Fibrin Monomer and Systemic Lupus Erythematosus Reactivation During Pregnancy: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Group
3.2. Laboratory Indices of the Participants
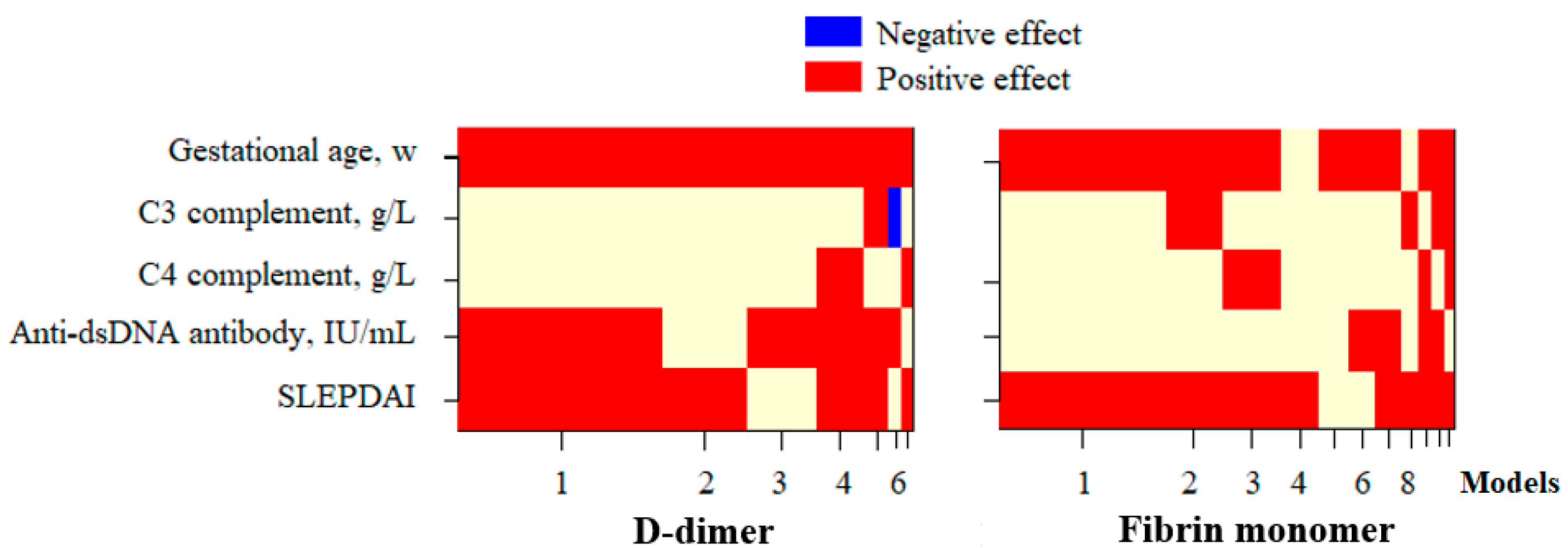
3.3. Factors Affecting the D-Dimer and FM Concentration in Pregnant Patients with SLE
3.4. Roles of D-Dimer and FM in Assessing SLE Disease Activity During Pregnancy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osio-Salido, E.; Manapat-Reyes, H.J.L. Epidemiology of systemic lupus erythematosus in Asia. Lupus 2010, 19, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef]
- Graham, J.J.; Longhi, M.S.; Heneghan, M.A. T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 2021, 121, 102651. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Jamison, M.; Myers, E.; James, A.H. A national study of the complications of lupus in pregnancy. Am. J. Obstet. Gynecol. 2008, 199, e121–e127. [Google Scholar] [CrossRef]
- Eudy, A.M.; Siega-Riz, A.M.; Engel, S.M.; Franceschini, N.; Howard, A.G.; Clowse, M.E.B.; Petri, M. Effect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 855–860. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Colón, I.; Langen, E.S.; Nix, D.A.; El-Sayed, Y.Y.; Genovese, M.C.; Druzin, M.L. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am. J. Obstet. Gynecol. 2005, 192, 1897–1904. [Google Scholar] [CrossRef]
- Stanhope, T.J.; White, W.M.; Moder, K.G.; Smyth, A.; Garovic, V.D. Obstetric Nephrology: Lupus and Lupus Nephritis in Pregnancy. Clin. J. Am. Soc. Nephrol. 2012, 7, 2089–2099. [Google Scholar] [CrossRef]
- Linkins, L.A.; Takach Lapner, S. Review of D-dimer testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39, 98–103. [Google Scholar] [CrossRef]
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am. J. Hematol. 2019, 94, 833–839. [Google Scholar] [CrossRef]
- Wu, H.; Birmingham, D.J.; Rovin, B.; Hackshaw, K.V.; Haddad, N.; Haden, D.; Yu, C.-Y.; Hebert, L.A. D-dimer level and the risk for thrombosis in systemic lupus erythematosus. Clin. J. Am. Soc. Nephrol. 2008, 3, 1628–1636. [Google Scholar] [CrossRef]
- Li, C.; Mu, R.; Li-min, R.; Fan, W.; Ren, C.; Li, Z. The clinical significance of D-dimer in systemic lupus erythematosus. Chin. J. Intern. Med. 2010, 49, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, A.H.; Petersen, P.H.; Bjørge, L.; Røraas, T.; Sandberg, S. Concentration of fibrin monomer in pregnancy and during the postpartum period. Ann. Clin. Biochem. 2019, 56, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Kaniyu, K.; Iwashita, M.; Tanaka, A.; Watanabe, T. Fibrin monomer complex in normal pregnant women: A potential thrombotic marker in pregnancy. Ann. Clin. Biochem. 2007, 44, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Fukuda, N.; Suzuki, A.; Kobayashi, Y.; Matsuda, M.; Kanda, R.; Kiseki, H.; Tsukahara, Y.; Hashimura, N. Use of fibrin monomer complex for screening for venous thromboembolism in the late pregnancy and post-partum period. J. Obstet. Gynaecol. Res. 2014, 40, 700–704. [Google Scholar] [CrossRef]
- Hamano, A.; Umeda, M.; Ueno, Y.; Tanaka, S.; Mimuro, J.; Sakata, Y. Latex immunoturbidimetric assay for soluble fibrin complex. Clin. Chem. 2005, 51, 183–188. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Buyon, J.P.; Kalunian, K.C.; Ramsey-Goldman, R.; Petri, M.A.; Lockshin, M.D.; Ruiz-Irastorza, G.; Khamashta, M.J.L. Assessing disease activity in SLE patients during pregnancy. Lupus 1999, 8, 677–684. [Google Scholar] [CrossRef]
- Buyon, J.P.; Petri, M.A.; Kim, M.Y.; Kalunian, K.C.; Grossman, J.; Hahn, B.H.; Merrill, J.T.; Sammaritano, L.; Lockshin, M.; Alarcón, G.S. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann. Intern. Med. 2005, 142, 953–962. [Google Scholar] [CrossRef]
- Afeltra, A.; Vadacca, M.; Conti, L.; Galluzzo, S.; Mitterhofer, A.P.; Ferri, G.M.; Del Porto, F.; Caccavo, D.; Gandolfo, G.M.; Amoroso, A. Thrombosis in systemic lupus erythematosus: Congenital and acquired risk factors. Arthritis Care Res. 2005, 53, 452–459. [Google Scholar] [CrossRef]
- Hansen, A.T.; Andreasen, B.H.; Salvig, J.D.; Hvas, A. Changes in fibrin D-dimer, fibrinogen, and protein S during pregnancy. Scand. J. Clin. Lab. Investig. 2011, 71, 173–176. [Google Scholar] [CrossRef]
- Wang, M.; Lu, S.; Li, S.; Shen, F. Reference intervals of D-dimer during the pregnancy and puerperium period on the STA-R evolution coagulation analyzer. Clin. Chim. Acta 2013, 425, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ercan, Ş.; Özkan, S.; Yücel, N.; Orçun, A. Establishing reference intervals for D-dimer to trimesters. J. Matern. Fetal Neonatal Med. 2015, 28, 983–987. [Google Scholar] [CrossRef]
- Gutiérrez García, I.; Pérez Cañadas, P.; Martínez Uriarte, J.; García Izquierdo, O.; Angeles Jódar Pérez, M.; García de Guadiana Romualdo, L. D-dimer during pregnancy: Establishing trimester-specific reference intervals. Scand. J. Clin. Lab. Investig. 2018, 78, 439–442. [Google Scholar] [CrossRef]
- Chan, W.S.; Lee, A.; Spencer, F.; Chunilal, S.; Crowther, M.; Wu, W.; Johnston, M.; Rodger, M.; Ginsberg, J.S. D-dimer testing in pregnant patients: Towards determining the next ‘level’ in the diagnosis of deep vein thrombosis. J. Thromb. Haemost. 2010, 8, 1004–1011. [Google Scholar] [CrossRef]
- van der Pol, L.M.; Tromeur, C.; Bistervels, I.M.; Ni Ainle, F.; van Bemmel, T.; Bertoletti, L.; Couturaud, F.; van Dooren, Y.P.A.; Elias, A.; Faber, L.M.; et al. Pregnancy-Adapted YEARS Algorithm for Diagnosis of Suspected Pulmonary Embolism. N. Engl. J. Med. 2019, 380, 1139–1149. [Google Scholar] [CrossRef]
- Orita, Y.; Hamada, T.; Togami, S.; Douchi, T.; Kobayashi, H. The Optimal Cutoff Level of D-Dimer during Pregnancy to Exclude Deep Vein Thrombosis, and the Association between D-Dimer and Postpartum Hemorrhage in Cesarean Section Patients. Kurume Med. J. 2021, 66, 107–114. [Google Scholar] [CrossRef]
- Ferreira, K.S.; Cicarini, W.B.; Alves, L.C.V.; Loures, C.d.M.G.; Campos, F.M.F.; Dos Santos, L.I.; da Silva, M.V.F.; Guimarães, T.M.D.; Reis, E.A.; Neiva, C.L.S.; et al. Correlation between active disease and hypercoagulability state in patients with systemic lupus erythematosus. Clin. Chim. Acta 2019, 490, 107–112. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Park, E.H.; Park, J.W.; Song, Y.W.; Lee, E.B. Practical utility of D-dimer test for venous thromboembolism in systemic lupus erythematosus depends on disease activity: A retrospective cohort study. J. Korean Med. Sci. 2020, 35, e356. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, S.-B.; Wu, C.-H.; Hu, Y.; Zhang, Q.; Li, S.; Fan, Y.-G.; Leng, R.-X.; Pan, H.-F.; Xiong, H.-B. Coagulation cascade and complement system in systemic lupus erythematosus. Oncotarget 2018, 9, 14862–14881. [Google Scholar] [CrossRef]



| Characteristics | Value |
|---|---|
| Age, y, mean ± SD median (IQR) | 27.9 ± 4.7 27.5 (25.0–32.0) |
| Gestational age, w, mean ± SD median (IQR) | 21.0 ± 10.3 22.0 (10.25–30.0) |
| Trimesters, n (%) | |
| 1st | 23 (30.3) |
| 2nd | 30 (39.4) |
| 3rd | 23 (30.3) |
| Disease duration, y, mean ± SD median (IQR) | 6.3 ± 4.2 6.0 (3.0–9.75) |
| Clinical and laboratory manifestations, n (%) | |
| Skin lesions, n (%) | 7 (9.2) |
| Mucocutaneous lesions, n (%) | 3 (3.9) |
| Arthritis, n (%) | 7 (9.2) |
| Alopecia, n (%) | 8 (10.5) |
| Hematologic disorders, n (%) | 26 (34.2) |
| Serositis, n (%) | 6 (7.9) |
| Nephritis, n (%) | 36 (47.4) |
| SLEPDAI, mean ± SD median (IQR) | 5.5 ± 5.4 4.0 (1.3–9.0) |
| SLE flare, n (%) | 25 (32.8) |
| Laboratory Indices | Value | 1st Trimester (n = 23) | 2nd Trimester (n = 30) | 3rd Trimester (n = 23) | p * | P1-2 † | P2-3 † | P1-3 † |
|---|---|---|---|---|---|---|---|---|
| C3 complement, g/L Median (IQR) | 0.96 (0.70–1.21) | 0.82 (0.69–1.02) | 0.98 (0.61–1.24) | 1.07 (0.91–1.22) | 0.311 | 0.478 | 0.667 | 0.072 |
| C4 complement, g/L Median (IQR) | 0.17 (0.12–0.28) | 0.21 (0.13–0.36) | 0.13 (0.09–0.23) | 0.18 (0.12–0.27) | 0.062 | 0.022 | 0.161 | 0.373 |
| Anti-dsDNA antibody, IU/mL Median (IQR) | 33.8 (10.0–149.9) | 33.5 (10.0–150.0) | 34.0 (15.5–111.5) | 77.7 (10.0–150.0) | 0.921 | 0.743 | 0.876 | 0.723 |
| Prothrombin, s Median (IQR) | 11.0 (10.1–11.6) | 11.5 (10.8–11.9) | 10.8 (10.2–11.4) | 11.0 (10.1–11.6) | 0.074 | 0.030 | 0.886 | 0.078 |
| APTT, s Median (IQR) | 29.4 (27.4–30.8) | 30.3 (28.9–34.8) | 28.9 (26.7–30.4) | 29.1 (26.0–30.8) | 0.001 | 0.298 | 0.001 | 0.001 |
| Fibrinogen, g/L Median (IQR) | 4.26 (3.56–4.96) | 3.83 (2.97–4.38) | 4.04 (3.60–4.57) | 4.94 (4.37–5.78) | 0.032 | 0.009 | 0.733 | 0.068 |
| Platelet, ×109/L Median (IQR) | 244 (189–280) | 246 (156–298) | 242 (193.5–283.7) | 230 (186–270) | 0.908 | 0.900 | 0.596 | 0.930 |
| D-dimer, µg/mL Median (IQR) | 1.229 (0.722–1.953) | 0.370 (0.262–0.954) | 1.198 (0.762–1.952) | 1.840 (1.324–2.146) | <0.001 | <0.001 | 0.006 | <0.001 |
| FM, µg/mL Median (IQR) | 4.17 (3.01–5.34) | 3.61 (2.33–4.76) | 3.98 (2.76–6.08) | 5.03 (3.38–8.69) | 0.048 | 0.206 | 0.161 | 0.016 |
| FM > 2 × Ref, % | 21.1% | - | - | - | - | |||
| Parameter | Log10-Transformed D-Dimer | Log10-Transformed FM | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Gestational age, w | 0.669 | <0.001 | 0.310 | 0.006 |
| C3 complement, g/L | −0.186 | 0.107 | −0.054 | 0.644 |
| C4 complement, g/L | −0.218 | 0.058 | −0.020 | 0.867 |
| Anti-dsDNA antibody, IU/mL | 0.389 | 0.001 | 0.246 | 0.032 |
| SLEPDAI | 0.378 | 0.001 | 0.317 | 0.005 |
| Variable | Log10-Transformed D-Dimer | Log10-Transformed FM | ||
|---|---|---|---|---|
| Estimate (95%CI) | p-Value | Estimate (95%CI) | p-Value | |
| Gestational age, w | 0.023 (0.017–0.028) | <0.001 | 0.011 (0.003–0.018) | 0.008 |
| SLEPDAI | 0.015 (0.003–0.027) | 0.013 | 0.021 (0.006–0.035) | 0.007 |
| Anti-dsDNA antibody, IU/mL | 0.001 (0.000–0.002) | 0.016 | - | - |
| R2 | 0.591 | <0.001 | 0.183 | 0.001 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | p-Value | Estimate | 95%CI | p-Value | |
| Gestational age, w | 0.039 | −0.083–0.160 | 0.529 | |||
| C3 complement, g/L | −9.665 | (−12.503)–(−6.826) | <0.001 | −7.763 | (−10.729)–(−5.080) | <0.001 |
| C4 complement, g/L | −14.752 | (−24.768)–(−4.737) | 0.004 | |||
| Anti-dsDNA antibody, IU/mL | 0.047 | 0.028–0.065 | <0.001 | 0.022 | 0.005–0.039 | 0.012 |
| LtDD | 5.586 | 2.419–8.753 | 0.001 | |||
| LtFM | 4.568 | 1.407–7.729 | 0.005 | 3.319 | 0.875–5.764 | 0.009 |
| Prothrombin, s | −1.521 | (−2.903)–(−0.139) | 0.031 | |||
| APTT, s | −0.056 | (−0.283)–0.172 | 0.628 | |||
| Fibrinogen, g/L | 0.025 | (−1.023)–1.073 | 0.962 | |||
| Platelet, ×109/L | −0.014 | (−0.030)–0.002 | 0.094 | |||
| R2 | - | - | 0.551 | <0.001 | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Estimate | OR (95%CI) | p-Value | Estimate | OR (95%CI) | p-Value | |
| Gestational age, w | 0.002 | 1.002 (0.956–1.050) | 0.924 | |||
| C3 complement, g/L | −3.601 | 0.027 (0.004–0.189) | <0.001 | −3.247 | 0.039 (0.004–0.340) | 0.003 |
| C4 complement, g/L | −6.190 | 0.002 (0.000–0.358) | 0.019 | |||
| Anti-dsDNA antibody, IU/mL | 0.018 | 1.019 (1.009–1.028) | <0.001 | 0.012 | 1.012 (1.002–1.023) | 0.024 |
| LtDD | 2.160 | 8.670 (1.713–43.876) | 0.009 | |||
| LtFM | 1.823 | 6.192 (1.481–25.880) | 0.012 | 1.821 | 6.177 (1.259–30.308) | 0.025 |
| Prothrombin, s | −0.583 | 0.558 (0.311–1.001) | 0.051 | |||
| APTT, s | −0.012 | 0.988 (0.906–1.077) | 0.778 | |||
| Fibrinogen, g/L | 0.069 | 1.071 (0.718–1.599) | 0.736 | |||
| Platelet, ×109/L | −0.002 | 0.998 (0.992–1.004) | 0.537 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
My, T.T.K.; Ha, H.T.; Truong, N.H.; Thiet, D.T.; Ha, N.K.; Xoay, T.D.; Olson, L.; Khanh, B.Q. Fibrin Monomer and Systemic Lupus Erythematosus Reactivation During Pregnancy: A Retrospective Study. Diseases 2025, 13, 210. https://doi.org/10.3390/diseases13070210
My TTK, Ha HT, Truong NH, Thiet DT, Ha NK, Xoay TD, Olson L, Khanh BQ. Fibrin Monomer and Systemic Lupus Erythematosus Reactivation During Pregnancy: A Retrospective Study. Diseases. 2025; 13(7):210. https://doi.org/10.3390/diseases13070210
Chicago/Turabian StyleMy, Tran Thi Kieu, Hoang Thi Ha, Nguyen Huu Truong, Dao Thi Thiet, Nguyen Khanh Ha, Tran Dang Xoay, Linus Olson, and Bach Quoc Khanh. 2025. "Fibrin Monomer and Systemic Lupus Erythematosus Reactivation During Pregnancy: A Retrospective Study" Diseases 13, no. 7: 210. https://doi.org/10.3390/diseases13070210
APA StyleMy, T. T. K., Ha, H. T., Truong, N. H., Thiet, D. T., Ha, N. K., Xoay, T. D., Olson, L., & Khanh, B. Q. (2025). Fibrin Monomer and Systemic Lupus Erythematosus Reactivation During Pregnancy: A Retrospective Study. Diseases, 13(7), 210. https://doi.org/10.3390/diseases13070210





