Comparison of Chayote (Sechium edule (Jacq.) Sw.) Accessions from Mexico, Japan, and Myanmar Using Reproductive Characters and Microsatellite Markers
Abstract
1. Introduction
2. Results
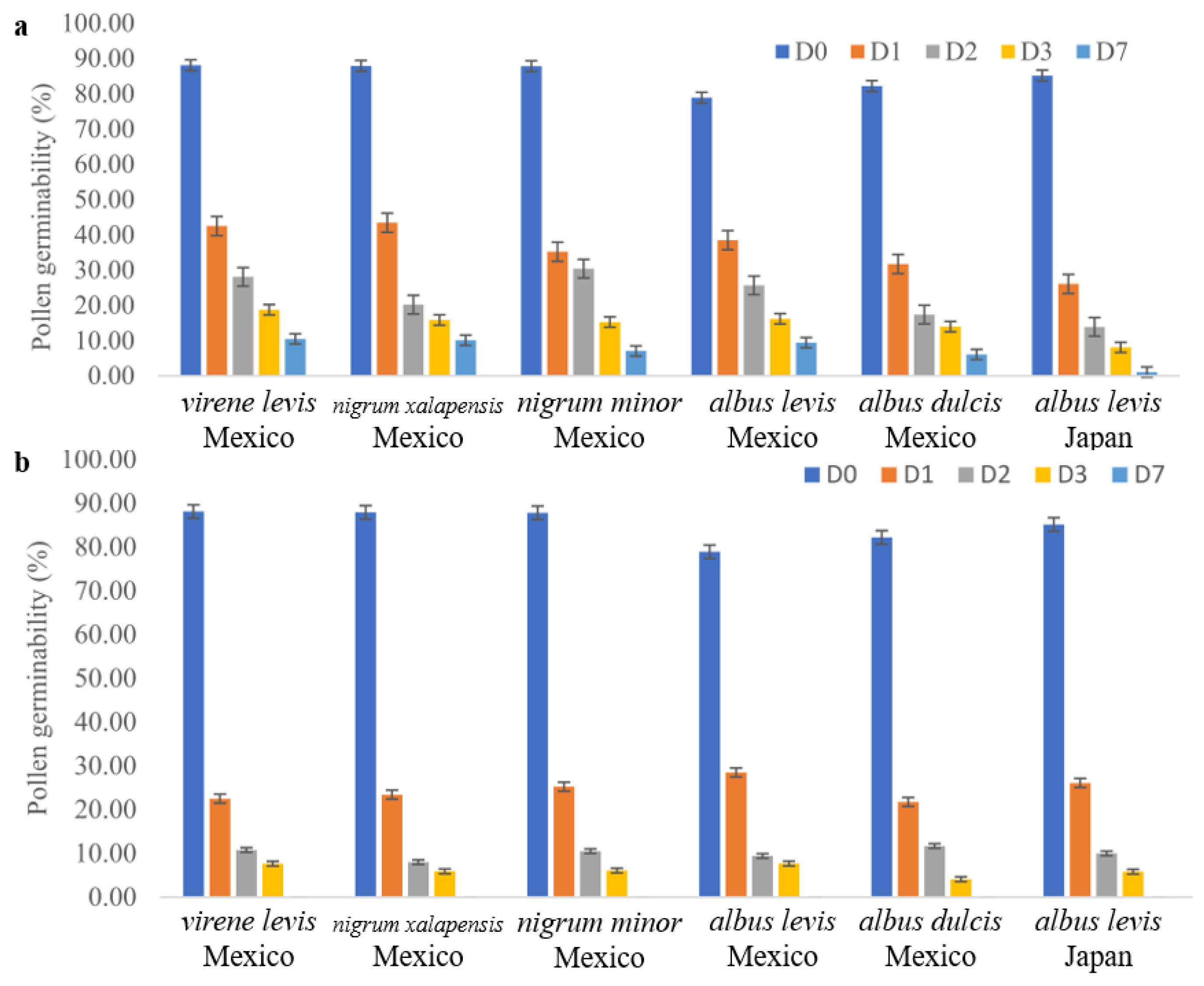
2.1. Palynology, Fruit, and Flower Morphology
2.2. Cytological Characterization
2.3. Microsatellite-Based Genetic Diversity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Palynology, Fruit, and Flower Morphology
4.2.1. Flower and Pollen Observation
4.2.2. Pollen Germination Medium Optimization
4.2.3. Pollen Longevity Test
4.2.4. Fruit Observation
4.3. Cytological Characterization
4.3.1. Experiment Reliability Performance
4.3.2. Flow Cytometry Experiment
4.3.3. Chromosomal Counting
4.4. Microsatellite-Based Genetic Diversity
4.4.1. DNA Isolation
4.4.2. SSR Genotyping
4.4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Feledyn-Szewczyk, B.; Kuś, J.; Stalenga, J.; Berbeć, A.K.; Radzikowski, P. The role of biological diversity in agroecosystems and organic farming. In Organic Farming-A Promising Way of Food Production; IntechOpen: London, UK, 2016; ISBN 953-51-2256-8. [Google Scholar]
- Collins, W.W.; Qualset, C.O. Biodiversity in Agroecosystems; CRC Press: Boca Raton, FL, USA, 1998; ISBN 1-4200-4924-0. [Google Scholar]
- Mayes, S.; Massawe, F.J.; Alderson, P.G.; Roberts, J.A.; Azam-Ali, S.N.; Hermann, M. The potential for underutilized crops to improve security of food production. J. Exp. Bot. 2012, 63, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, C. A review of the Cucurbitaceae. Bot. J. Linn. Soc. 1980, 81, 233–247. [Google Scholar] [CrossRef]
- Lira Saade, R. Promoting the conservation and use of underutilized and neglected crops. In Chayote, Sechium edule (Jacq.) Sw; Bioversity International: Rome, Italy, 1996. [Google Scholar]
- Wang, Y.-H.; Behera, T.K.; Kole, C. Genetics, Genomics and Breeding of Cucurbits; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1-57808-766-X. [Google Scholar]
- Olvera-Vazquez, S.G.; Cadena-Iñiguez, J.; Gilani, S.A.; Watanabe, K.N. The Cytological Studies on Neglected and Underutilized Cucurbit Species with Special Reference to Chayote, an Under-Exploited Species. Am. J. Plant Sci. 2019, 10, 1261–1279. [Google Scholar] [CrossRef]
- Bermejo, J.E.H.; León, J. Neglected Crops: 1492 from a Different Perspective; Food and Agriculture Organization: Rome, Italy, 1994; Volume 26, ISBN 92-5-103217-3. [Google Scholar]
- Hernandez-Uribe, J.P.; Agama-Acevedo, E.; Gonzalez-Soto, R.A.; Bello-Pérez, L.A.; Vargas-Torres, A. Isolation and characterization of Mexican chayote tuber (Sechium edule Sw.) starch. Starch Stärke 2011, 63, 32–41. [Google Scholar] [CrossRef]
- Newstrom, L.E. Evidence for the origin of chayote, Sechium edule (Cucurbitaceae). Econ. Bot. 1991, 45, 410–428. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, Genetics, Postharvest Management and Pharmacological Characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Atsumi, H. Studies on Protease in Sechium edule Swartz; Tokyo Kasei University: Tokyo, Japan, 2000; Volume 40, pp. 107–110. ISSN 03851214. [Google Scholar]
- Zaman, M.R. Pollen germination, viability and tube growth in fourteen cultivated and wild species of cucurbit grown in Bangladesh. J. Life Earth Sci. 2006, 1, 1–7. [Google Scholar]
- Perveen, A.; Ali, S. Pollen germination capacity and maintenance of pollen in Praecitrullus fistulosus (Stocks) Pangola (Cucurbitaceae). Pak. J. Bot. 2011, 43, 47–50. [Google Scholar]
- Akhtar, N.; Khan, S.A.; Khattak, U. Palynological characteristics of some selected species of family Cucurbitaceae (Juss.) using light and scanning electron microscopy techniques. Microsc. Res. Tech. 2019, 82, 1852–1861. [Google Scholar]
- Beevy, S.S.; Kuriachan, P. Chromosome numbers of south Indian Cucurbitaceae and a note on the cytological evolution in the family. J. Cytol. Genet. 1996, 31, 65–71. [Google Scholar]
- Bisognin, D.A. Origin and evolution of cultivated cucurbits. Ciênc. Rural. 2002, 32, 715–723. [Google Scholar] [CrossRef]
- Behera, T.K.; Sureja, A.K.; Islam, S.; Sidhu, A.D.M. and A.S. Minor Cucurbits. In Genetics, Genomics and Breeding of Cucurbits; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-429-06640-5. [Google Scholar]
- Robinson, R.W.; Decker-Walters, D.S. Cucurbits; Cab International: Wallingford, UK, 2006; p. 226. [Google Scholar]
- Dane, F. Cytogenetics of the genus Cucumis. Chromosome Eng. Plants Genet. Breed. Evol. 1991, 1, 201–214. [Google Scholar]
- Roy, R.P.; Saran, S.; Dutt, B. 10—Cytogenetics of the Cucurbitaceae. In Developments in Plant Genetics and Breeding; Tsuchiya, T., Gupta, P.K., Eds.; Chromosome Engineering in Plants; Elsevier: Amsterdam, The Netherlands, 1991; Volume 2, pp. 181–199. [Google Scholar]
- Fu, A.; Wang, Q.; Mu, J.; Ma, L.; Wen, C.; Zhao, X.; Gao, L.; Li, J.; Shi, K.; Wang, Y.; et al. Combined genomic, transcriptomic, and metabolomic analyses provide insights into chayote (Sechium edule) evolution and fruit development. Hortic. Res. 2021, 8, 35. [Google Scholar] [CrossRef]
- Ntuli, N.R.; Tongoona, P.B.; Zobolo, A.M. Genetic diversity in Cucurbita pepo landraces revealed by RAPD and SSR markers. Sci. Hortic. 2015, 189, 192–200. [Google Scholar] [CrossRef]
- Adeyemo, O.; Adegoke, S.; Oladapo, D.; Amaghereonu, C.C.; Thomas, A.; Ebirikwem, E.E.; Adeyinka, B.; Amoda, W. Transferability of SSR markers used for assessment of genetic relationship in five species/genera in Cucurbitaceae. Egypt. J. Bot. 2020, 60, 275–286. [Google Scholar]
- Hu, J.; Wang, L.; Li, J. Comparison of genomic SSR and EST-SSR markers for estimating genetic diversity in cucumber. Biol. Plant. 2011, 55, 577–580. [Google Scholar] [CrossRef]
- Tautz, D.; Renz, M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984, 12, 4127–4138. [Google Scholar] [CrossRef]
- Machida-Hirano, R.; Cortés-Cruz, M.; González, B.A.A.; Íñiguez, J.C.; Shirata, K.; Watanabe, K.N. Isolation and characterization of novel microsatellite markers in chayote [Sechium edule (Jacq.) Sw.]. Am. J. Plant Sci. 2015, 6, 2033. [Google Scholar] [CrossRef]
- Saw, O.M.; San Thein, M.; Hein, A.P.; Takei, E.; Osada, T.; Domon, E.; Watanabe, K.; Ebana, K.; Kawase, M. A Field Study Exploring Plant Genetic Resources in Kachin State and Chin State, Myanmar in 2017. In Annual Report on Exploration and Introduction of Plant Genetic Resources in FY2017; National Agriculture and Food Research Organization (NARO): Tsukuba, Japan; Volume 34, pp. 159–192. ISSN 2434-7485. [CrossRef]
- Yamamoto, S.; Hmwe, N.H.; Deuanhaksa, C.; Kyaw, M.T.; Suthiluk, P.; Watanabe, K. Preliminary field survey of cultivated crops in north eastern Myanmar, northern Laos and northern Thailand, 2013. Annu. Rep. Explor. Introd. Plant Genet. Resour. NIAS Tsukuba 2015, 31, 367–377. [Google Scholar]
- Domon, E.; Thein, M.S.; Takei, E.; Osada, T.; Kawase, M. A field study collecting cultivated crops and useful plants in Sagaing region of Myanmar in 2014. Annu. Rep. Explor. Introd. Plant Genet. Resour. 2015, 31, 343–365. [Google Scholar]
- Aung, L.H.; Ball, A.; Kushad, M. Developmental and nutritional aspects of chayote (Sechium edule, Cucurbitaceae). Econ. Bot. 1990, 44, 157–164. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Cisneros-Salgado, V.M.; ArévaloGalarza, L.; Ruiz-Posada, L.M.; Aguirre-Medina, J. Guía de Descriptores Varietales de Sechium edule (Jacq.) Sw. Para Protección Legal de su Variación; Colegio de Postograduado: Tescoco, Mexico, 2017; ISBN 978-607-715-361-0. [Google Scholar]
- Bhowmick, B.K.; Jha, S. Differential heterochromatin distribution, flow cytometric genome size and meiotic behavior of chromosomes in three Cucurbitaceae species. Sci. Hortic. 2015, 193, 322–329. [Google Scholar] [CrossRef]
- Bennett, M.D.; Smith, J.B. Nuclear DNA amounts in angiosperms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 274, 227–274. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D. Chromosome studies and nuclear DNA in relation to sex difference and plant habit in two species of Cucurbitaceae. Cytology 1991, 56, 409–417. [Google Scholar] [CrossRef]
- Pellicer, J.; Leitch, I.J. The Plant DNA C-values database (release 7.1): An updated online repository of plant genome size data for comparative studies. New Phytol. 2019, 226, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.W.; Davis, G.N. Cucurbits. Botany, cultivation, and utilization. Cucurbits Bot. Cultiv. Util. 1962. [Google Scholar]
- Varghese, B.M.; BM, V. Cytology of Sechium edule Sw. Curr. Sci. 1973. [Google Scholar]
- Singh, A.K. Cytogenetics and evolution in the Cucurbitaceae. In Biology and Utilization of Cucurbitaceae; Bates, D.M., Robinson, R.W., Jeffrey, C., Eds.; Comstock Publishing Associates; Cornell University Press: Ithaca, NY, USA, 1990. [Google Scholar]
- De Donato, M.; Cequea, H. A cytogenetic study of six cultivars of the chayote, Sechium edule Sw.(Cucurbitaceae). J. Hered. 1994, 85, 238–241. [Google Scholar] [CrossRef]
- Mercado, P.; Lira, R. Contribución al conocimiento de los números cromosómicos de los géneros Sechium P. Br. y Sicana Naudin (Cucurbitaceae). Acta Bot. Mex. 1994, 27, 7–13. [Google Scholar] [CrossRef]
- Barrera-Guzmán, L.A.; Cadena-Iñiguez, J.; Legaria-Solano, J.P.; Sahagún-Castellanos, J. Phylogenetics of the genus Sechium P. Brown: A review. Span. J. Agric. Res. 2021, 19, e07R01. [Google Scholar] [CrossRef]
- Kong, Q.; Chen, J.; Liu, Y.; Ma, Y.; Liu, P.; Wu, S.; Huang, Y.; Bie, Z. Genetic diversity of Cucurbita rootstock germplasm as assessed using simple sequence repeat markers. Sci. Hortic. 2014, 175, 150–155. [Google Scholar] [CrossRef]
- Lock, M. Plant Resources of Tropical Africa 2. Vegetables. Kew Bull. 2004, 59, 650. [Google Scholar] [CrossRef]
- Abdelnour, A.; Rocha, O.J. Genetic characterization of a collection of chayote, Sechium edule (Jacq.) Swartz, in Costa Rica by using isozyme markers. Genet. Resour. Crop Evol. 2008, 55, 163–170. [Google Scholar] [CrossRef]
- Verma, V.K.; Pandey, A.; Jha, A.K.; Ngachan, S.V. Genetic characterization of chayote [Sechium edule (Jacq.) Swartz.] landraces of North Eastern Hills of India and conservation measure. Physiol. Mol. Biol. Plants 2017, 23, 911–924. [Google Scholar] [CrossRef]
- Sucher, N.J.; Hennell, J.R.; Carles, M.C. (Eds.) Plant DNA Fingerprinting and Barcoding: Methods and Protocols; Humana: New York, NY, USA, 2012; ISBN 978-1-61779-608-1. [Google Scholar]
- Potter, D.; Gao, F.; Aiello, G.; Leslie, C.; Mcgranahan, G. Intersimple Sequence Repeat Markers for Fingerprinting and Determining Genetic Relationships of Walnut (Juglans regia) Cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 75–81. [Google Scholar] [CrossRef]
- Roundy, B.A.; McArthur, E.D.; Haley, J.S.; Mann, D.K. General Technical Report INT-GTR-315, Proceedings of the Wildland Shrub and Arid Land Restoration Symposium, Las Vegas, NV, USA, 19–21 October 1993; U.S. Department of Agriculture, Forest Service, Intermountain Research Station: Washington, DC, USA, 1995; 384p. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J. VARIACIÓN MORFOLÓGICA A NIVEL INTER E INFRAESPECÍFICO EN Sechium spp. Agro Product. 2017, 10, 58–63. [Google Scholar]
- Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Arévalo-Galarza, M.; de, L.; Cisneros-Solano, V.M.; Ruiz-Posadas, L.; del, M.; Aguirre-Medina, J.F.; Watanabe, K.; Machida-Hirano, R.; et al. Varietal Descriptors for the Distinction of Underutilized Varieties of Sechium edule (Jacq) Swartz. Plants 2022, 11, 3309. [Google Scholar] [CrossRef]
- Qureshi, S.J.; Khan, M.A.; Arshad, M.; Rashid, A.; Ahmad, M. Pollen fertility (viability) status in Asteraceae species of Pakistan. Trakia J. Sci. 2009, 7, 12–16. [Google Scholar]
- Vizintin, L.; Bohanec, B. In vitro manipulation of cucumber (Cucumis sativus L.) pollen and microspores: Isolation procedures, viability tests, germination, maturation. Acta Biol. Crac. Ser. Bot. 2004, 46, 177–183. [Google Scholar]
- Guia de Descriptors: Varietales de Sechium edule (Jacq.) Sw. Para Protección Legal de su Variacion. Available online: https://www.researchgate.net/publication/352901122_Guia_de_Descriptors_Varietales_de_Sechium_edule_Jacq_Sw_Para_Proteccion_Legal_de_su_Variacion (accessed on 24 June 2022).
- Villanueva-Jiménez, J.A. Las variedades del chayote (Sechium edule (Jacq.) Sw.) y su Comercio Mundial. Agric. Soc. Desarro. 2012, 9, 481–482. [Google Scholar]
- Cadena Iñiguez, J.; Soto Hernández, M.; Arévalo Galarza, M.; de, L.; Avendaño Arrazate, C.H.; Aguirre Medina, J.F.; Ruiz Posadas, L.; del, M. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Rev. Chapingo Ser. Hortic. 2011, 17, 45–55. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, J. Nuclear DNA content and genome size of trout and human. Cytom. A 2003, 51, 127–128. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small amounts of fresh leaf material. Photochem. Bull 1987, 19, 11–15. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research–An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Smouse, P.E.; Banks, S.C.; Peakall, R. Converting quadratic entropy to diversity: Both animals and alleles are diverse, but some are more diverse than others. PLoS ONE 2017, 12, e0185499. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3-319-24277-6. [Google Scholar]
- Yu, G. Using ggtree to Visualize Data on Tree-Like Structures. Curr. Protoc. Bioinforma. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Lam, T.T.-Y.; Zhu, H.; Guan, Y. Two Methods for Mapping and Visualizing Associated Data on Phylogeny Using Ggtree. Mol. Biol. Evol. 2018, 35, 3041–3043. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]



| Flower Morphological Characteristics | Palynological Characteristics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession | Variety Name | Origin | Colour | Number of Petals | Petal Length (cm) | Petal Width (cm) | Number of Stamens | Polar Axis (µm) | Equatorial Axis (µm) | P/E Ratio | Shape | Pollen Wall (µm) |
| KW014 | virens levis | Mexico | Light green | 4, 5 | 0.7–0.9 | 0.3–0.5 | 5 | 100.16 | 85.76 | 1.17 | Subprolate | 8.18 |
| 98.40 | 99.31 | 0.99 | Oblate-spheroidal | |||||||||
| KW015 | nigrum xalapensis | Mexico | Yellowish | 5, 6 | 0.7–0.9 | 0.3–0.5 | 4, 5 | 97.06 | 86.12 | 1.13 | Prolate-spheroidal | 4.70 |
| 95.54 | 94.26 | 1.01 | Oblate-spheroidal | |||||||||
| KW016 | nigrum minor | Mexico | Dark green | 5 | 0.7–0.8 | 0.3–0.5 | 4, 5 | 92.71 | 85.35 | 1.09 | Prolate-spheroidal | 5.47 |
| 85.75 | 82.68 | 1.04 | Oblate-spheroidal | |||||||||
| KW017 | albus levis | Mexico | Yellowish | 5, 6 | 0.7–0.9 | 0.3–0.5 | 5 | 85.65 | 75.58 | 1.13 | Subprolate | 5.96 |
| 84.29 | 82.44 | 1.02 | Oblate-spheroidal | |||||||||
| KW018 | albus dulcis | Mexico | Yellowish | 5 | 0.8–0.9 | 0.3–0.5 | 5 | 94.58 | 83.48 | 1.13 | Subprolate | 6.14 |
| 96.17 | 93.62 | 1.03 | Oblate-spheroidal | |||||||||
| KW019 | albus levis | Japan | White | 4, 5 | 0.3–0.5 | 0.3–0.4 | 4, 5 | 104.83 | 88.94 | 1.18 | Subprolate | 3.73 |
| 103.80 | 102.69 | 1.01 | Oblate-spheroidal | |||||||||
| Locus | N | Na | Ne | I | Ho | He | uHe | F |
|---|---|---|---|---|---|---|---|---|
| Sed11 | 7.000 | 3 | 1.535 | 0.467 | 0.217 | 0.284 | 0.307 | 0.140 |
| Sed09 | 7.000 | 3 | 1.960 | 0.737 | 0.500 | 0.490 | 0.530 | −0.014 |
| Sed08 | 7.000 | 3 | 1.905 | 0.692 | 0.125 | 0.458 | 0.494 | 0.758 |
| Sed07 | 6.333 | 3 | 1.477 | 0.394 | 0.000 | 0.270 | 0.294 | 1.000 |
| Sed06 | 7.000 | 3 | 1.977 | 0.723 | 0.633 | 0.471 | 0.507 | −0.364 |
| Sed03 | 7.000 | 5 | 2.116 | 0.654 | 0.238 | 0.477 | 0.382 | 0.122 |
| Average | 6.889 | 3.333 | 1.828 | 0.611 | 0.286 | 0.408 | 0.419 | 0.274 |
| Fst | Japan | Mexico | Myanmar | D | Japan | Mexico | Myanmar |
|---|---|---|---|---|---|---|---|
| Japan | 0.000 | Japan | 0.000 | ||||
| Mexico | 0.137 | 0.000 | Mexico | 0.303 | 0.000 | ||
| Myanmar | 0.199 | 0.274 | 0.000 | Myanmar | 0.422 | 0.440 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Wang, Y.; Olvera-Vazquez, S.G.; Iñiguez, J.C.; Thein, M.S.; Watanabe, K.N. Comparison of Chayote (Sechium edule (Jacq.) Sw.) Accessions from Mexico, Japan, and Myanmar Using Reproductive Characters and Microsatellite Markers. Plants 2023, 12, 476. https://doi.org/10.3390/plants12030476
Shi M, Wang Y, Olvera-Vazquez SG, Iñiguez JC, Thein MS, Watanabe KN. Comparison of Chayote (Sechium edule (Jacq.) Sw.) Accessions from Mexico, Japan, and Myanmar Using Reproductive Characters and Microsatellite Markers. Plants. 2023; 12(3):476. https://doi.org/10.3390/plants12030476
Chicago/Turabian StyleShi, Miao, Yihang Wang, Sergio Gabriel Olvera-Vazquez, Jorge Cadena Iñiguez, Min San Thein, and Kazuo N. Watanabe. 2023. "Comparison of Chayote (Sechium edule (Jacq.) Sw.) Accessions from Mexico, Japan, and Myanmar Using Reproductive Characters and Microsatellite Markers" Plants 12, no. 3: 476. https://doi.org/10.3390/plants12030476
APA StyleShi, M., Wang, Y., Olvera-Vazquez, S. G., Iñiguez, J. C., Thein, M. S., & Watanabe, K. N. (2023). Comparison of Chayote (Sechium edule (Jacq.) Sw.) Accessions from Mexico, Japan, and Myanmar Using Reproductive Characters and Microsatellite Markers. Plants, 12(3), 476. https://doi.org/10.3390/plants12030476







