Analgesic and Neuroprotective Roles of Dexmedetomidine in Spine Surgery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Research
2.2. Selection Criteria
2.3. Data Extraction
2.4. Evaluation Analysis
3. Results
3.1. Included Studies
3.2. Quality Assessment
4. Discussion
4.1. Dexmedetomidine and Anti-Inflammatory Activity in Pain Control
4.2. Impact of Dexmedetomidine on Postoperative Cognitive Function and Delirium
4.3. Dexmedeomidine, Pain Management, and Delirium
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blaudszun, G.; Lysakowski, C.; Elia, N.; Tramèr, M.R. Effect of Perioperative Systemic A2 Agonists on Postoperative Morphine Consumption and Pain Intensity: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Anesthesiology 2012, 116, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Boezaart, A.P.; Davis, G.; Le-Wendling, L. Recovery after Orthopedic Surgery: Techniques to Increase Duration of Pain Control. Curr. Opin. Anaesthesiol. 2012, 25, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.K.; Durieux, M.E.; Nemergut, E.C. Non-Opioid Analgesics: Novel Approaches to Perioperative Analgesia for Major Spine Surgery. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 79–89. [Google Scholar] [CrossRef]
- Devin, C.J.; Mcgirt, M.J. Best Evidence in Multimodal Pain Management in Spine Surgery and Means of Assessing Postoperative Pain and Functional Outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef]
- Grosu, I.; de Kock, M. New Concepts in Acute Pain Management: Strategies to Prevent Chronic Postsurgical Pain, Opioid-Induced Hyperalgesia, and Outcome Measures. Anesthesiol. Clin. 2011, 29, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S. Effect of Intravenous Infusion of Dexmedetomidine on Perioperative Haemodynamic Changes and Postoperative Recovery: A Study with Entropy Analysis. Indian J. Anaesth. 2012, 56, 542–546. [Google Scholar] [CrossRef]
- Carollo, D.S.; Nossaman, B.D.; Ramadhyani, U. Dexmedetomidine: A Review of Clinical Applications. Curr. Opin. Anesthesiol. 2008, 21, 457–461. [Google Scholar] [CrossRef]
- Khan, Z.P.; Ferguson, C.N.; Jones, R.M. Alpha-2 and Imidazoline Receptor Agonists Therapeutic Role. Anaesthesia 1999, 54, 146–165. [Google Scholar] [CrossRef]
- Takahiko, K.; Mervyn, M. Clinical Uses of A2-Adrenergic Agonists. Anesthesiology 2000, 93, 1345–1349. [Google Scholar]
- Evered, L.A.; Silbert, B.S. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth. Analg. 2018, 127, 496–505. [Google Scholar] [CrossRef]
- Brown, C.H.; LaFlam, A.; Max, L.; Wyrobek, J.; Neufeld, K.J.; Kebaish, K.M.; Cohen, D.B.; Walston, J.D.; Hogue, C.W.; Riley, L.H. Delirium After Spine Surgery in Older Adults: Incidence, Risk Factors, and Outcomes. J. Am. Geriatr. Soc. 2016, 64, 2101–2108. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, B.J.; Mu, D.L.; Hu, J.; Guo, C.; Li, X.Y.; Ma, D.; Wang, D.X. Randomized Clinical Trial of Intraoperative Dexmedetomidine to Prevent Delirium in the Elderly Undergoing Major Non-Cardiac Surgery. Br. J. Surg. 2020, 107, e123–e132. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Nadler, J.W.; Preud, X.A.; Fang, E.; Daughtry, R.L.; Chapman, J.B.; Attarian, D.; Wellman, S.; Krystal, A.D. Clinical Neurophysiology Pilot Prospective Study of Post-Surgery Sleep and EEG Predictors of Post-Operative Delirium. Clin. Neurophysiol. 2017, 128, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Constan, E.; Stovall, C.; Matlock, R.; Steverson, C.; Wilbanks, B.; McMullan, S.; Yerdon, A. The Perception and Use of Dexmedetomidine Among Anesthesia Providers: A Quality Improvement Project. J. Perianesthesia Nurs. 2024, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, Ottawa Health Research Institute. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 29 June 2025).
- Niu, J.Y.; Yang, N.; Tao, Q.Y.; He, Y.; Hou, Y.B.; De Ning, R.; Yu, J.M. Effect of Different Administration Routes of Dexmedetomidine on Postoperative Delirium in Elderly Patients Undergoing Elective Spinal Surgery: A Prospective Randomized Double-Blinded Controlled Trial. Anesth. Analg. 2023, 136, 1075–1083. [Google Scholar] [CrossRef]
- Ahn, H.; Chae, Y.J.; Bin Choi, G.; Lee, M.G.; Yoo, J.Y. Determining the Optimal Dosage of Dexmedetomidine for Smooth Emergence in Older Patients Undergoing Spinal Surgery: A Study of 44 Cases. Med. Sci. Monit. 2024, 30, 1–8. [Google Scholar] [CrossRef]
- Tao, Q.Y.; Liu, D.; Yu, J.M.; Wang, S.J.; Wang, X.; Ouyang, R.N.; Ning, R.; Niu, J.Y. Effects of Esketamine Combined with Dexmedetomidine on Early Postoperative Cognitive Function in Elderly Patients Undergoing Lumbar Spinal Surgery: A Double-Blind Randomized Controlled Clinical Trial. Drug Des. Devel. Ther. 2024, 18, 5461–5472. [Google Scholar] [CrossRef]
- Xie, B.L.; Nie, L.Z.; Zhong, B.; Xiong, J.; Nie, M.; Ai, Q.X.; Yang, D. Longitudinal Trends in the Incidence of Hyperactive Delirium and Its Causes of Change After Surgery for Degenerative Lumbar Disease: A Population-Based Study of 7250 Surgical Patients Over 11 Years. Orthop. Surg. 2024, 17, 714–723. [Google Scholar] [CrossRef]
- Ye, C.; Shen, J.; Zhang, C.; Hu, C. Impact of Intraoperative Dexmedetomidine on Postoperative Delirium and Pro-Inflammatory Cytokine Levels in Elderly Patients Undergoing Thoracolumbar Compression Fracture Surgery: A Prospective, Randomized, Placebo-Controlled Clinical Trial. Medicine 2024, 103, E37931. [Google Scholar] [CrossRef]
- Turgut, N.; Turkmen, A.; Gökkaya, S.; Altan, A.; Hatiboglu, M.A. Fentanyl-Based Total Intravenous Anesthesia for Lumbar Laminectomy. Minerva Anestesiol. 2008, 74, 469–474. [Google Scholar]
- Kim, M.H.; Lee, K.Y.; Bae, S.J.; Jo, M.; Cho, J.S. Intraoperative Dexmedetomidine Attenuates Stress Responses in Patients Undergoing Major Spine Surgery. Minerva Anestesiol. 2019, 85, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Jannatmakan, F.; Nassajian, N.; Jarirahmadi, S.; Tabatabaee, K.; Zafari, M. Comparison of the Effect of Dexmedetomidine and Remifentanil on Pain Control after Spinal Surgery: A Double-Blind, Randomized Clinical Trial. Anesthesiol. Pain Med. 2021, 11, 111533. [Google Scholar] [CrossRef]
- Nikoubakht, N.; Alimian, M.; Faiz, S.H.R.; Derakhshan, P.; Sandri, M.S. Effects of Ketamine versus Dexmedetomidine Maintenance Infusion in Posterior Spinal Fusion Surgery on Acute Postoperative Pain. Surg. Neurol. Int. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Inokuchi, R.; Hanaoka, K.; Suka, M.; Yanagisawa, H. Dexmedetomidine Use during Epiduroscopy Reduces Fentanyl Use and Postoperative Nausea and Vomiting: A Single-Center Retrospective Study. SAGE Open Med. 2018, 6, 2050312118756804. [Google Scholar] [CrossRef]
- Waelkens, P.; Alsabbagh, E.; Sauter, A.; Joshi, G.P.; Beloeil, H. Pain Management after Complex Spine Surgery: A Systematic Review and Procedure-Specific Postoperative Pain Management Recommendations. Eur. J. Anaesthesiol. 2021, 38, 985–994. [Google Scholar] [CrossRef]
- Koh, W.S.; Leslie, K. Postoperative Analgesia for Complex Spinal Surgery. Curr. Opin. Anaesthesiol. 2022, 35, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Baek, J.; Kim, D.Y. Effect of Dexmedetomidine and Remifentanil Infusion on Postoperative Sore Throat after Lumbar Spine Surgery in the Prone Position. Medicine 2023, 102, E33506. [Google Scholar] [CrossRef]
- Naik, B.I.; Nemergut, E.C.; Kazemi, A.; Fernández, L.; Cederholm, S.K.; Mcmurry, T.L.; Durieux, M.E. The Effect of Dexmedetomidine on Postoperative Opioid Consumption and Pain After Major Spine Surgery. Anesth. Analg. 2016, 122, 1646–1653. [Google Scholar] [CrossRef]
- Rahimzadeh, P.; Faiz, S.H.R.; Alimian, M.; Mohammadian Erdi, A. Remifentanil versus Dexmedtomidine for Posterior Spinal Fusion Surgery. Med. J. Islam. Repub. Iran 2015, 29, 215. [Google Scholar]
- Hwang, W.; Lee, J.; Park, J.; Joo, J. Dexmedetomidine versus Remifentanil in Postoperative Pain Control after Spinal Surgery: A Randomized Controlled Study. BMC Anesthesiol. 2015, 15, 21. [Google Scholar] [CrossRef]
- Bojaraaj, D.R.K.; Senthilkumar, S.; Vijayaragavan, S.; Gnanavelrajan, A. Effect of Intravenous Use of Dexmedetomidine on Anesthetic Requirements in Patients Undergoing Elective Spine Surgery: A Double Blinded Randomized Controlled Trail. Int. J. Sci. Study 2016, 4, 251–255. [Google Scholar] [CrossRef]
- Garg, N.; Panda, N.; Gandhi, K.; Bhagat, H.; Batra, Y.; Grover, V.; Chhabra, R. Comparison of Small Dose Ketamine and Dexmedetomidine Infusion for Postoperative Analgesia in Spine Surgery—A Prospective. J. Neurosurg. Anesthesiol. 2016, 28, 27–31. [Google Scholar] [CrossRef]
- Song, Y.; Shim, J.K.; Song, J.W.; Kim, E.K.; Kwak, Y.L. Dexmedetomidine Added to an Opioid-Based Analgesic Regimen for the Prevention of Postoperative Nausea and Vomiting in Highly Susceptible Patients. Eur. J. Anaesthesiol. 2016, 33, 75–83. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, J.; Shen, Y.; Kang, S.; Zong, Y. Effects of Dexmedetomidine on CD42a+/CD14+, HLADR+/CD14+ and Inflammatory Cytokine Levels in Patients Undergoing Multilevel Spinal Fusion. Clin. Neurol. Neurosurg. 2017, 160, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, G.; Pourzitaki, C.; Aloisio, S.; Federico, B. Dexmedetomidine as a Sedative and Analgesic Adjuvant in Spine Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Pharmacol. 2018, 74, 1377–1389. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z. Analgesic Mechanism of Dexmedetomidine and Esketamine in Rats with Spinal Cord Injury. Discov. Med. 2024, 36, 714. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xing, Q.; Li, Y.; Han, X.; Sun, L. Dexmedetomidine Protects against Ischemia-Reperfusion Injury in Rat Skeletal Muscle. J. Surg. Res. 2014, 186, 240–245. [Google Scholar] [CrossRef]
- Gao, J.; Sun, Z.; Xiao, Z.; Du, Q.; Niu, X.; Wang, G.; Chang, Y.W.; Sun, Y.; Sun, W.; Lin, A.; et al. Dexmedetomidine Modulates Neuroinflammation and Improves Outcome via Alpha2-Adrenergic Receptor Signaling after Rat Spinal Cord Injury. Br. J. Anaesth. 2019, 123, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, D.; Guo, Y.; Sun, X.; Chen, C.; Zhai, X.; Jin, X.; Zhu, H.; Li, P.; Yu, W. Recent Advances and Perspectives of Postoperative Neurological Disorders in the Elderly Surgical Patients. CNS Neurosci. Ther. 2022, 28, 470–483. [Google Scholar] [CrossRef]
- Caumo, W.; Schmidt, A.P.; Schneider, C.N.; Bergmann, J.; Iwamoto, C.W.; Bandeira, D.; Ferreira, M.B.C. Risk Factors for Preoperative Anxiety in Adults. Acta Anaesthesiol. Scand. 2001, 45, 298–307. [Google Scholar] [CrossRef]
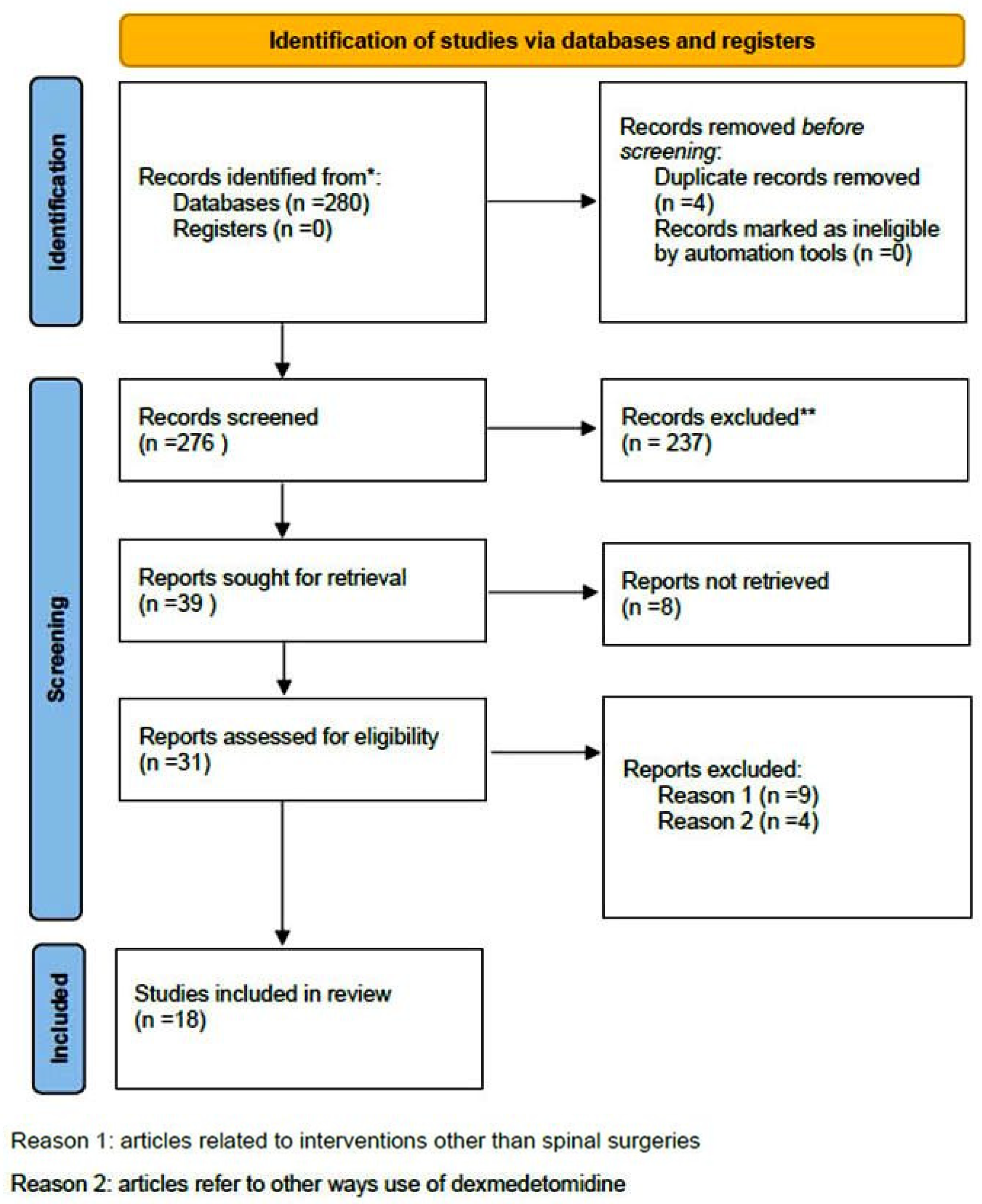
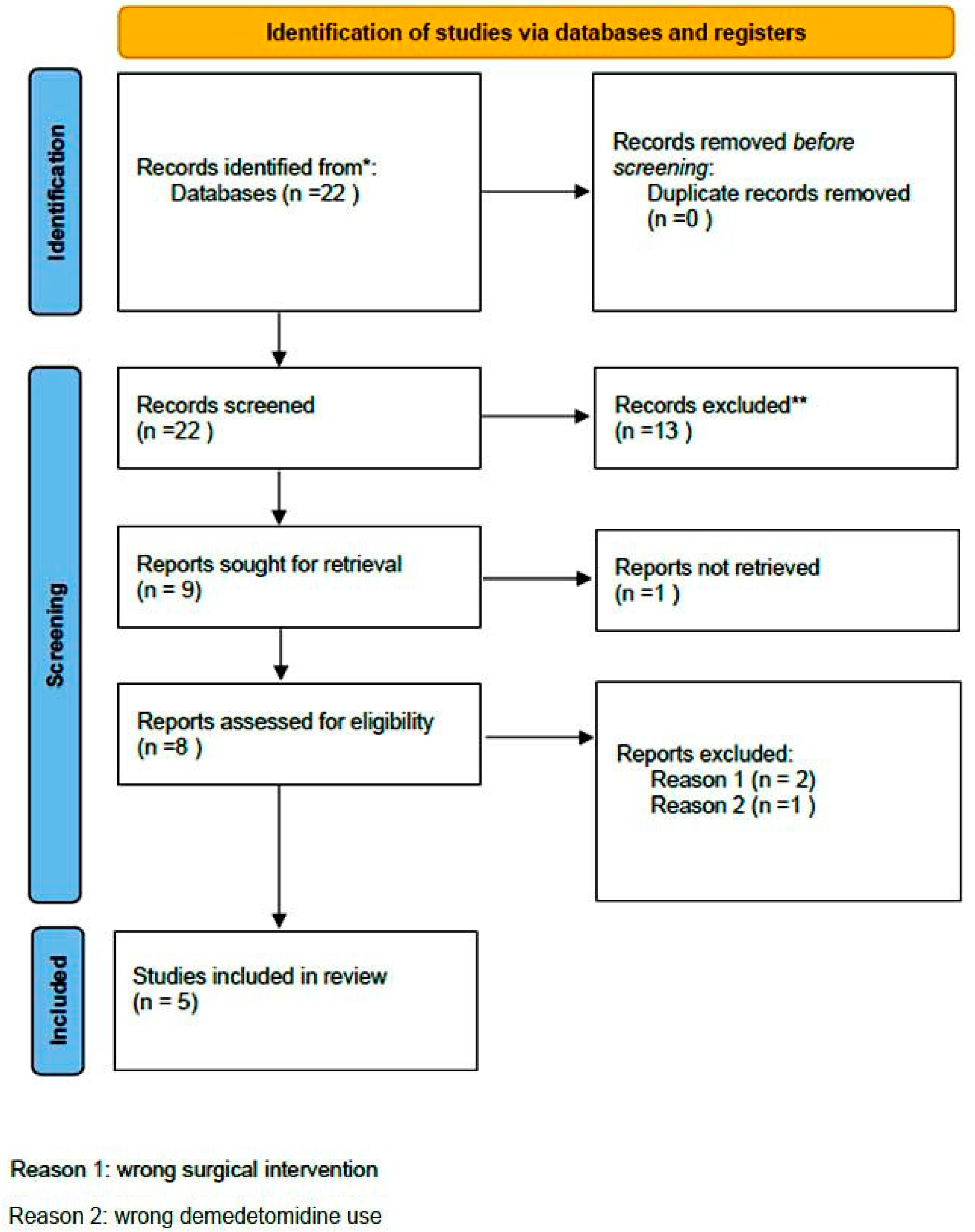
| Study | Type of Study | Number of Patients/Number of Studies | Type of Surgery | Purpose of Study | Newcastle–Ottawa Score | Results | Comments |
|---|---|---|---|---|---|---|---|
| Turgut et al., 2008 [21] | Clinical article | 50 | Lumbar laminectomy | Compare fentanyl/DEX * on perioperative haemodynamic conditions, propofol need, and side effects | 08 (Good) | Earlier need for analgesics in fentanyl group (median time 35 min vs. 60 min); greater incidence of vomiting and nausea in the same group | DEX * offers more rapid recovery and reduced need for pain medication, thus reducing total length of stay |
| Naik et al., 2014 [29] | Clinical article | 142 | >3 level of thoracic/lumbar spine deformity surgery | Evaluate impact of DEX * on postoperative pain management and need for opioids | 09 (Good) | Lower intraoperative opioid consumption, but not statistically significantly lower postoperative consumption or improved pain scores (up to 3 days) | Methadone mask beneficial effect of DEX * |
| Rahimzadeh et al., 2014 [30] | Original article | 60 | Posterior spinal fusion | Compare remifentanil with DEX * in spinal surgery | 08 (Good) | Lower intraoperative and especially postoperative consumption of analgesics | Single-centre study with small population |
| Hwang et al., 2015 [31] | Clinical research article | 40 | Posterior lumbar interbody fusion | Compare remifentanil with DEX * on postoperative need for analgesics | 08 (Good) | VAS ** score of remifentanil group significantly greater than DEX * one, every time following surgery (p < 0.05) | DEX * more efficient in pain control at first 48 h, but effective for extended period, too |
| Bojaraaj et al., 2016 [32] | Original article | 60 | Elective spine surgery | Evaluate efficacy and safety of intravenous DEX * on haemodynamic changes and anaesthetic challenges | 07 (Good) | Significant lower amount of propofol in the dexmedetomidine group intraoperatively, to maintain haemodynamics | Statistically significantly better pain scores in dexmedetomidine group at 1 h and 2 h postoperative (p = 0.0001) |
| Garg et al., 2016 [33] | Clinical research article | 66 | Elective spine surgery | Compare safety of opioid sparing analgesics | 08 (Good) | Pain score statistically significantly lower in drug groups (except 0 h) and lower in ketamine group | Comparable results between ketamine and dexmedetomidine group (except 6 h postoperatively) |
| Song et al., 2016 [34] | Original article | 108 | Posterior lumbar spine fusion (one of two levels) | Compare impact of DEX * added to a fentanyl-based patient-controlled mixture on nausea (postoperative) and analgesia | 09 (Good) | DEX * with reduced risk of postoperative nausea at first 1 to 3 h; consumption of fentanyl at each time greater in control group up to 6 h; total amount of pethidine during 48 h also greater | VAS ** scores without significant difference between groups; lower use of opioids in DEX * group |
| Zhou et al., 2017 [35] | Clinical research article | 48 | Multilevel fusion | Assess impact of DEX * on CD42a +, CD14+, and HLADR +/CD14+ levels and inflammatory cytokines | 08 (Good) | No significant difference in VAS ** scores post-surgery. T2, T3, T4, T5, WBC, and CRP decreased significantly in DEX * group, with IL-6 and TNF-a markedly reduced. | Pain not related with inflammation level in both groups |
| Suzuki et al., 2018 [25] | Clinical article | 45 | Minimal invasive epiduroscopy | Compare conventional anaesthetics with DEX * | 08 (Good) | Use of DEX * limits need for fentanyl compared with groups under fentanyl and dropiridol | Avoiding high doses of fentanyl with the use of DEX * can prevent respiratory depression or aspiration of elderly |
| Tsaousi et al., 2016 [36] | Review article | 15 (studies) | Emergency or elective spinal surgery | Evaluate efficacy and safety of dexmedetomidine as sedative and analgesic agent in spine surgery | - | More efficient on intraoperative action when compared with placebo | Heterogeneity of clinical trials means that postoperative efficacy should be interpreted with extreme caution |
| Kim et al., 2019 [22] | Original article | 52 | Spine fusion Surgery | Impact of DEX * on stress responses | 08 (Good) | Lower pain score at 1 h postoperatively | Similar pain scores at 1–6 h, 6–24 h, and 24–48 h postoperatively |
| Janatmankan et al., 2021 [23] | Research article | 60 | Lumbar discectomy | Compare DEX * and remifentanil regarding pain control | 08 (Good) | Significant lower pain levels in DEX * group. | More stable haemodynamic conditions in the same group |
| Nikoubakht et al., 2021 [24] | Original article | 87 | Posterior spine fusion | Postoperative pain control in ketamine versus DEX * group | 08 (Good) | Difference (significant) between groups regarding opioids prescribed during recovery and at 2, 4, 6, 12, and 24 h after surgery (p < 0.05) | No significant differences in ketamine and DEX * groups regarding pain intensity (low dose of DEX *) |
| Waelkens et al., 2021 [26] | Review article | 35 (studies) | Complex spine surgery | Evaluate known research and recommendations regarding pain control | - | Lower need for perioperative opioids; better postoperative pain scores with DEX * | Limited procedure specific evidence on the use of intravenous DEX *; lower incidence of side effects (nausea, bradycardia, hypotension, and shivering) |
| Koh and Leslie, 2022 [27] | Review article | Not applicable | Complex spine surgery | Review research regarding agents and techniques for pain control | - | Emerging evidence for DEX * use for pain control and lower opioid requirement | No differences regarding hypotension and bradycardia; need for multimodal analgesia |
| Choi et al., 2023 [28] | Clinical trial | 98 | Lumbar spine surgery | Evaluate DEX * and remifentanil regarding postoperative sore throat | 08 (Good) | Comparable results in pain scores and need for analgesics in both groups (1, 6, and 24 h postoperatively). | Results explained due to different surgical settings (time both agents stopped) |
| Tao et al., 2024 [18] | Clinical research article | 162 | Lumbar spine surgery | Evaluate of esketamine and DEX * on postoperative cognitive dysfunction | 09 (Good) | VAS ** scores at 2 and 24 h significantly lower in esketamine DEX * group (p < 0.05); no significant differences in scores at 48 h postoperatively (p > 0.05) | Combination of drugs more effective in postoperative pain management |
| Ye et al., 2024 [20] | Clinical trial | 218 | Thoracolumbar compression fracture surgery | Assess influence of DEX * on delirium following surgery and inflammatory biomarkers | 09 (Good) | Similar pain assessment scores between DEX * and control group at first (p = 0.748), second (p = 0.862), and third (p = 0.509); no statistically significant differences at these time points (p values > 0.05) | DEX * significantly mitigates short term elevation of IL-6 and TNF-α levels, contributes to short-term recovery in elderly patients; short term follow-up (three days) |
| Study | Type of Study | Number of Patients | Type of Surgery | Purpose of Study | Newcastle–Ottawa Score | Results | Comments |
|---|---|---|---|---|---|---|---|
| Niu et al., 2023 [16] | Clinical research article | 150 | Elective spinal surgery | Study efficacy of administration routes of DEX * on POD ** | 09 (Good) | Incidence of POD ** in intravenous group significantly lower than in intranasal within 3 days (3 of 49 [6.1%] vs. 14 of 50 [28.0%]; p < 0.017); no difference between intratracheal and intravenous groups (5 of 49 [10.2%] vs. 3 of 49 [6.1%]; OR, 1.74; 95% CI, 0.40–7.73; p > 0.017) | Better sleep quality postoperatively in intravenous group; mild adverse events in all three groups |
| Ahn et al., 2024 [17] | Clinical article | 44 | Spine surgery (type not applicable) | Determine effective dose of DEX * intraoperative infusion to prevent emergence agitation | 07 (Good) | At admission at the post-anaesthesia unit: one patient anxious, agitated, or restless; one with signs of excitement (quickly returned to tranquil state) | Effectiveness of DEX * on emergence agitation; stable recovery |
| Tao et al., 2024 [18] | Clinical research article | 162 | Lumbar spine surgery | Evaluate of esketamine and DEX * on POCD *** | 09 (Good) | Incidence of POCD ** on first postoperative day significantly lower in esketamine–DEX * group compared to esketamine but not statistically different for DEX * alone compared with combination; on third postoperative day, differences between groups not significant | Combination eliminated serologic markers compared to individual drugs, revealing effective reduction in biomarkers of neuro inflammation and neuronal damage |
| Xie et al., 2024 [19] | Clinical article | 7250 | Lumber surgical treatment/degenerative diseases | Search changes in incidence of delirium in patients with lumbar degenerative disease and potential causes of changes | 09 (Good) | Delirium related to age, number of elements, duration of surgical, remifentanil, benzodiazepines, and DEX * (p < 0.05) | Increase in dosage of DEX * reduces risk of delirium |
| Ye et al., 2024 [20] | Clinical trial | 218 | Thoracolumbar compression fracture surgery | Assess influence of DEX * on delirium following surgery | 08 (Good) | On first postoperative day, DEX * group with significant reduction in POD ** compared with control group; overall incidence of POD lower in DEX * group; no significant differences in POD ** on second and third postoperative days | DEX * mitigates short-term elevation of IL-6 and TNF-α levels, and contributes to short-term recovery in elderly patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrati, S.R.; Lianou, I.; Kaspiris, A.; Marougklianis, V.; Kotanidou, A.; Pneumaticos, S.G. Analgesic and Neuroprotective Roles of Dexmedetomidine in Spine Surgery: A Systematic Review. Diseases 2025, 13, 212. https://doi.org/10.3390/diseases13070212
Afrati SR, Lianou I, Kaspiris A, Marougklianis V, Kotanidou A, Pneumaticos SG. Analgesic and Neuroprotective Roles of Dexmedetomidine in Spine Surgery: A Systematic Review. Diseases. 2025; 13(7):212. https://doi.org/10.3390/diseases13070212
Chicago/Turabian StyleAfrati, Spyridoula Roberta, Ioanna Lianou, Angelos Kaspiris, Vasileios Marougklianis, Anastasia Kotanidou, and Spiros G. Pneumaticos. 2025. "Analgesic and Neuroprotective Roles of Dexmedetomidine in Spine Surgery: A Systematic Review" Diseases 13, no. 7: 212. https://doi.org/10.3390/diseases13070212
APA StyleAfrati, S. R., Lianou, I., Kaspiris, A., Marougklianis, V., Kotanidou, A., & Pneumaticos, S. G. (2025). Analgesic and Neuroprotective Roles of Dexmedetomidine in Spine Surgery: A Systematic Review. Diseases, 13(7), 212. https://doi.org/10.3390/diseases13070212







