.; Dem, F.; Diniz, I.R.; et al. The global distribution of diet breadth in insect herbivores. Proc. Natl. Acad. Sci. USA 2015, 112, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.C.; Ozaki, K.; Makino, T.; Uchiyama, H.; Yajima, S.; Kawata, M. Evolution of Gustatory Receptor Gene Family Provides Insights into Adaptation to Diverse Host Plants in Nymphalid Butterflies. Genome Biol. Evol. 2018, 10, 1351–1362. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 19, 117–154. [Google Scholar] [CrossRef]
- Eisner, T.; Meinwald, J. Chemical Ecology: The Chemistry of Biotic Interaction; National Academies Press: Washington, DC, USA, 1995; p. 4979. [Google Scholar] [CrossRef]
- McCoy, V.E.; Gee, C.T.; Michalski, J.M.; Wings, O. Oldest fossil evidence of latex sabotaging behavior by herbivorous insects. Rev. Palaeobot. Palynol. 2022, 300, 104631. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.E. Host-Plant Selection by Phytophagous Insects; Springer: Boston, MA, USA, 1994. [Google Scholar] [CrossRef]
- Jones, P.L.; Petschenka, G.; Flacht, L.; Agrawal, A.A. Cardenolide Intake, Sequestration, and Excretion by the Monarch Butterfly along Gradients of Plant Toxicity and Larval Ontogeny. J. Chem. Ecol. 2019, 45, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Engler-Chaouat, H.S.; Gilbert, L.E. De novo Synthesis vs. Sequestration: Negatively Correlated Metabolic Traits and the Evolution of Host Plant Specialization in Cyanogenic Butterflies. J. Chem. Ecol. 2007, 33, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Lampert, E.C.; Dyer, L.A.; Bowers, M.D. Chemical Defense Across Three Trophic Levels: Catalpa bignonioides, the Caterpillar Ceratomia catalpae, and its Endoparasitoid Cotesia congregata. J. Chem. Ecol. 2011, 37, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Winde, I.; Wittstock, U. Insect herbivore counteradaptations to the plant glucosinolate–myrosinase system. Phytochemistry 2011, 72, 1566–1575. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Zangerl, A.R. Furanocoumarin metabolism in Papilio polyxenes: Biochemistry, genetic variability, and ecological significance. Oecologia 1993, 95, 370–375. [Google Scholar] [CrossRef]
- Frankfater, C.; Schühly, W.; Fronczek, F.R.; Slattery, M. Processing of a Sesquiterpene Lactone by Papilio glaucus Caterpillars. J. Chem. Ecol. 2005, 31, 2541–2550. [Google Scholar] [CrossRef]
- Barny, L.A.; Tasca, J.A.; Sanchez, H.A.; Smith, C.R.; Koptur, S.; Livshultz, T.; Minbiole, K.P.C. Chemotaxonomic investigation of Apocynaceae for retronecine-type pyrrolizidine alkaloids using HPLC-MS/MS. Phytochemistry 2021, 185, 112662. [Google Scholar] [CrossRef]
- Prabhadevi, V.; Sahaya, S.S.; Johnson, M.; Venkatramani, B.; Janakiraman, N. Phytochemical studies on Allamanda cathartica L. using GC–MS. Asian Pac. J. Trop. Biomed. 2012, 2, S550–S554. [Google Scholar] [CrossRef]
- Marques, J.; Alves, J.; Torres-Rêgo, M.; Furtado, A.; Siqueira, E.; Galinari, E.; Araújo, D.; Guerra, G.; Azevedo, E.; Fernandes-Pedrosa, M.; et al. Phytochemical Analysis by HPLC–HRESI-MS and Anti-Inflammatory Activity of Tabernaemontana catharinensis. IJMS 2018, 19, 636. [Google Scholar] [CrossRef]
- Palmeira-Mello, M.V.; Souza, J.L.; Pérez, A.F.; Cavalcanti, A.D.S.; Kahn, S.A.; Passe-Coutrin, N.; Guerra, I.R.; Doménech-Carbó, A.; Jauregui-Haza, U.J.; de Souza, A.M.T.; et al. Insights of Tris(2-pyridylmethyl)amine as anti-tumor agent for osteosarcoma: Experimental and in silico studies. J. Mol. Struct. 2021, 1228, 129773. [Google Scholar] [CrossRef]
- Rozoy, E.; Araya-Farias, M.; Simard, S.; Kitts, D.; Lessard, J.; Bazinet, L. Redox properties of catechins and enriched green tea extracts effectively preserve l-5-methyltetrahydrofolate: Assessment using cyclic voltammetry analysis. Food Chem. 2013, 138, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Kablan, L.; Ferreira, M.E.; Rodríguez de la Cruz, D.; Doménech-Carbó, A.; Vera de Bilbao, N.; Rojas de Arias, A.; Figadère, B.; Poupon, E.; Fournet, A. Harvesting canthinones: Identification of the optimal seasonal point of harvest of Zanthoxylum chiloperone leaves as a source of 5-methoxycanthin-6-one. Nat. Prod. Res. 2015, 29, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Cebrián-Torrejón, G.; Lopes-Souto, A.; Martins-de-Moraes, M.; Jorge-Kato, M.; Fechine-Tavares, J.; Barbosa-Filho, J.M. Electrochemical ecology: VIMP monitoring of plant defense against external stressors. RSC Adv. 2015, 5, 61006–61011. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Labuda, J.; Scholz, F. Electroanalytical chemistry for the analysis of solids: Characterization and classification (IUPAC Technical Report). Pure Appl. Chem. 2012, 85, 609–631. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. IJMS 2021, 22, 1442. [Google Scholar] [CrossRef]
- Mannan, M.; Alam, M.; Mustari, F.; Kudrat-E-Zahan, M.; Ali, R.; Haque, A.; Zaman, S.; Talukder, D. In vitro Antioxidant, Antimicrobial, Insecticidal and Cytotoxic Activities of the Medicinal Plants: Allamanda cathartica and Mimusops elengi. EJMP 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Speed, M.P.; Fenton, A.; Jones, M.G.; Ruxton, G.D.; Brockhurst, M.A. Coevolution can explain defensive secondary metabolite diversity in plants. New Phytol. 2015, 208, 1251–1263. [Google Scholar] [CrossRef]
- Petschenka, G.; Agrawal, A.A. Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc. R. Soc. B. 2015, 282, 20151865. [Google Scholar] [CrossRef]
- Duffey, S.S. Sequestration of Plant Natural Products by Insects. Annu. Rev. Entomol. 1980, 25, 447–477. [Google Scholar] [CrossRef]
- Dyer, L.A. Tasty Generalists and Nasty Specialists? Antipredator Mechanisms in Tropical Lepidopteran Larvae. Ecology 1995, 76, 1483–1496. [Google Scholar] [CrossRef]
- Karban, R. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 2011, 25, 339–347. [Google Scholar] [CrossRef]
- Ramos, M.V.; Pereira, D.A.; Souza, D.P.; Silva, M.-L.S.; Alencar, L.M.R.; Sousa, J.S.; Queiroz, J.-F.N.; Freitas, C.D.T. Peptidases and peptidase inhibitors in gut of caterpillars and in the latex of their host plants. Planta 2015, 241, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for Metabolomics: Access to The Metabolome: Extraction for Metabolomics: Access to the Metabolome. Phytochem. Anal. 2014, 25, 291–306. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, N.G.; Genenbacher, J.L.; Patti, G.J. A roadmap for the XCMS family of software solutions in metabolomics. Curr. Opin. Chem. Biol. 2016, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis. In Encyclopedia of Statistics in Behavioral Science; Everitt, B.S., Howell, D.C., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2005; p. bsa501. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Rodrigues, D.; Muller, A.F.; Bonomini, T.; Klein-Júnior, L.; Lucinda-Silva, R.; Malheiros, A. Chemical variability of allamanda cathartica l. flowers assessed by multivariate data analysis. Quím. Nova 2020, 43, 201–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).