Atypical Rapid Onset of Olmesartan-Induced Enteropathy with Recurrence After Rechallenging
Abstract
1. Introduction
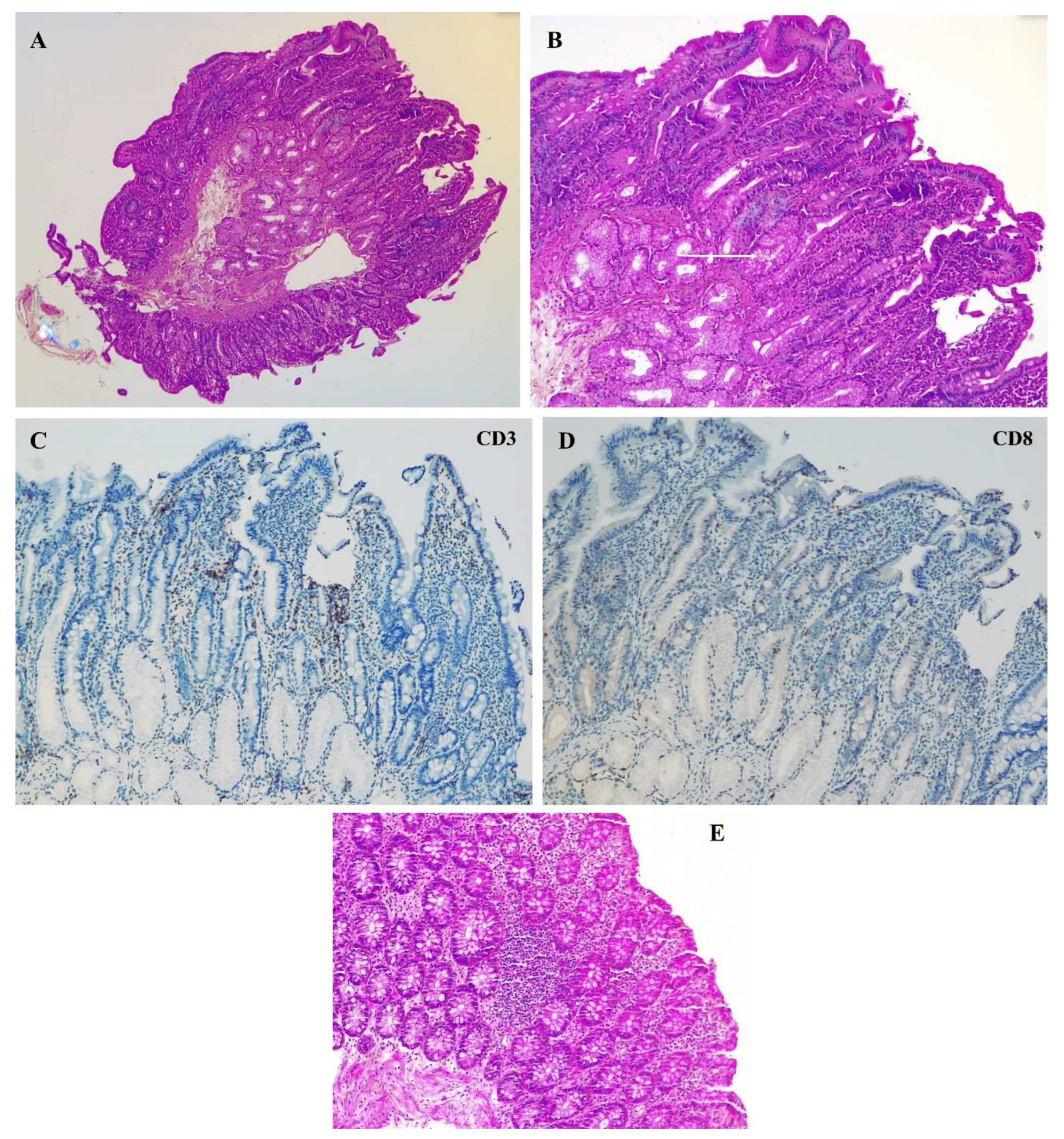
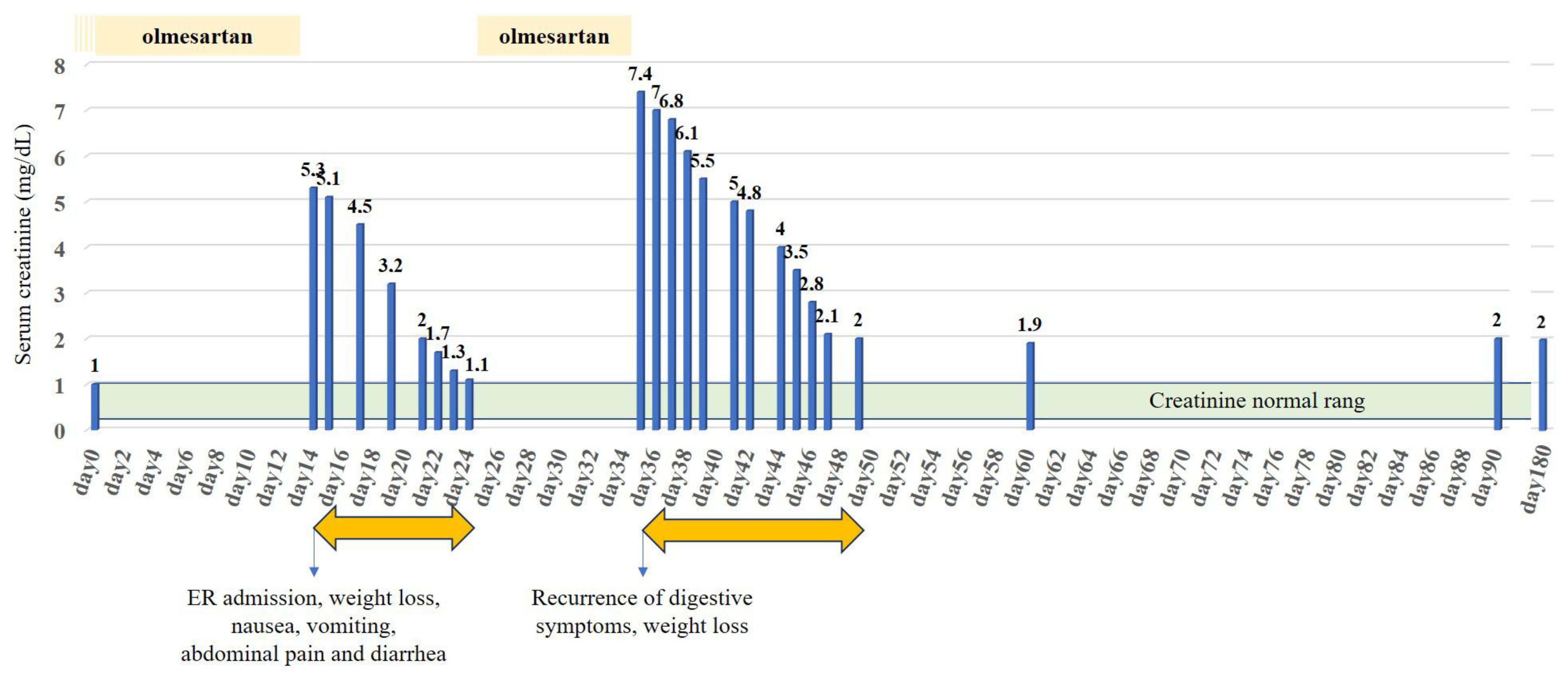
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| ARB | Angiotensin II receptors blocker |
| AT | Angiotensin II receptor type 1 |
| CKD | Chronic kidney disease |
| GI | Gastrointestinal |
| OIE | Angiotensin II receptor type 1 (AT1 receptor) |
References
- Rubio-Tapia, A.; Herman, M.L.; Ludvigsson, J.F.; Kelly, D.G.; Mangan, T.F.; Wu, T.T.; Murray, J.A. Severe spruelike enteropathy associated with olmesartan. Mayo Clin. Proc. 2012, 87, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Fain, C.; Park, A.; Wang, P.; Gonzalez-Velez, E.; Leffler, D.A.; Hutfless, S.M. Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue-like enteropathy: A systematic review. Gastroenterol. Rep. 2019, 7, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Van Horebeek, N.; Croes, R.; Vonck, A.; Colpaert, E. Olmesart0061n induced enteropathy affecting the entire gastrointestinal tract: A case report. Acta. Gastro-Enterol. Belg. 2023, 86, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Storozuk, T.; Brown, I.; Lagana, S.; Westerhoff, M.; Setia, N.; Hart, J.; Alpert, L. The histological spectrum of ARB-induced gastritis. Histopathology 2022, 81, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbò, S.; Montalto, M.; Ricci, R.; Gasbarrini, A.; Cammarota, G. Systematic review: Sprue-like enteropathy associated with olmesartan. Aliment. Pharmacol. Ther. 2014, 40, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Burbure, N.; Lebwohl, B.; Arguelles-Grande, C.; Green, P.H.R.; Bhagat, G.; Lagana, S. Olmesartan-associated sprue-like enteropathy: A systematic review with emphasis on histopathology. Hum. Pathol. 2016, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Abdelazeem, B.; Alnounou, M. Olmesartan-Induced Ischemic Enteritis Complicated With Bowel Perforation: A Case Report and Literature Review. Cureus 2023, 15, e36660. [Google Scholar] [CrossRef] [PubMed]
- Holtgrewe, L.M.L.; Dippel, H.; Weckauf, H.; Linnemüller, S.; Schuppert, F. Candesartan-Induced Enteropathy That Mimics Celiac Disease in a 90-Year-Old Patient. Case Rep. Gastroenterol. 2023, 17, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kulai, T.; Arnason, T.; MacIntosh, D.; Igoe, J. Duodenal Villous Atrophy in a TTG-Negative Patient Taking Olmesartan: A Case Report and Review of the Literature. Can. J. Gastroenterol. Hepatol. 2016, 2016, 6091571. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Min, K.H.; Kim, H.W.; Park, S.B.; Kang, D.H.; Choi, C.W. Olmesartan-associated Enteropathy with Acute Kidney Injury. Korean J. Gastroenterol. Taehan Sohwagi Hakhoe Chi. 2022, 79, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; the Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Roca-Argente, L.; Diaz-Jaime, F.C.; López-Romero, L.C.; Manzur-Aguilar, Y.; Moll-Guillem, J.L.; Del Val-Antoñana, A.; Hernandez-Jaras, J. Acute kidney injury secondary to diarrhea caused by « sprue-like » enteropathy associated with olmesartan. Nefrol Publ. Soc. Esp. Nefrol. 2017, 37, 461–562. [Google Scholar]
- Mauloni, P.A.; Capuani, F.; Paone, C.; Marasco, G.; Bellacosa, L.; Cogliandro, R.F.; Cremon, C.; Barbara, G.; Vasuri, F.; Stanghellini, V. An unusual cause of diarrhoea: Case report and literature review of olmesartan-associated enteropathy. Eur. J. Gastroenterol. Hepatol. 2021, 33 (Suppl. 1), e1060–e1066. [Google Scholar] [CrossRef] [PubMed]
- Kodama, E.; Kawata, Y.; Yamazaki, S.; Igarashi, T.; Kojima, Y.; Tominaga, K.; Yokoyama, J.; Honma, T.; Terai, S. Diagnosis and resolution of olmesartan-associated sprue-like enteropathy confirmed by capsule endoscopy: A case report and literature review. Clin. J. Gastroenterol. 2024, 17, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Briongos-Figuero, L.S.; Cuevas-González, J. Olmesartan-associated sprue-like enteropathy. Lancet 2023, 402, 1660. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Li, H. Olmesartan and Drug-Induced Enteropathy. Pharm. Ther. 2014, 39, 47–50. [Google Scholar]
- Dong, Y.H.; Jin, Y.; Tsacogianis, T.N.; He, M.; Hsieh, P.H.; Gagne, J.J. Use of olmesartan and enteropathy outcomes: A multi-database study. Aliment. Pharmacol. Ther. 2018, 47, 792–800. [Google Scholar] [CrossRef] [PubMed]
- DeGaetani, M.; Tennyson, C.A.; Lebwohl, B.; Lewis, S.K.; Abu Daya, H.; Arguelles-Grande, C.; Bhagat, G.; Green, P.H.R. Villous atrophy and negative celiac serology: A diagnostic and therapeutic dilemma. Am. J. Gastroenterol. 2013, 108, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Daines, B.S.; Kankam, A.; Tanami, S.; Nambiar, R. A Case Report of Olmesartan-Induced Enteropathy. Cureus 2021, 13, e20722. [Google Scholar] [CrossRef] [PubMed]
- Gamelas, V.; Borges, V.; Santos, S.; Santos, J.; Silva, M.; Bernardes, C.; Ramos, J. Gastrointestinal: Olmesartan-induced enterocolopathy: A new presentation of a known entity. J. Gastroenterol. Hepatol. 2021, 36, 1150. [Google Scholar] [CrossRef] [PubMed]
- Bashari, D.R. Severe Sprue-Like Enteropathy and Colitis due to Olmesartan: Lessons Learned From a Rare Entity. Gastroenterol. Res. 2020, 13, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Scialom, S.; Malamut, G.; Meresse, B.; Guegan, N.; Brousse, N.; Verkarre, V.; Derrieux, C.; Macintyre, E.; Seksik, P.; Savoye, G.; et al. Gastrointestinal Disorder Associated with Olmesartan Mimics Autoimmune Enteropathy. PLoS ONE 2015, 10, e0125024. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.K.; McKenna, B.J. Olmesartan-Associated Enteropathy: A Review of Clinical and Histologic Findings. Arch. Pathol. Lab. Med. 2015, 139, 1242–1247. [Google Scholar] [CrossRef] [PubMed]

| 1st Admission Values | 2nd Admission Values | Normal Values | |
|---|---|---|---|
| BLOOD | |||
| Hemoglobin (g/dL) | 14.3 | 15.6 | 13–18 |
| MCV (fL) | 90 | 93 | 80–100 |
| Thrombocytes ×103/µL | 387 | 553 | 150–440 |
| Leucocytes ×103/µL | 10.68 | 15.56 | 3.5–11 |
| CRP (mg/dL) | 32 | 24 | <5 |
| Urea (mg/dL) | 437 | 127 | 17–48 |
| Creatinine (mg/dL) | 5.30 | 7.44 | 0.7–1.2 |
| K/ HCO3 (mmol/L) | 3.3/12 | 3.4/8 | 3.5–4.5/23–29 |
| URINE | |||
| Erythrocytes /µL | 34 | <12 | <12 |
| Leucocytes /µL | 359 | 50 | <10 |
| Pathological cylinders | Absence | Presence (>6/2.25 µL) | Absence |
| Fractional excretion of urea (%) | 11.9 | 13.2 | |
| Fractional excretion of Sodium (%) | 0.2 | 0.1 | |
| Potassium (mmol/L) | 9 | 40 | <20 |
| - Rarity and underlooked disease [2] |
| - Heterogeneous clinical presentation, with a variety of symptoms ranging from mild enteropathy to severe diarrhea and malabsorption [4,5] |
| - Heterogeneous histological presentation, ranging from unremarkable intestinal biopsy to classical “sprue like enteropathy” with villous atrophy and intraepithelial lymphocyte infiltrate [6] |
| - Patchy distribution of histological lesions, requiring multiple biopsies from various gastrointestinal [4,18,20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekkai, L.; Ibn Majah, A.; Verset, L.; Jacobs, L.; Danneel, C.; Elkilic, O.; Collart, F.; Nortier, J.; Taghavi, M. Atypical Rapid Onset of Olmesartan-Induced Enteropathy with Recurrence After Rechallenging. Diseases 2025, 13, 223. https://doi.org/10.3390/diseases13070223
Bekkai L, Ibn Majah A, Verset L, Jacobs L, Danneel C, Elkilic O, Collart F, Nortier J, Taghavi M. Atypical Rapid Onset of Olmesartan-Induced Enteropathy with Recurrence After Rechallenging. Diseases. 2025; 13(7):223. https://doi.org/10.3390/diseases13070223
Chicago/Turabian StyleBekkai, Lila, Aymen Ibn Majah, Laurine Verset, Lucas Jacobs, Charline Danneel, Okyay Elkilic, Frédéric Collart, Joëlle Nortier, and Maxime Taghavi. 2025. "Atypical Rapid Onset of Olmesartan-Induced Enteropathy with Recurrence After Rechallenging" Diseases 13, no. 7: 223. https://doi.org/10.3390/diseases13070223
APA StyleBekkai, L., Ibn Majah, A., Verset, L., Jacobs, L., Danneel, C., Elkilic, O., Collart, F., Nortier, J., & Taghavi, M. (2025). Atypical Rapid Onset of Olmesartan-Induced Enteropathy with Recurrence After Rechallenging. Diseases, 13(7), 223. https://doi.org/10.3390/diseases13070223






