Abstract
Objectives: The incidence of postoperative pain in patients that undergo spinal interventions is significantly increased, affecting their functional outcomes and quality of life. Dexmedetomidine (DEX) belongs to the category of centrally acting nonopioid agents with highly selective α2 adrenoreceptor agonist activity that are frequently applied in spinal surgery based on its antinociceptive and anxiolytic properties. Although many studies displayed the effectiveness of DEX in postoperative pain management, the impact of DEX on functional improvement after spinal surgeries is still debatable. Purpose: This systematic review focuses on the intraoperative and postoperative role of dexmedetomidine (DEX) as an analgesic agent in elective and emergency adult spine surgery. Methods: An electronic literature review search was conducted via Web of Science and PubMed to assess the impact of DEX on postoperative pain management, postoperative delirium (POD), and postoperative cognitive dysfunction (POCD). Discussion: Twenty-one studies were retrieved, three of which were review articles. The effects of DEX were studied for up to 48 h postoperatively. In most cases, its administration was associated with reduced intraoperative and postoperative opioid consumption. However, findings on pain control were less conclusive due to heterogeneity in dosing protocols, concomitant medications, the timing of administration, and pain scoring systems. DEX appears to reduce the incidence of POD and POCD, particularly when used in combination with other drugs. Conclusions: Although the present study supports that the intraoperative administration of dexmedetomidine decreases the pain intensity and/or opioid consumption as well as the development of POD and POCD in patients undergoing spinal surgeries during the first 24 h postoperatively, the current literature should be expanded to allow for the safe generalisation of findings over longer follow-up periods. Further research into the neuroprotective, analgesic, and anti-inflammatory roles of DEX is warranted.
1. Introduction
The range of spinal surgeries performed in patients with significant comorbidities, including heart failure, respiratory disease, and vascular conditions, poses considerable challenges for both anaesthesia and surgical teams. Perioperative and postoperative pain management significantly affects patients’ haemodynamic stability, rehabilitation, complication rates, and overall quality of life, including cognitive status [1]. Non-effective pain management can cause various complications, including ileus, nausea, prolonged hospital stay, poor surgical outcome, and chronic pain syndromes [2]. Opioids, which have been mainly used for treating chronic spinal pain, are commonly used as first-line agents of perioperative analgesia [3]. However, the chronic use of this therapy seems to be associated with hyperalgesia and postoperative pain, due to peripheral and central sensitisation, cognitive impairment, and respiratory depression [4]. A multimodal approach incorporating both opioid and nonopioid agents has been effectively employed to mitigate postoperative pain and reduce opioid-related side effects [5]. Nonopioid anaesthesia methods may help eliminate iatrogenic postoperative pain and the need for opioid analgesia [1].
The use of α2 adrenergic receptor agonists (dexmedetomidine (DEX) and clonidine) can contribute to haemodynamic stability and a variety of therapeutic effects [6]. DEX is a highly selective α2-adrenergic receptor agonist, exhibiting seven to eight times greater selectivity than clonidine [7]. The activation of these receptors, located in the central venous system and the spinal cord, leads to the inhibition of adenylyl cyclase, which catalyses cAMP (Cyclic AMP) formation, an important second messenger in various cellular catabolic pathways. Moreover, alterations in cellular ion conductance and membrane hyperpolarisation diminish noradrenergic activity, stress responses and sympathetic outflow, resulting in improved myocardial oxygenation and blood supply, and enhanced recovery quality [8,9]. DEX exhibits anxiolytic, sedative, anti-inflammatory, and analgesic effects, while also promoting respiratory and haemodynamic stability. Exceptions include patients with volume depletion, severe heart block, or vasoconstriction, in whom DEX should be used cautiously due to the risks of bradycardia and hypotension [7]. Ultimately, when combined with commonly used analgesics, DEX may enhance postoperative pain control and improve overall quality of life.
Postoperative cognitive dysfunction (POCD), a common perioperative neurological complication in the elderly, is characterised by impairments in language, memory, task execution, and perceptual functions. In contrast, postoperative delirium (POD) typically presents in the early postoperative period and is marked by disorientation, purposeless movements, and restlessness. POD results from acute cerebral dysfunction and is defined by disturbances in attention, awareness, and cognition [10]. This type of delirium, presented in patients undergoing spinal surgery, is reported with an incidence up to 40.5% [11]. DEX has been shown to improve N3 sleep, leading to the enhancement of neurocognitive function and a reduction in postoperative delirium [12]. Moreover, the impact of DEX on postoperative pain management is reported to affect cognition, as sleep disorders on the first postoperative night, often caused by severe pain, can be predictive factors for delirium [13]. Consequently, DEX appears to be effective in maintaining and improving neurocognitive function and reducing the risk of postoperative delirium.
Although dexmedetomidine is an effective alternative to opioid analgesics used in anaesthesia care and is associated with fewer opioid-related complications, its integration into anaesthetic practice remains limited. According to the literature, its limited intraoperative use is due to a lack of clinical experience and knowledge [14].
The aim of this review is to enhance current understanding and encourage further research into the beneficial effects of perioperative DEX on pain management and cognitive recovery following spinal surgery.
2. Materials and Methods
2.1. Literature Research
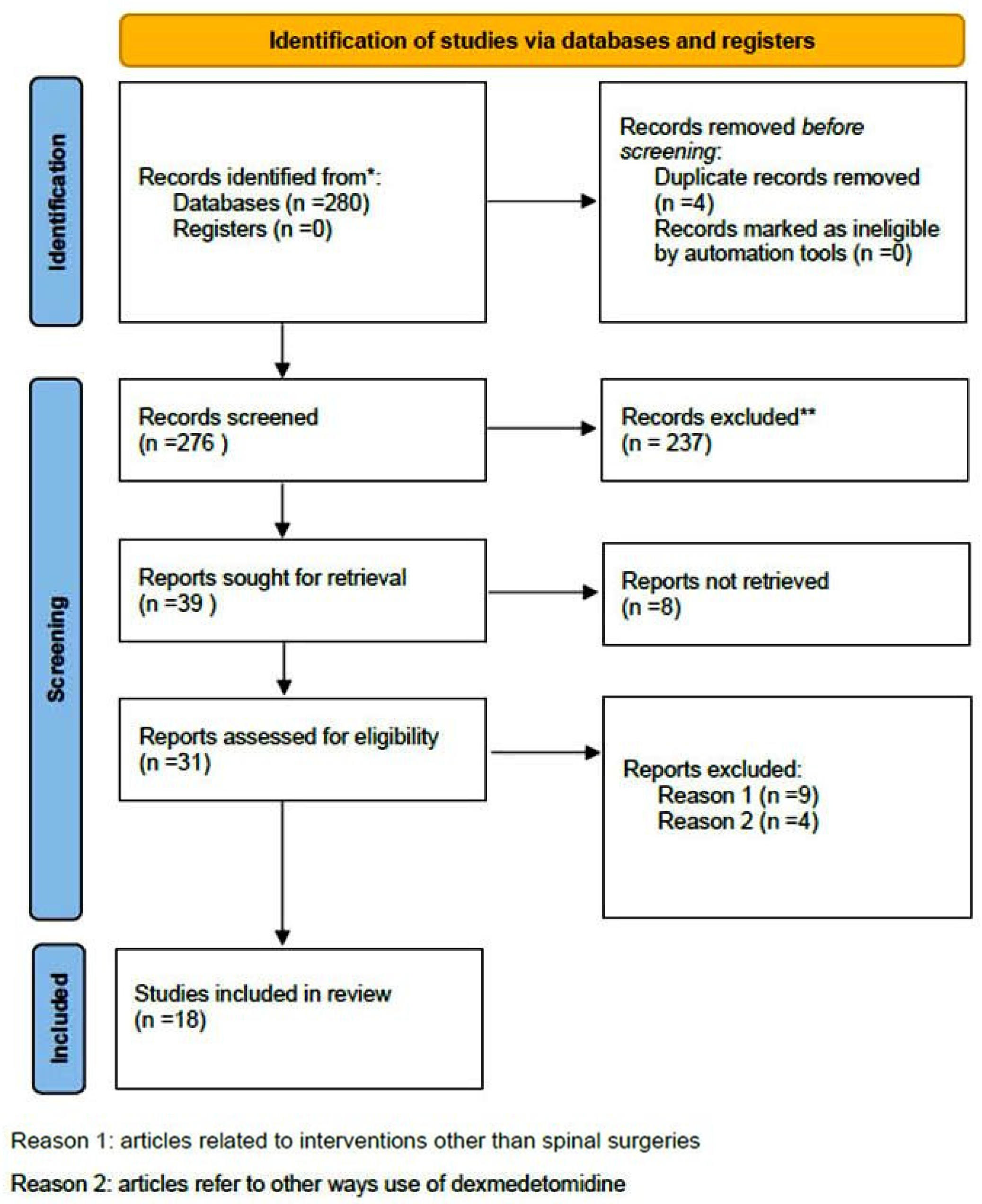
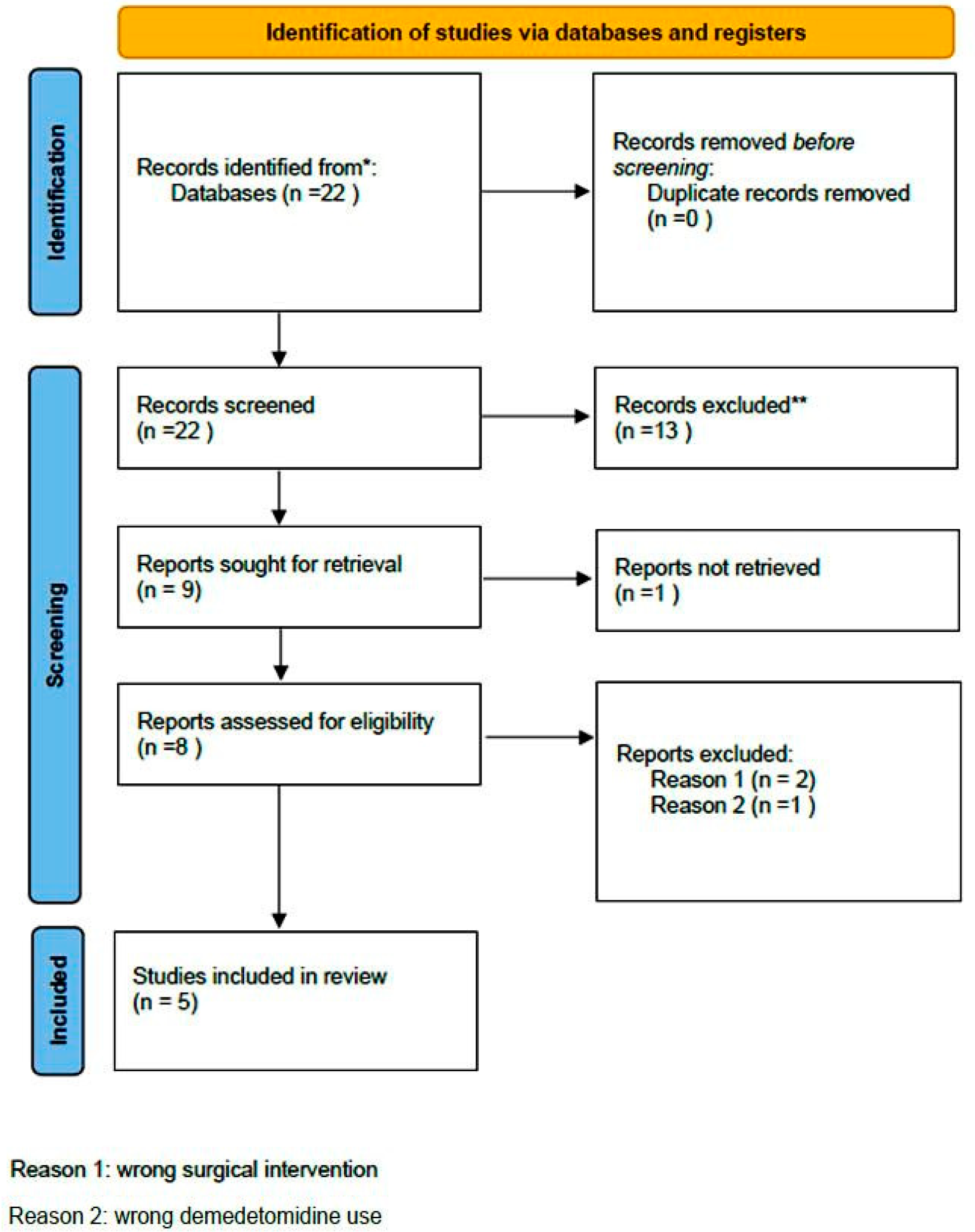
A comprehensive computer-based literature search was conducted on 02/05/2025 to investigate the use of dexmedetomidine (DEX) in relation to postoperative pain control, cognitive function, and spinal surgery. The databases searched were Web of Science (1900 to present) and PubMed (1947 to present). The search methodology used a combination of the terms “dexmedetomidine [All Fields]”, “spinal surgery [All Fields]”, “pain control [All Fields]”, and “delirium” [All Fields]. Duplicate records were removed using Zotero version 6.0.10 (Digital Scholar, https://digitalscholar.org/, accessed on 20 April 2025 ). The systematic review was conducted according to PRISMA guidelines and is presented in the following PRISMA flow charts (Figure 1 and Figure 2). Moreover, the study was registered in the PROSPERO (International Prospective Register of Systematic Reviews) database with registration number CRD420251082437, and full access to the protocol is provided.
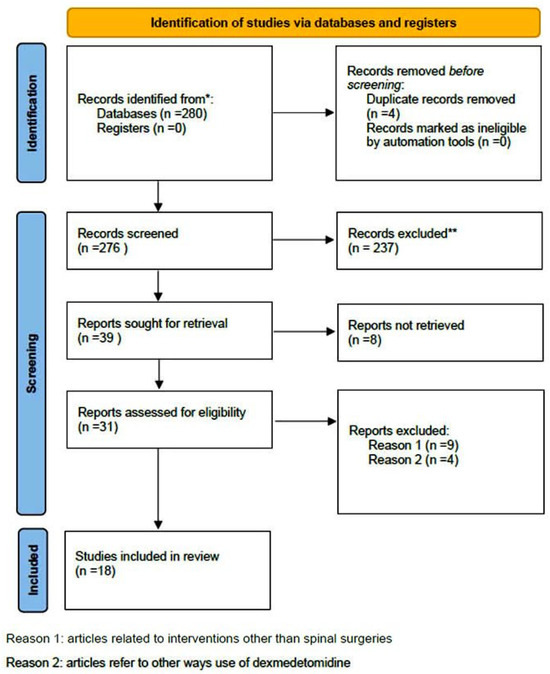
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers regarding pain management. (* Records identified in both databases (Pubmed and Web of Science), ** Records excluded by the authors without automation tools).
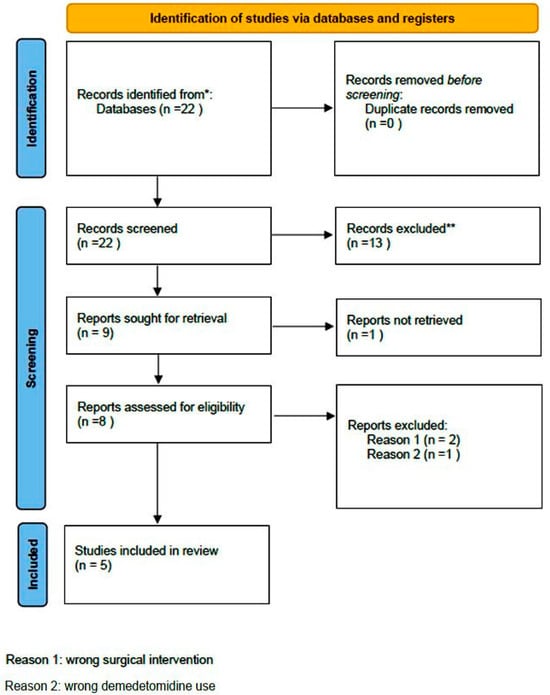
Figure 2.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers regarding delirium. (* Records identified in both databases (Pubmed and Web of Science, ** Records excluded by the authors without automation tools).
2.2. Selection Criteria
The initial search included only studies published in English. All qualitative studies relevant to the main topics were retrieved. Additional inclusion criteria included (a) only full-text articles, (b) comparative studies assessing the systematic (intravenous) perioperative use of dexmedetomidine and other anaesthetics in all types of spinal surgeries in adults (aged over 18 years), focusing on pain control and cognitive function, and (c) studies evaluating outcomes of perioperative dexmedetomidine administration in spinal procedures. No restrictions were placed on the date of publication. Exclusion criteria were (a) studies based on in vitro or in vivo animal models, (b) case reports, case series, letters to the editor, or studies with insufficient data regarding the period of use and clinical outcomes, (c) studies focusing on wound infiltration with dexmedetomidine rather than its systematic administration, and (d) studies written in languages other than English.
2.3. Data Extraction
The literature research and data extraction were conducted independently by two authors (S.R.A. and I.L.) and an experienced librarian. The authors screened titles and abstracts in accordance with the inclusion and exclusion criteria and identified articles relevant to the favourable effects of DEX on perioperative or postoperative analgesia and cognitive function or delirium. Disagreements regarding inclusion were resolved by the senior author (S.G.P.). Relevant data were then extracted and recorded in a Microsoft Excel spreadsheet (Microsoft Office 365, Redmond, WA). Any disagreements between the two authors on the data extracted were also resolved by the senior author after the re-evaluation of the full texts.
This study adheres to the principles of systematic reviews, aiming to synthesise the existing literature conscientiously.
2.4. Evaluation Analysis
The methodology of each original study was assessed independently by two authors (A.K. and R.S.A.) using the Newcastle–Ottawa quality assessment scale [15]. Included studies were graded using a three-category scale. Studies displaying a total score of 0–3, 4–6, and 7–9 were classified as poor-, fair-, or good-quality, respectively. Review articles were not evaluated by the above assessment scale.
3. Results
3.1. Included Studies
A total of 21 studies met the inclusion criteria and are presented in two tables: Table 1 [16,17,18,19,20], and Table 2 [17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Three of these were review articles [25,26,35].

Table 1.
The literature on the intraoperative and postoperative role of DEX in pain management.

Table 2.
The literature on the role of DEX on cognitive function. (* dexmedetomidine, ** postoperative delirium, *** postoperative cognitive dysfunction).
3.2. Quality Assessment
4. Discussion
4.1. Dexmedetomidine and Anti-Inflammatory Activity in Pain Control
The wide range of spinal surgeries performed today presents significant challenges for anaesthetic teams aiming to maintain perioperative haemodynamic and respiratory stability, achieve effective pain control, and facilitate recovery. Surgery and anaesthesia stress combined with excessive bleeding cause an inflammatory response, which leads to impaired body function and side effects [35]. Several drugs, including dexmedetomidine, have been used in order to suppress this response.
DΕΧ acts at the level of the spinal cord as a highly selective a2 adrenergic receptor agonist, which primarily inhibits signal transmission in the dorsal horns by activating pre-synaptic a2 adrenergic receptors. The activation of this receptor leads to internal calcium ion flow, resulting in decreased neurotransmitter release and the inhibition of signal transduction from peripheral nerve fibres [37]. Moreover, findings from animal experiments suggest that DEX can activate anti-apoptotic agents and thus protect cells, by decreasing CD14+ and CD42a+ (T2, T3, and T4 cells) and increasing HLDRA+ and CD14+ (T4 and T5 cells) [38]. The changes in the concentration of this agent contribute to reducing lipid peroxidation and ischemia–reperfusion injury and suppressing the inflammatory response [39].
Animal studies have shown that DEX can block the transmission of neural signals from peripheral neurons and thus contribute to perioperative pain control [36]. Its short duration of action and plasma half-life (approximately 2.3 h) compared to clonidine make it an effective adjuvant for immediate postoperative analgesia. The short- and long-term action of DEX has been widely studied in clinical research [18,20,21,22,23,24,25,26,27,29,30,31,32,34,35].
Hwang et al. [31] reported the superior analgesic efficacy of DEX compared with remifentanil for up to 48 h postoperatively. These results are attributed to its action on nociceptors, located in laminae I-III of the dorsal horns, where it modulates pain thresholds, resulting in improved postoperative pain management. Similarly, when the combined use of DEX and fentanyl (first group) was compared to the use of fentanyl plus droperidol (second group), a lower need for intraoperative fentanyl was reported in the first group, indicating greater efficacy compared to other anaesthetics [25]. Rahimzadeh et al. also noted decreased perioperative and postoperative analgesic use when DEX was used [30]. Moreover, patients receiving perioperative DEX required supplemental analgesia later than those receiving fentanyl [21]. Bojaaraj et al. reported better pain scores at 1 and 2 h postoperatively in patients undergoing elective spine surgery with DEX compared with controls receiving saline [32]. Results on a more extended postoperative period were reported in a study by Nikhoubakht et al. [24], who revealed a statistically significant difference regarding the use of opioids between dexmedetomidine and the control group after posterior spinal fusion until 24 h following surgery. Similarly, Kim et al. extended the postoperative follow-up to 48 h and observed better pain scores in the DEX group at 1 h postoperatively. However, no statistically significant difference in the long-term results was reported between the two groups (1–6 h, 6–24 h, and 24–48 h postoperatively) [22].
Results from other studies regarding the impact of dexmedetomidine on postoperative pain perception and requirements for opioid analgesics remain inconclusive, as no statistically significant outcomes were revealed in various clinical cases [18,24,26,28,29,34,35]. The different duration of the follow-up periods for postoperative pain evaluation (after drug withdrawal), under 48 h in most cases, resulted in heterogeneity and incomplete data on the long-term response to DEX administration [24,28,35].
Differences in surgical settings the drugs used may also explain inconsistencies in the findings, as reported by Choi et al., where the comparable efficacy of dexmedetomidine and remifentanil within 48 h postoperatively was observed [28]. Even though a lower opioid consumption was noted in patients receiving dexmedetomidine, Naik et al. did not find statistically significant differences in postoperative opioid use or pain scores during a three-day follow-up after major spinal surgery [29]. This effect may be attributed to the perioperative use of methadone, a long-acting, opioid-sparing analgesic, which could have masked the action of DEX. Similarly, the opioid-sparing effect of DEX on pain management could be masked by the different ketorolac consumption and analgesics mix, through patient-controlled intravenous anaesthesia, as seen in the clinical outcomes reported by Song et al. [34].
The diversity in DEX dosages used can affect the results reported in some studies [24]. The comparison between groups under ketamine or DEX revealed statistically significant differences in results in the immediate postoperative period (less than 24 h), regarding the need for additional analgesia with opioids, but no significant differences in pain control. These results may reflect the unusually relative low dose of dexmedetomidine used in this study [24]. Even in studies with longer follow-up periods (up to five days), small sample sizes and varied subjective pain assessment tools contributed to a lack of statistically significant findings in postoperative pain scores. According to Zhou et al. [35], although dexmedetomidine can limit immunosuppression and the inflammatory response, postoperative pain does not appear to affect sympathetic response [35]. In contrast with other studies, Garg et al. reported better pain scores in the group treated with ketamine compared with those under DEX, although both groups outperformed the control group [33].
According to the existing literature reviewed by Tsaousi et al. and Waelkens et al., over the past years, DEX has emerged as an efficient opioid-sparing agent during the intraoperative period, but its postoperative efficacy remains unclear [26,36]. The lack of consensus on the results of dexmedetomidine on pain management and opioid consumption following spinal surgeries could be explained by the heterogeneity of the parameters of these clinical trials. DEX could be combined with various other pharmacological agents, used in different dosages and models of drug administration. Moreover, inconsistencies in subjective pain scoring systems, the usually short-term follow-up periods, and opioid-induced hyperalgesia limit the interpretation of its analgesic role to a short postoperative period (up to 6 h) [36].
4.2. Impact of Dexmedetomidine on Postoperative Cognitive Function and Delirium
Postoperative cognitive dysfunction (POCD) is characterised by impaired task assembly ability, memory, perceptual or language functions and differs from postoperative delirium (POD), which mainly presents with disturbance in memory, attention, awareness, cognition, and consciousness. Postoperative delirium presents with three subtypes: hypoactive, hyperactive, and mixed [40]. Both POCD and POD are common perioperative and postoperative neurological disorders [41]. Their notable association can be explained by the elevation of pro-inflammatory cytokines in the plasma and cerebrospinal fluid of elderly patients presenting with delirium post-surgically (first postoperative day). The normalisation of their trend on the third postoperative day often coincides with the resolution of delirium symptoms.
According to Ye et al., DEX may modulate the immune response and reduce postoperative delirium occurrence, by mitigating the early elevation of IL-6 and TNF-α. This agent inhibits signals related with inflammation and cell death (the downregulation of phospho-JNK-phosphorylated c-Jun N-terminal kinase) and enhances neuroprotection pathways (the upregulation of phosphor-ERK1/2-phosphorylated extracellular signal regulated kinase 1/2 expression) [20]. Niu et al. reported superior outcomes on postoperative delirium with intratracheal or intravenous DEX compared to intranasal use [16]. Intravenous use was also associated with better sleeping patterns. Similarly, DEX appears to reduce the incidence of hyperactive postoperative delirium in patients undergoing spine surgery for lumbar degenerative diseases [19].
The efficacy of DEX combined with esketamine on POCD in patients treated with elective lumbar spine surgery was studied by Tao et al. [18]. The combination of these drugs can be more effective in reduction in POCD incidence on the first postoperative day than either agent alone. No statistically significant difference was observed between the esketamine–DEX group and the DEX-only group. However, these results could be influenced by the relatively low presence of POCD in these patients, due to the advanced surgical techniques that reduce trauma effect. Moreover, pain scoring scales presented better results in the group of patients under esketamine and DEX at 2 and 24 h postoperatively, which is in line with results regarding the incidence of POCD, as this possibly indicates their role in the suppression of inflammation and neuroprotection [37]. Finally, various side effects, including emergence agitation or delirium, which are presented with disorientation, uncontrolled movements, and excitation during early aesthesia recovery, could be avoided with a well-controlled dosage of DEX, which reduces haemodynamic adverse events, especially in older patients (aged over 65 years) [17].
4.3. Dexmedeomidine, Pain Management, and Delirium
Postoperative pain is a recognised risk factor for the development of POD [40]. However, pain assessment scales were incorporated in only two of the studies focusing on POD or POCD [18,20]. Although DEX has been shown to significantly reduce the incidence of POD and attenuate short-term elevations in IL-1, IL-6, and TNF-α, no statistically significant differences in pain scores were observed between patients receiving DEX and those who did not, according to Ye et al. [20]. These results derive from a single-centre study conducted in a secondary hospital, which suffered from considerable patient loss during a relatively short follow-up period, resulting in incomplete pain assessment data that may have influenced the outcomes. Similarly, Tao et al. studied the effect of esketamine combined with DEX on VAS (visual analogue scale) scores, in addition to its impact on POCD. The combination of these drugs revealed superior outcomes on pain control at 2 and 24 h than each drug alone, but not at the long-term follow-up (48 h) [18]. Further studies are warranted to clarify the direct relationship between perioperative and postoperative pain control, inflammatory processes, and cognitive function. These investigations should aim to determine the optimal DEX dosage for achieving both analgesic and neuroprotective effects.
5. Strengths and Limitations
To the best of our knowledge, this review represents the most up-to-date synthesis on the analgesic effect of DEX, incorporating the latest clinical trial data on this issue and is the only one focusing on its outcomes both on pain management and postoperative cognitive dysfunction or delirium. However, our study has several limitations. Although 21 entries of high quality were included in this review, the studies’ designs and methods were heterogeneous as different clinical protocols were used and no standardised methods were applied in order to evaluate the reproducibility of the outcomes. Moreover, the limited experience in clinical settings raises concerns about the long-term results of DEX infusion in patients undergoing spinal surgeries. Finally, a language bias could be present as only studies written in English were reviewed.
6. Conclusions
Recent studies have explored the pain-relieving and neuroprotective effects of DEX in spinal surgery. However, the heterogeneity of dosing protocols, surgical procedures, and the tools used to assess pain and cognitive outcomes has led to discrepancies in findings and a lack of statistically significant results across several trials. Moreover, the association between pain control, inflammation response, and delirium or postoperative cognitive dysfunction remains insufficiently studied. The present review study supports the intraoperative administration of dexmedetomidine in decreasing the pain intensity and/or opioid consumption as well as the development of POD and POCD in haemodynamically stable patients undergoing spinal surgeries during the first 24 h postoperatively. The outcomes of this study should be interpreted with caution and should not yet be generalised. Future studies on different parameters of dexmedetomidine administration and its impact on pain management and cognitive function over longer follow-up periods could contribute to more efficient and safe outcomes.
Author Contributions
Conceptualization, formal analysis, methodology, and writing—original draft preparation S.R.A.; writing—original draft preparation, I.L.; writing—review and editing, A.K. (Angelos Kaspiris); writing—review and editing, V.M.; writing—review and editing, A.K. (Anastasia Kotanidou); supervision, S.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blaudszun, G.; Lysakowski, C.; Elia, N.; Tramèr, M.R. Effect of Perioperative Systemic A2 Agonists on Postoperative Morphine Consumption and Pain Intensity: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Anesthesiology 2012, 116, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Boezaart, A.P.; Davis, G.; Le-Wendling, L. Recovery after Orthopedic Surgery: Techniques to Increase Duration of Pain Control. Curr. Opin. Anaesthesiol. 2012, 25, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.K.; Durieux, M.E.; Nemergut, E.C. Non-Opioid Analgesics: Novel Approaches to Perioperative Analgesia for Major Spine Surgery. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 79–89. [Google Scholar] [CrossRef]
- Devin, C.J.; Mcgirt, M.J. Best Evidence in Multimodal Pain Management in Spine Surgery and Means of Assessing Postoperative Pain and Functional Outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef]
- Grosu, I.; de Kock, M. New Concepts in Acute Pain Management: Strategies to Prevent Chronic Postsurgical Pain, Opioid-Induced Hyperalgesia, and Outcome Measures. Anesthesiol. Clin. 2011, 29, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S. Effect of Intravenous Infusion of Dexmedetomidine on Perioperative Haemodynamic Changes and Postoperative Recovery: A Study with Entropy Analysis. Indian J. Anaesth. 2012, 56, 542–546. [Google Scholar] [CrossRef]
- Carollo, D.S.; Nossaman, B.D.; Ramadhyani, U. Dexmedetomidine: A Review of Clinical Applications. Curr. Opin. Anesthesiol. 2008, 21, 457–461. [Google Scholar] [CrossRef]
- Khan, Z.P.; Ferguson, C.N.; Jones, R.M. Alpha-2 and Imidazoline Receptor Agonists Therapeutic Role. Anaesthesia 1999, 54, 146–165. [Google Scholar] [CrossRef]
- Takahiko, K.; Mervyn, M. Clinical Uses of A2-Adrenergic Agonists. Anesthesiology 2000, 93, 1345–1349. [Google Scholar]
- Evered, L.A.; Silbert, B.S. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth. Analg. 2018, 127, 496–505. [Google Scholar] [CrossRef]
- Brown, C.H.; LaFlam, A.; Max, L.; Wyrobek, J.; Neufeld, K.J.; Kebaish, K.M.; Cohen, D.B.; Walston, J.D.; Hogue, C.W.; Riley, L.H. Delirium After Spine Surgery in Older Adults: Incidence, Risk Factors, and Outcomes. J. Am. Geriatr. Soc. 2016, 64, 2101–2108. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, B.J.; Mu, D.L.; Hu, J.; Guo, C.; Li, X.Y.; Ma, D.; Wang, D.X. Randomized Clinical Trial of Intraoperative Dexmedetomidine to Prevent Delirium in the Elderly Undergoing Major Non-Cardiac Surgery. Br. J. Surg. 2020, 107, e123–e132. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Nadler, J.W.; Preud, X.A.; Fang, E.; Daughtry, R.L.; Chapman, J.B.; Attarian, D.; Wellman, S.; Krystal, A.D. Clinical Neurophysiology Pilot Prospective Study of Post-Surgery Sleep and EEG Predictors of Post-Operative Delirium. Clin. Neurophysiol. 2017, 128, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Constan, E.; Stovall, C.; Matlock, R.; Steverson, C.; Wilbanks, B.; McMullan, S.; Yerdon, A. The Perception and Use of Dexmedetomidine Among Anesthesia Providers: A Quality Improvement Project. J. Perianesthesia Nurs. 2024, 39, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, Ottawa Health Research Institute. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 29 June 2025).
- Niu, J.Y.; Yang, N.; Tao, Q.Y.; He, Y.; Hou, Y.B.; De Ning, R.; Yu, J.M. Effect of Different Administration Routes of Dexmedetomidine on Postoperative Delirium in Elderly Patients Undergoing Elective Spinal Surgery: A Prospective Randomized Double-Blinded Controlled Trial. Anesth. Analg. 2023, 136, 1075–1083. [Google Scholar] [CrossRef]
- Ahn, H.; Chae, Y.J.; Bin Choi, G.; Lee, M.G.; Yoo, J.Y. Determining the Optimal Dosage of Dexmedetomidine for Smooth Emergence in Older Patients Undergoing Spinal Surgery: A Study of 44 Cases. Med. Sci. Monit. 2024, 30, 1–8. [Google Scholar] [CrossRef]
- Tao, Q.Y.; Liu, D.; Yu, J.M.; Wang, S.J.; Wang, X.; Ouyang, R.N.; Ning, R.; Niu, J.Y. Effects of Esketamine Combined with Dexmedetomidine on Early Postoperative Cognitive Function in Elderly Patients Undergoing Lumbar Spinal Surgery: A Double-Blind Randomized Controlled Clinical Trial. Drug Des. Devel. Ther. 2024, 18, 5461–5472. [Google Scholar] [CrossRef]
- Xie, B.L.; Nie, L.Z.; Zhong, B.; Xiong, J.; Nie, M.; Ai, Q.X.; Yang, D. Longitudinal Trends in the Incidence of Hyperactive Delirium and Its Causes of Change After Surgery for Degenerative Lumbar Disease: A Population-Based Study of 7250 Surgical Patients Over 11 Years. Orthop. Surg. 2024, 17, 714–723. [Google Scholar] [CrossRef]
- Ye, C.; Shen, J.; Zhang, C.; Hu, C. Impact of Intraoperative Dexmedetomidine on Postoperative Delirium and Pro-Inflammatory Cytokine Levels in Elderly Patients Undergoing Thoracolumbar Compression Fracture Surgery: A Prospective, Randomized, Placebo-Controlled Clinical Trial. Medicine 2024, 103, E37931. [Google Scholar] [CrossRef]
- Turgut, N.; Turkmen, A.; Gökkaya, S.; Altan, A.; Hatiboglu, M.A. Fentanyl-Based Total Intravenous Anesthesia for Lumbar Laminectomy. Minerva Anestesiol. 2008, 74, 469–474. [Google Scholar]
- Kim, M.H.; Lee, K.Y.; Bae, S.J.; Jo, M.; Cho, J.S. Intraoperative Dexmedetomidine Attenuates Stress Responses in Patients Undergoing Major Spine Surgery. Minerva Anestesiol. 2019, 85, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Jannatmakan, F.; Nassajian, N.; Jarirahmadi, S.; Tabatabaee, K.; Zafari, M. Comparison of the Effect of Dexmedetomidine and Remifentanil on Pain Control after Spinal Surgery: A Double-Blind, Randomized Clinical Trial. Anesthesiol. Pain Med. 2021, 11, 111533. [Google Scholar] [CrossRef]
- Nikoubakht, N.; Alimian, M.; Faiz, S.H.R.; Derakhshan, P.; Sandri, M.S. Effects of Ketamine versus Dexmedetomidine Maintenance Infusion in Posterior Spinal Fusion Surgery on Acute Postoperative Pain. Surg. Neurol. Int. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Inokuchi, R.; Hanaoka, K.; Suka, M.; Yanagisawa, H. Dexmedetomidine Use during Epiduroscopy Reduces Fentanyl Use and Postoperative Nausea and Vomiting: A Single-Center Retrospective Study. SAGE Open Med. 2018, 6, 2050312118756804. [Google Scholar] [CrossRef]
- Waelkens, P.; Alsabbagh, E.; Sauter, A.; Joshi, G.P.; Beloeil, H. Pain Management after Complex Spine Surgery: A Systematic Review and Procedure-Specific Postoperative Pain Management Recommendations. Eur. J. Anaesthesiol. 2021, 38, 985–994. [Google Scholar] [CrossRef]
- Koh, W.S.; Leslie, K. Postoperative Analgesia for Complex Spinal Surgery. Curr. Opin. Anaesthesiol. 2022, 35, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Baek, J.; Kim, D.Y. Effect of Dexmedetomidine and Remifentanil Infusion on Postoperative Sore Throat after Lumbar Spine Surgery in the Prone Position. Medicine 2023, 102, E33506. [Google Scholar] [CrossRef]
- Naik, B.I.; Nemergut, E.C.; Kazemi, A.; Fernández, L.; Cederholm, S.K.; Mcmurry, T.L.; Durieux, M.E. The Effect of Dexmedetomidine on Postoperative Opioid Consumption and Pain After Major Spine Surgery. Anesth. Analg. 2016, 122, 1646–1653. [Google Scholar] [CrossRef]
- Rahimzadeh, P.; Faiz, S.H.R.; Alimian, M.; Mohammadian Erdi, A. Remifentanil versus Dexmedtomidine for Posterior Spinal Fusion Surgery. Med. J. Islam. Repub. Iran 2015, 29, 215. [Google Scholar]
- Hwang, W.; Lee, J.; Park, J.; Joo, J. Dexmedetomidine versus Remifentanil in Postoperative Pain Control after Spinal Surgery: A Randomized Controlled Study. BMC Anesthesiol. 2015, 15, 21. [Google Scholar] [CrossRef]
- Bojaraaj, D.R.K.; Senthilkumar, S.; Vijayaragavan, S.; Gnanavelrajan, A. Effect of Intravenous Use of Dexmedetomidine on Anesthetic Requirements in Patients Undergoing Elective Spine Surgery: A Double Blinded Randomized Controlled Trail. Int. J. Sci. Study 2016, 4, 251–255. [Google Scholar] [CrossRef]
- Garg, N.; Panda, N.; Gandhi, K.; Bhagat, H.; Batra, Y.; Grover, V.; Chhabra, R. Comparison of Small Dose Ketamine and Dexmedetomidine Infusion for Postoperative Analgesia in Spine Surgery—A Prospective. J. Neurosurg. Anesthesiol. 2016, 28, 27–31. [Google Scholar] [CrossRef]
- Song, Y.; Shim, J.K.; Song, J.W.; Kim, E.K.; Kwak, Y.L. Dexmedetomidine Added to an Opioid-Based Analgesic Regimen for the Prevention of Postoperative Nausea and Vomiting in Highly Susceptible Patients. Eur. J. Anaesthesiol. 2016, 33, 75–83. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, J.; Shen, Y.; Kang, S.; Zong, Y. Effects of Dexmedetomidine on CD42a+/CD14+, HLADR+/CD14+ and Inflammatory Cytokine Levels in Patients Undergoing Multilevel Spinal Fusion. Clin. Neurol. Neurosurg. 2017, 160, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, G.; Pourzitaki, C.; Aloisio, S.; Federico, B. Dexmedetomidine as a Sedative and Analgesic Adjuvant in Spine Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Pharmacol. 2018, 74, 1377–1389. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z. Analgesic Mechanism of Dexmedetomidine and Esketamine in Rats with Spinal Cord Injury. Discov. Med. 2024, 36, 714. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xing, Q.; Li, Y.; Han, X.; Sun, L. Dexmedetomidine Protects against Ischemia-Reperfusion Injury in Rat Skeletal Muscle. J. Surg. Res. 2014, 186, 240–245. [Google Scholar] [CrossRef]
- Gao, J.; Sun, Z.; Xiao, Z.; Du, Q.; Niu, X.; Wang, G.; Chang, Y.W.; Sun, Y.; Sun, W.; Lin, A.; et al. Dexmedetomidine Modulates Neuroinflammation and Improves Outcome via Alpha2-Adrenergic Receptor Signaling after Rat Spinal Cord Injury. Br. J. Anaesth. 2019, 123, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, D.; Guo, Y.; Sun, X.; Chen, C.; Zhai, X.; Jin, X.; Zhu, H.; Li, P.; Yu, W. Recent Advances and Perspectives of Postoperative Neurological Disorders in the Elderly Surgical Patients. CNS Neurosci. Ther. 2022, 28, 470–483. [Google Scholar] [CrossRef]
- Caumo, W.; Schmidt, A.P.; Schneider, C.N.; Bergmann, J.; Iwamoto, C.W.; Bandeira, D.; Ferreira, M.B.C. Risk Factors for Preoperative Anxiety in Adults. Acta Anaesthesiol. Scand. 2001, 45, 298–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).