Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects
Abstract
1. Introduction
2. Common and Disparate Characteristics Between Longvax and Long COVID
2.1. Clinical: Symptoms, Pathophysiology, and Biomarkers
2.1.1. Shared Background
2.1.2. Persistent Ipsilateral Lymphadenopathy
2.1.3. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
2.1.4. Nucleocapsid and Membrane Protein-Driven Effects Contribute to Differentiate Long COVID from Longvax
2.1.5. Differential Biomarker Panel
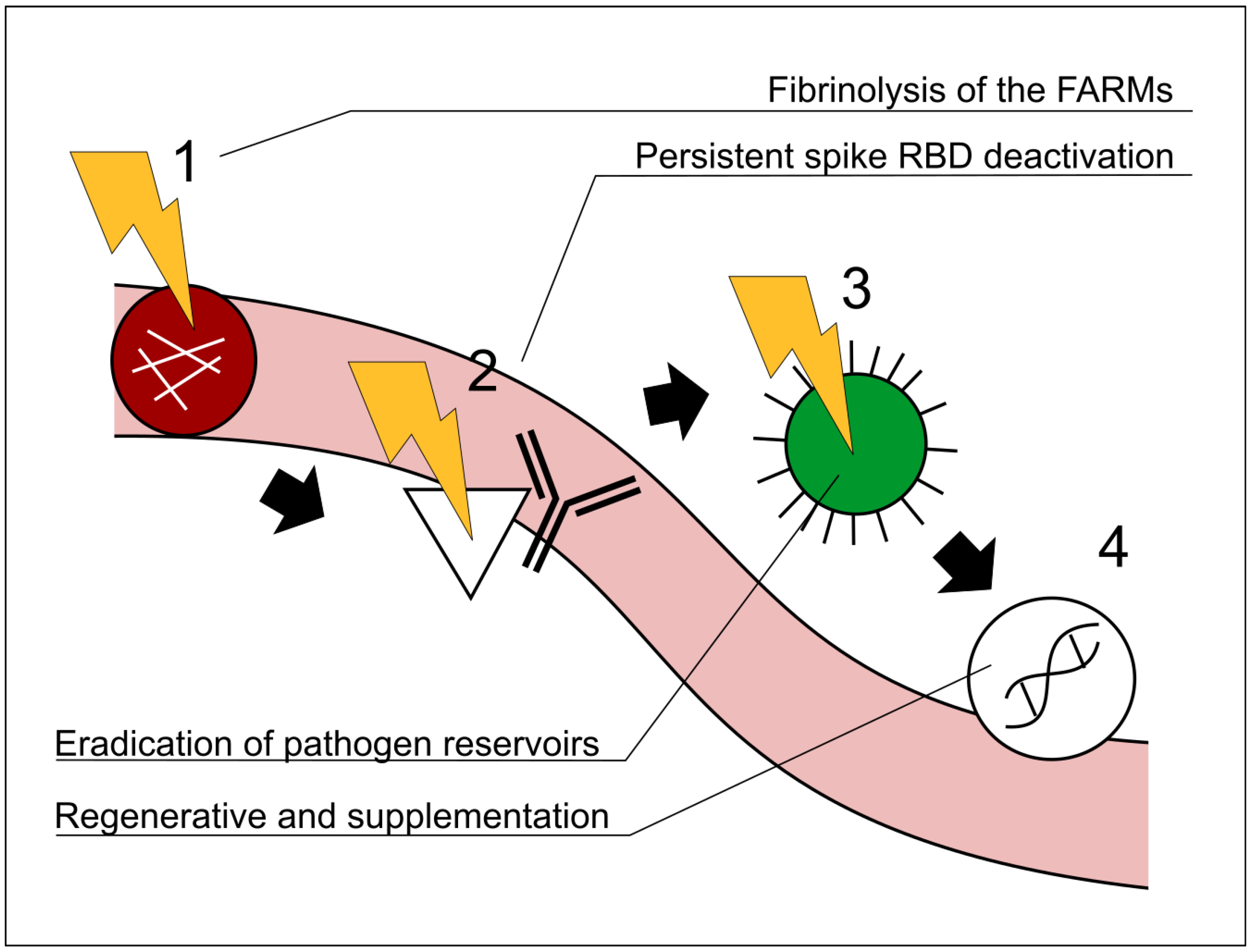
3. Description of the Model for the Vaxtherapy Protocol
3.1. First Step: Ameliorating Microvascular Hypoperfusion with Fibrinolytic Agents
3.2. Second Step:Neutralization of the Spikes with Multimodal Monoclonal Approach
3.3. Third Step: Pathogen Clearance
3.4. Fourth Step: Supplementation and Regenerative
4. Pathophysiological and Clinical Experience Background of the Protocol
4.1. Pathophysiology
4.1.1. Perfusion Recovery as a Prerequisite for Therapeutic Efficacy
4.1.2. Neutralization of the Spike Protein
4.1.3. Elimination of Pathogenic Reservoirs
4.1.4. Adjuvant Therapies for Regeneration and Recovery
4.2. Clinical Experience: Reproducibility
4.2.1. Clinical Experiences with Fibrinolytics
4.2.2. Clinical Experiences with Monoclonal Antibodies Targeting the Spike Protein
4.2.3. Therapeutic Targets for Reactivated Reservoirs: The Case of EBV
4.2.4. Cardiovascular Regeneration
4.2.5. Supplementation: Improvement Through the Use of PQQ, Ubiquinol, Resveratrol, and Others
5. Regulatory Status, Contraindications, and Safety Considerations
6. Considering Alternative Mechanisms Beyond the Proposed Model as a Basis for Future Therapeutic Strategies
7. Limitations of the Model for Vaxtherapy Protocol
8. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| ADCP | Antibody-Dependent Cellular Phagocytosis |
| EBV | Epstein–Barr virus, a herpesvirus associated with infectious mononucleosis and various chronic/lymphoproliferative conditions. |
| FARMs | Fibrin Amyloid–Resistant Microclots: persistent, therapy-resistant microc-lots that reduce blood flow and cell signaling. |
| Fibrinolytic agents | Compounds (e.g., nattokinase, serrapeptase, lumbrokinase) that break down fibrin clots, restoring microvascular circulation in longvax. |
| Hypoperfusion | Reduced blood supply, especially at the microvascular level, contributing to nutrient, immune, and signaling deficits. |
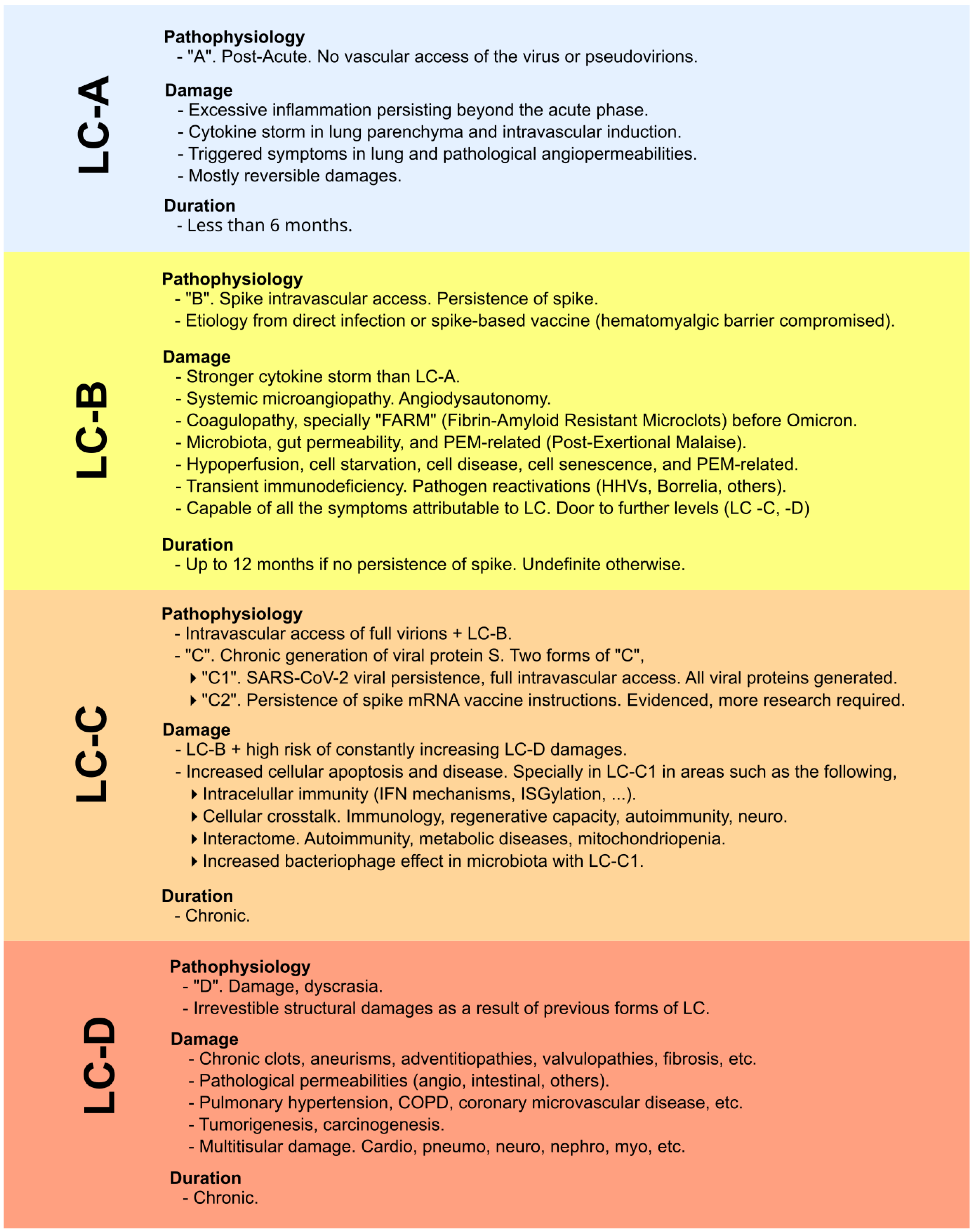
| LC-B | A category of long COVID or longvax involving spike-protein persistence but no ongoing viral replication. |
| LC-C2 | A classification from preliminary work in 2022, with chronic generation of SARS-CoV-2 spike proteins via lingering mRNA instructions, requiring further research. LC-C1 in the same classification is reserved for the chronic generation of spikes via SARS-CoV-2 viral persistence. |
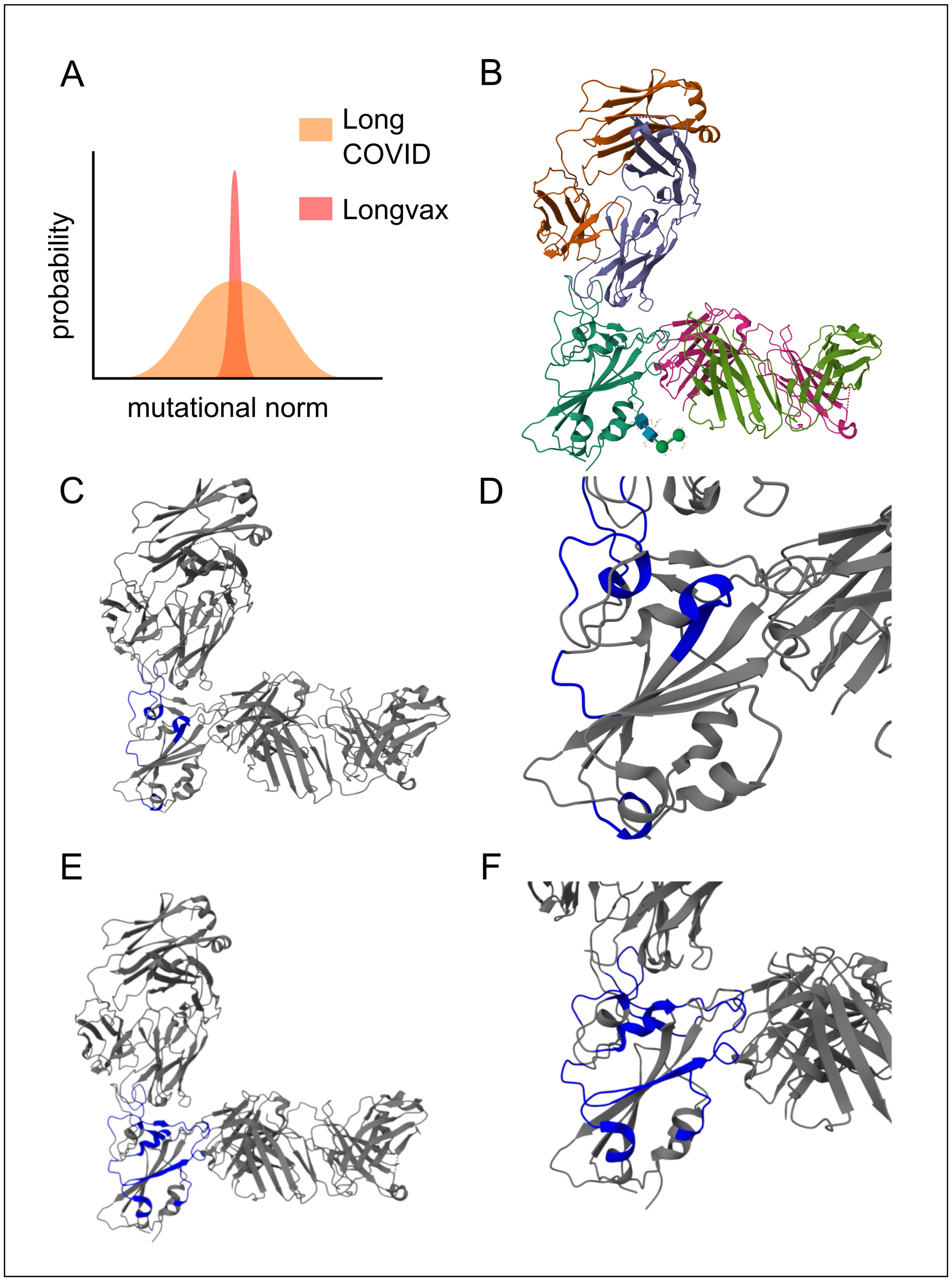
| longvax | An effective term describing COVID vaccine-induced disease, characterized by spike-protein persistence and multisystem damage, in parallel with long COVID. |
| Pathogen reactivation | When dormant pathogens (e.g., EBV, VZV) become active due to immune disruption. |
| Multimodal monoclonal | Therapeutic strategy deploying multiple monoclonal antibodies (e.g., sotrovimab, casirivimab, imdevimab) to neutralize the spike; different from a polyclonal approach. |
| RBD | Receptor-binding domain of the spike protein, critical for binding to ACE2 and central to spike-mediated pathogenesis. |
| VZV | Varicella zoster virus or the human herpesvirus 3. |
References
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Nuovo, G.J.; Magro, C.; Shaffer, T.; Awad, H.; Suster, D.; Mikhail, S.; He, B.; Michaille, J.J.; Liechty, B.; Tili, E.; et al. Endothelial cell damage is central to COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2021, 51, 151682. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A.; et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Barrios, J. #Longcovid. Patogénesis. Fenotipos. #Vacuna: A Quién Afecta y por qué [Video]. YouTube. Published March 2022. Available online: https://www.youtube.com/watch?v=K1B_bjIklZo (accessed on 18 June 2025).
- Tziolos, N.R.; Ioannou, P.; Baliou, S.; Kofteridis, D.P. Long COVID-19 pathophysiology: What do we know so far? Microorganisms 2023, 11, 2458. [Google Scholar] [CrossRef]
- Bohmwald, K.; Diethelm-Varela, B.; Rodríguez-Guilarte, L.; Rivera, T.; Riedel, C.A.; González, P.A.; Kalergis, A.M. Pathophysiological, immunological, and inflammatory features of long COVID. Front. Immunol. 2024, 15, 1341600. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Crespo-Barrios, J. Understanding the cure strategy for the SARS-CoV-2 viral persistence behind Long COVID [preprint]. ResearchGate 2024. [Google Scholar] [CrossRef]
- Patterson, B.K.; Guevara-Coto, J.; Yogendra, R.; Francisco, E.B.; Long, E.; Pise, A.; Rodrigues, H.; Parikh, P.; Mora, J.; Mora-Rodríguez, R.A.; et al. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front. Immunol. 2021, 12, 700782. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vasc. Pharmacol. 2021, 137, 106823. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating severe acute respiratory syndrome coronavirus 2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Dima, F.; Salvagno, G.L.; Lippi, G. Effects of recombinant SARS-CoV-2 spike protein variants on red blood-cell parameters and red-blood-cell distribution width. Biomed. J. 2024, 47, 100787. [Google Scholar] [CrossRef] [PubMed]
- Rajah, M.M.; Bernier, A.; Buchrieser, J.; Schwartz, O. The mechanism and consequences of SARS-CoV-2 spike-mediated fusion and syncytia formation. J. Mol. Biol. 2022, 434, 167280. [Google Scholar] [CrossRef] [PubMed]
- Kalkeri, R.; Goebel, S.; Sharma, G.D. SARS-CoV-2 shedding from asymptomatic patients: Contribution of potential extrapulmonary tissue reservoirs. Am. J. Trop. Med. Hyg. 2020, 103, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome: SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef]
- Buonsenso, D.; Piazza, M.; Boner, A.L.; Bellanti, J.A. Long COVID: A proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022, 43, 187–193. [Google Scholar] [CrossRef]
- Dotan, A.; David, P.; Arnheim, D.; Shoenfeld, Y. The autonomic aspects of the post-COVID-19 syndrome. Autoimmun. Rev. 2022, 21, 103071. [Google Scholar] [CrossRef]
- Bellanti, J.A.; Novak, P.; Faitelson, Y.; Bernstein, J.A.; Castells, M.C. The long road of long COVID: Specific considerations for the allergist/immunologist. J. Allergy Clin. Immunol. Pract. 2023, 11, 3335–3345. [Google Scholar] [CrossRef]
- Ciliberti, V.; Maffei, E.; Giudice, V.; Ciancia, G.; Zeppa, P.; Caputo, A. COVID-19 vaccine-associated lymphadenopathy: A review. Infez. Med. 2024, 32, 119–130. [Google Scholar] [CrossRef]
- Mema, E.; Lane, E.G.; Drotman, M.B.; Dodelzon, K.; Weinreb, J.C.; Kolokythas, O.; Eathiraj, A.; Longo, M.L.; Tran, H.; Shah, S.; et al. Axillary lymphadenopathy after a COVID-19 vaccine booster dose: Time to resolution on ultrasound follow-up and associated factors. AJR Am. J. Roentgenol. 2023, 221, 175–183. [Google Scholar] [CrossRef]
- Netherlands Pharmacovigilance Centre Lareb. Prolonged Duration of COVID-19 Vaccine-Induced Lymphadenopathy; Netherlands Pharmacovigilance Centre Lareb: Lareb, The Netherlands, 2024; Available online: https://www.lareb.nl/en/news/prolongedduration- of-covid-19-vaccine-induced-lymphadenopathy (accessed on 15 June 2025).
- Stewart, C.D.; Baker, M.H. Post-COVID lymphadenopathy in a 26-year-old patient four months after mild SARS-CoV-2 infection. In Proceedings of the Southern Medical Association Annual Scientific Assembly, Virtual, 6 May 2021. [Google Scholar]
- Karsulovic, C.; Hojman, L.P.; Seelmann, D.L.; Wurmann, P.A. Diffuse lymphadenopathy flare in systemic lupus erythematosus and mixed connective-tissue disease after mild COVID-19 infection: A three-patient case series. Am. J. Case. Rep. 2021, 22, e932751. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Boyd, R.J.; Sarkar, D.; Teijeira-Curbelo, T.; Chan, C.K.; Bates, E.; Waraich, K.; Vant, J.; Wilson, E.; Truong, C.D.; et al. ChAdOx1 adenovirus vector interacts with CAR and platelet factor 4: Implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021, 7, eabl8213. [Google Scholar] [CrossRef]
- Greinacher, A.; Schönborn, L.; Siegerist, F.; Endlich, K.; Weisser, K.; Potzsch, B.; Handtke, S.; Reder, A.; Thiele, T.; Aurich, K.; et al. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 97–107. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef]
- Ling, V.W.T.; Fan, B.E.; Lau, S.L.; Lee, X.H.; Tan, C.W.; Lee, S.Y. Severe thrombocytopenia, thrombosis and anti-PF4 antibody after Pfizer-BioNTech COVID-19 mRNA booster—Is it vaccine-induced immune thrombotic thrombocytopenia? Vaccines 2022, 10, 2023. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madan, R.; McCoy, S.R.; Wan, E.Y.; Chung, M.K.; Mehdi, S.; Liu, A.; Fitzgerald, J.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Gao, T.; Qin, Z.; Cao, X.; Li, K.; Lin, J.; Yu, J.; Wang, Y.; Liu, Q.; Gong, Y.; Du, J.; et al. SARS-CoV-2 nucleocapsid protein drives MASP-2–mediated complement over-activation and microvascular injury. Sci. Immunol. 2022, 7, eabh2438. [Google Scholar]
- Pan, P.; Sun, X.; Wu, Y.; Wang, W.; Zhang, L.; Liang, H.; Gao, C.; Wang, J.; Shi, W.; Luo, X.; et al. SARS-CoV-2 nucleocapsid protein promotes NLRP3 inflammasome activation and pyroptosis. Cell Rep. 2021, 35, 109272. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, B.; Li, R.; Yang, G.; Zhang, Y.; Deng, Y.; Zhang, Y.; Li, M.; Wang, T.; Liu, R.; et al. The membrane protein of SARS-CoV-2 hijacks TBK1 for ubiquitin-mediated degradation and abolishes type-I interferon signalling. EMBO Rep. 2021, 22, e52225. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Bente, D.A.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via Toll-like receptor 4 signalling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef]
- Villacampa, A.; Alfaro, E.; Morales, C.; Peiró, C.; Escribano, J.; Ortega-Rubio, P.; Cervera, C.; Jiménez-Blanco, J.; de Oña, J.; Vidal, F.; et al. SARS-CoV-2 S protein activates NLRP3 inflammasome and deregulates coagulation factors in endothelial and immune cells. Cell Commun. Signal. 2024, 22, 38. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Radia, D.; Choi, P.; Gregory, A.; Boyd-Carson, H.; et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Almeida, B.; Dias, T.R.; Teixeira, A.L.; Medeiros, R. Plasma EV-miRNAs as potential biomarkers of COVID-19 vaccine immune response in cancer patients. Vaccines 2024, 12, 848. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Nikitin, A.O.; Nevinsky, G.A. Circulating miRNAs in the plasma of post-COVID-19 patients with typical recovery and those with long COVID symptoms: Regulation of immune-response pathways. Noncoding RNA 2024, 10, 48. [Google Scholar] [CrossRef]
- Mone, P.; Jankauskas, S.S.; Manzi, M.V.; Gambardella, J.; Coppola, A.; Kansakar, U.; Izzo, R.; Fiorentino, G.; Lombardi, A.; Varzideh, F.; et al. Endothelial extracellular vesicles enriched in microRNA-34a predict new-onset diabetes in COVID-19 patients: Novel insights for long COVID metabolic sequelae. J. Pharmacol. Exp. Ther. 2024, 389, 34–39. [Google Scholar] [CrossRef]
- Huang, K.; Wang, C.; Vagts, C.; Raguveer, V.; Finn, P.W.; Perkins, D.L. Long non-coding RNAs NEAT1 and MALAT1 are differentially expressed in severe COVID-19 patients: An integrated single-cell analysis. PLoS ONE 2022, 17, e0261242. [Google Scholar] [CrossRef]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines 2023, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-lasting biochemically modified mRNA and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Karalis, V.; Drakoulis, N.; Malandrerou, K.; Paraskevis, M.; Dalagiorgou, G.N.; Mavroudis, K.; Dimopoulos, K.; et al. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends. Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 2021, 41, BSR20210611. [Google Scholar] [CrossRef]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef]
- Proal, A.D.; Aleman, S.; Bomsel, M.; Brodin, P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; Deeks, S.G.; et al. Targeting the SARS-CoV-2 reservoir in long COVID. Lancet Infect. Dis. 2025, 25, e294–e306. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Chew, M.C.; Wiryasaputra, S.; Wu, M.; Khor, W.B.; Chan, A.S.Y. Incidence of COVID-19 vaccination-related uveitis and effects of booster dose in a tertiary uveitis referral center. Front. Med. 2022, 9, 925683. [Google Scholar] [CrossRef]
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhães-de-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.E.B.S.; de Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.D.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022, 80, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, A.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999, 116, 28–32. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kase, M.; Sano, H.; Kamijima, R.; Sano, S. Persistent varicella-zoster virus infection after mRNA COVID-19 vaccination associated with spike protein in the lesion. J. Dermatol. 2022, 49, 588–591. [Google Scholar] [CrossRef]
- Rong, Z.; Mai, H.; Ebert, G.; Kapoor, S.; Puelles, V.G.; Czogalla, J.; Hu, S.; Su, J.; Prtvar, D.; Singh, I.; et al. Persistence of spike protein at the skull–meninges–brain axis may contribute to the neurological sequelae of COVID-19. Cell Host Microbe 2024, 32, 2112–2130.e10. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Remuzzi, G. SARS-CoV-2 and the spike protein in endotheliopathy. Nat. Rev. Nephrol. 2024, 20, 205–216. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Nagai, A. The role of the vascular system in degenerative diseases: Mechanisms and implications. Int. J. Mol. Sci. 2024, 25, 2169. [Google Scholar] [CrossRef]
- ElAli, A.; Thériault, P.; Préfontaine, P.; Rivest, S. Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral β-amyloid brain entry and aggregation. Acta Neuropathol. Commun. 2013, 1, 75. [Google Scholar] [CrossRef]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of blood–brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef]
- Miyazato, Y.; Yamamoto, K.; Nakaya, Y.; Morioka, S.; Takeuchi, J.S.; Takamatsu, Y.; Maeda, K.; Kimura, M.; Sugiura, W.; Mitsuya, H.; et al. Successful use of casirivimab/imdevimab anti-spike monoclonal antibodies to enhance neutralizing antibodies in a woman on anti-CD20 treatment with refractory COVID-19. J. Infect. Chemother. 2022, 28, 991–994. [Google Scholar] [CrossRef]
- Hsia, C.H.; Shen, M.C.; Lin, J.S.; Wen, Y.K.; Hwang, K.L.; Cham, T.M.; Yang, N.C. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr. Res. 2009, 29, 190–196. [Google Scholar] [CrossRef]
- Mei, J.F.; Cai, S.F.; Yi, Y.; Wang, X.D.; Ying, G.Q. Study of the fibrinolytic activity of serrapeptase and its in vitro thrombolytic effects. Braz. J. Pharm. Sci. 2022, 58, e201004. [Google Scholar] [CrossRef]
- Cao, Y.J.; Zhang, X.; Wang, W.H.; Zhai, W.Q.; Qian, J.F.; Wang, J.S.; Chen, J.; You, N.X.; Zhao, Z.; Wu, Q.Y.; et al. Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: Results from a multicenter, randomized, parallel-group and controlled clinical trial. Chin. Med. J. 2013, 126, 4060–4065. [Google Scholar] [CrossRef] [PubMed]
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994, 343, 311–322. [Google Scholar] [CrossRef]
- Touret, F.; Baronti, C.; Bouzidi, H.S.; de Lamballerie, X. In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate. Sci. Rep. 2022, 12, 4683. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Subramanian, S.; Schnell, G.; di Iulio, J.; Gupta, A.K.; Shapiro, A.E.; Sarkis, E.H.; Lopuski, A.; Peppercorn, A.; Aldinger, M.; Hebner, C.M.; et al. Resistance analysis following sotrovimab treatment in participants with COVID-19 during the phase III COMET-ICE study. Future Virol. 2023, 18, 975–990. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S.; et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Letter of Revocation: Emergency Use Authorization 091 (REGEN-COV); US Food and Drug Administration: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/media/184465/download (accessed on 29 June 2025).
- European Medicines Agency. Ronapreve (Casirivimab/Imdevimab) Summary of Product Characteristics; EMA: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/documents/product-information/ronapreve-epar-product-information_en.pdf (accessed on 27 May 2025).
- Rivera, J.A.; Aragon, D.; Gomez, J.; Arredondo, H.; Thomas, P.M.; Dominici, P.; Akala, O.O.; Menowsky, M. Acute hypersensitivity reaction after casirivimab/imdevimab infusion in a COVID-19-positive young male: Myopericarditis or Kounis syndrome? Cureus 2022, 14, e31125. [Google Scholar] [CrossRef]
- Dippel, A.; Gallegos, A.; Aleti, V.; Barnes, A.; Chen, X.; Christian, E.; Delmar, J.; Du, Q.; Esfandiary, R.; Farmer, E.; et al. Developability profiling of a panel of Fc-engineered SARS-CoV-2 neutralizing antibodies. mAbs 2023, 15, 2152526. [Google Scholar] [CrossRef]
- de Campos-Mata, L.; Trinité, B.; Modrego, A.; Tejedor Vaquero, S.; Pradenas, E.; Pons-Grífols, A.; Melero, N.R.; Carlero, D.; Marfil, S.; Santiago, C.; et al. A monoclonal antibody targeting a large surface of the receptor-binding motif shows pan-neutralizing SARS-CoV-2 activity. Nat. Commun. 2024, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Malouf, M.A.; Chhajed, P.N.; Hopkins, P.; Plit, M.; Turner, J.; Glanville, A.R. Antiviral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J. Heart Lung Transplant. 2002, 21, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Höcker, B.; Böhm, S.; Fickenscher, H.; Küsters, U.; Schnitzler, P.; Pohl, M.; John, U.; Kemper, M.J.; Fehrenbach, H.; Wigger, M.; et al. (Val-)ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transpl. Int. 2012, 25, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Hwang, Y.Y.; Chan, T.S.Y.; Pang, A.W.; Leung, A.Y.; Tse, E.; Kwong, Y.L. Valganciclovir suppressed Epstein-Barr virus reactivation during immunosuppression with alemtuzumab. J. Clin. Virol. 2014, 59, 255–258. [Google Scholar] [CrossRef]
- Yager, J.E.; Magaret, A.S.; Kuntz, S.R.; Selke, S.; Huang, M.L.; Corey, L.; Wald, A. Valganciclovir for the suppression of Epstein-Barr virus replication. J. Infect. Dis. 2017, 216, 198–202. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Wakisaka, N.; Kondo, S.; Murono, S.; Shimizu, Y.; Nakashima, M.; Furukawa, M. Treatment of locally recurrent Epstein-Barr virus-associated nasopharyngeal carcinoma using the antiviral agent cidofovir. J. Med. Virol. 2008, 80, 879–882. [Google Scholar] [CrossRef]
- Neyts, J.; Sadler, R.; De Clercq, E.; Raab-Traub, N.; Pagano, J.S. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998, 58, 384–388. [Google Scholar]
- Abdulkarim, B.; Sabri, S.; Zelenika, D.; Deutsch, E.; Frascogna, V.; Klijanienko, J.; Bourhis, J. Cidofovir induces apoptosis and cell cycle arrest in human nasopharyngeal carcinoma cells. Int. J. Cancer 2003, 103, 475–483. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Afshar, K.; Rao, A.P.; Patel, V.; Forrester, K.; Ganesh, S. Successful foscarnet therapy for Epstein-Barr virus infection following control of PTLD with enhancement of cellular immunity in a lung-transplant recipient. Transpl. Infect. Dis. 2011, 13, 104–108. [Google Scholar] [CrossRef]
- Schneider, U.; Ruhnke, M.; Delecluse, H.J.; Stein, H.; Huhn, D. Regression of Epstein-Barr virus–associated lymphoproliferative disorders in AIDS during therapy with foscarnet. Blood 2000, 96, 1105–1107. [Google Scholar] [CrossRef]
- Zacny, V.L.; Gershburg, E.; Davis, M.G.; Biron, K.K.; Pagano, J.S. Inhibition of Epstein-Barr virus replication by the benzimidazole L-riboside BDCRB. J. Virol. 1999, 73, 6208–6216. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Roy, D.; Gershburg, E.; Whitehurst, C.B.; Dittmer, D.P.; Pagano, J.S. Maribavir inhibits Epstein-Barr virus transcription in addition to viral DNA replication. J. Virol. 2009, 83, 12108–12117. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, C.B.; Sanders, M.K.; Law, M.; Wang, F.Z.; Xiong, J.; Dittmer, D.P.; Pagano, J.S. Maribavir inhibits Epstein-Barr virus transcription through the EBV protein kinase BGLF4. J. Virol. 2013, 87, 5486–5494. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, S.Y.; Noh, K.W.; Joo, E.H.; Zhao, B.; Kieff, E.; Kang, M.S. Small-molecule inhibition of Epstein-Barr virus nuclear antigen-1 DNA-binding activity interferes with replication and persistence of the viral genome. Proc. Natl. Acad. Sci. USA 2014, 111, 3146–3151. [Google Scholar] [CrossRef]
- Kawamoto, A.; Gwon, H.C.; Iwaguro, H.; Yamaguchi, J.I.; Uchida, S.; Masuda, H.; Asahara, T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001, 103, 634–637. [Google Scholar] [CrossRef]
- Suh, W.; Kim, K.L.; Kim, J.M.; Shin, I.S.; Lee, Y.S.; Lee, J.Y.; Jang, H.S.; Lee, J.S.; Byun, J.; Choi, J.H.; et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005, 23, 1571–1578. [Google Scholar] [CrossRef]
- Ii, M.; Nishimura, H.; Iwakura, A.; Wecker, A.; Eaton, E.; Asahara, T.; Losordo, D.W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via imported nitric oxide synthase activity. Circulation 2005, 111, 1114–1120. [Google Scholar] [CrossRef]
- Jujo, K.; Ii, M.; Losordo, D.W. Endothelial progenitor cells in neovascularization of infarcted myocardium. J. Mol. Cell. Cardiol. 2008, 45, 530–544. [Google Scholar] [CrossRef]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Lee, J.S.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef]
- Marçola, M.; Rodrigues, C.E. Endothelial progenitor cells in tumor angiogenesis: Another brick in the wall. Stem Cells Int. 2015, 2015, 832649. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Xu, L.; Qian, Y.; Liu, N.; Zhou, C.; Liu, J.; Zhou, L.; Xu, Z.; Jia, R.; et al. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res. Ther. 2022, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, M.; Klomp, J.; Merrill, B.J.; Rehman, J.; Malik, A.B. Method for dual viral-vector-mediated CRISPR/Cas9 gene disruption in primary human endothelial cells. Sci. Rep. 2017, 7, 42127. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.; Reschke, M.; Pierce, R.W.; Qin, L.; Spindler, S.; Baltazar, T.; Manes, T.D.; Lopez-Giraldez, F.; Li, G.; Bracaglia, L.G.; et al. Progenitor-derived human endothelial cells evade alloimmunity by CRISPR/Cas9-mediated complete ablation of MHC expression. JCI Insight 2019, 4, e129739. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.J.; Park, J.H.; Lee, S.; Park, B.W.; Lee, S.M.; Hwang, J.W.; Kim, J.J.; Kang, B.; Sim, W.S.; et al. Enhancement strategy for effective vascular regeneration following myocardial infarction through a dual stem cell approach. Exp. Mol. Med. 2022, 54, 1165–1178. [Google Scholar] [CrossRef]
- Dai, Y.; Ashraf, M.; Zuo, S.; Uemura, R.; Dai, Y.S.; Wang, Y.; Haider, H.K.; Li, T.; Xu, M. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J. Mol. Cell. Cardiol. 2008, 44, 607–617. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Londono, P.; Cao, Y.; Sharpe, E.J.; Proenza, C.; O’Rourke, R.; Jones, K.L.; Jeong, M.Y.; Walker, L.A.; Buttrick, P.M.; et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 2015, 6, 8243. [Google Scholar] [CrossRef]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Żak, M.M.; Gehrke, L.; Zangi, L. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Gao, Y.; Kamogashira, T.; Fujimoto, C.; Iwasaki, S.; Yamasoba, T. Pyrroloquinoline quinone protects mitochondrial function of HEI-OC1 cells under premature senescence. Front. Cell Dev. Biol. 2022, 10, 858774. [Google Scholar] [CrossRef]
- Yan, T.; Nisar, M.F.; Hu, X.; Chang, J.; Wang, Y.; Wu, Y.; Liu, Z.; Cai, Y.; Jia, J.; Xiao, Y.; et al. Pyrroloquinoline quinone (PQQ): Its impact on human health and potential benefits. Curr. Res. Food Sci. 2024, 9, 100889. [Google Scholar] [CrossRef]
- García-Carpintero, S.; Domínguez-Bértalo, J.; Pedrero-Prieto, C.; Frontiñán-Rubio, J.; Amo-Salas, M.; Durán-Prado, M.; García-Pérez, E.; Vaamonde, J.; Alcain, F.J. Ubiquinol Supplementation Improves Gender-Dependent Cerebral Vasoreactivity and Ameliorates Chronic Inflammation and Endothelial Dysfunction in Patients with Mild Cognitive Impairment. Antioxidants 2021, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Sarkaki, A.; Rashidi, M.; Ranjbaran, M.; Asareh Zadegan Dezfuli, A.; Shabaninejad, Z.; Behzad, E.; Adelipour, M. Therapeutic Effects of Resveratrol on Ischemia–Reperfusion Injury in the Nervous System. Neurochem. Res. 2021, 46, 3085–3102. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The role of taurine in mitochondrial health: More than just an antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective Role of Taurine against Oxidative Stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, Y.; Tian, Z.; Zhu, M.; Zhang, B.; Du, S.; Li, Y.; Liu, Z.; Hou, S.; Yang, Y.; et al. Coenzyme Q10 attenuates human platelet aggregation induced by SARS-CoV-2 spike protein via reducing oxidative stress in vitro. Int. J. Mol. Sci. 2022, 23, 12345. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Ordonez, J.V.; Herzog, W.R.; Rohde, M.; Mortensen, S.A.; Folkers, K.; Gurbel, P.A. Dietary coenzyme Q10 alters platelet size and inhibits human aggregation in healthy volunteers. Int. J. Vitam. Nutr. Res. 1997, 67, 324–329. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Qasem, R.J. The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Nutrients 2020, 12, 2226. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yu, C.P.; Hsieh, Y.W.; Lin, S.P.; Hou, Y.C. Resveratrol stereoselectively affects warfarin pharmacokinetics and enhances its anticoagulation effect. Food Chem. Toxicol. 2020, 141, 111422. [Google Scholar] [CrossRef]
- Fujita, T.; Sato, Y. Hypotensive effect of taurine: Possible involvement of the sympathetic nervous system and endogenous opiates. Circulation 1988, 77, I298–I302. [Google Scholar] [CrossRef]
- Genentech Inc. Activase (Alteplase) for Injection; Genentech Inc.: South San Francisco, CA, USA, 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103172s5203lbl.pdf (accessed on 18 June 2025).
- Sanofi-Aventis, US. Streptase (Streptokinase) for Injection; Sanofi-Aventis US: Bridgewater, NJ, USA, 2021; Available online: https://www.rxlist.com/streptase-drug.htm (accessed on 18 June 2025).
- Ota, N.; Itani, M.; Aoki, T.; Sakuraid, A.; Fujisawae, T.; Okadaa, Y.; Nodaa, K.; Arakawac, Y.; Tokudaa, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral arteries: Implications for hemorrhagic stroke post-mRNA vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef]
- Florek, K.; Sokolski, M. Myocarditis associated with COVID-19 vaccination. Vaccines 2024, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Health Resources & Services Administration. National Vaccine Injury Compensation Program. Updated June 2025. Available online: https://www.hrsa.gov/vaccine-compensation (accessed on 16 June 2025).
- Chu, C.F.; Chang, T.H.; Ho, J.J. Comparative analysis of fourteen COVID-19 vaccine injury compensation systems and claim approval rates. Vaccine 2025, 52, 126830. [Google Scholar] [CrossRef]
- Bender Ignacio, R.A.; Chew, K.W.; Moser, C.; Currier, J.S.; Eron, J.J.; Javan, A.C.; Giganti, M.J.; Ritz, J.; Gibbs, M.; Kouekam, H.T.; et al. Tixagevimab/cilgavimab or placebo for COVID-19 in ACTIV-2: Safety, pharmacokinetics and neutralizing and anti-drug antibodies. iScience 2025, 28, 111938. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Chan, K.; Oudghiri, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Extensions of Marketing Authorisations: Questions and Answers; Updated 25 March 2025. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/variations-including-extensions-marketing-authorisations/extensions-marketing-authorisations-questions-answers (accessed on 16 June 2025).
- Chertow, D.S.; Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Smith, L.; Banakis, S.; Tai, D.S.; Yang, S.; Vanhove, L.; Reddy, R.; et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Nature 2023, 614, 521–529. [Google Scholar] [CrossRef]
- Stein, S.R.; Hosie, H.; Chertow, D.S.; Banakis, S.; Yang, S.; Taubenberger, J.K.; Suffredini, A.F.; Lin, A.E.; Singh, M.; Gladfelter, A.S.; et al. Evidence of SARS-CoV-2 replication in the human gastrointestinal tract months after infection. Nat. Commun. 2023, 14, 2025. [Google Scholar]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Dai, L.; Oppo, F.; Quinn, J.; Wu, Z.; Rouchka, E.C.; Mallipattu, S.K.; Romero, J.; et al. Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Arévalo-Cortés, A.; Rodriguez-Pinto, D.; Aguilar-Ayala, L. Evidence for molecular mimicry between SARS-CoV-2 and human antigens: Implications for autoimmunity in COVID-19. Autoimmune Dis. 2024, 2024, 8359683. [Google Scholar] [CrossRef]
- Goetzke, C.C.; Massoud, M.; Frischbutter, S.; Guerra, G.M.; Ferreira-Gomes, M.; Heinrich, F.; von Stuckrad, A.S.L.; Wisniewski, S.; Licha, J.R.; Bondareva, M.; et al. TGFβ links Epstein–Barr virus to multisystem inflammatory syndrome in children. Nature 2025, 640, 762–771. [Google Scholar] [CrossRef]
- Lin, C.; Wu, P.; Huang, Y.; Wang, J.; Lee, C. Disseminated herpes zoster following mRNA COVID-19 vaccination: Case series and literature review. J. Dermatol. Sci. 2023, 111, 27–31. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Patel, A.B.; Babady, N.E.; Olsen, M.R.; Nordvig, A.S.; Edlow, A.G.; Son, M.B.F.; Fasano, A.; et al. Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccination: The role of G-quadruplexes, exosomes and microRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 spike protein and human tissue proteins: Implications for autoimmune disease and vaccine safety. Clin. Immunol. 2021, 226, 108700. [Google Scholar]
- Heidecker, B.; Cooper, L.T.; Kelle, S.; Schultheiss, H.P.; Bhatt, D.L.; Adler, Y.; Klugman, N.; Klingel, K.; Sinning, J.M.; Caforio, A.L.P.; et al. Myocarditis following COVID-19 vaccination: Current evidence and suggested diagnostic work-up. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e58–e63. [Google Scholar] [CrossRef]
- Nakamura, H.; Noh, J.Y.; Ito, Y.; Takamura, Y.; Okamoto, S.; Miyauchi, A.; Ito, K. New-onset Graves disease following SARS-CoV-2 mRNA vaccination: A case series and literature review. Endocr. J. 2023, 70, 513–520. [Google Scholar]
- Zheng, K.I.; Wang, X.B.; Jin, X.H.; Wang, T.Y.; Liu, W.Y.; Gao, F.; Chen, Y.P.; Zheng, M.H. Autoimmune hepatitis after COVID-19 vaccination: New variant or coincidence? J. Hepatol. 2022, 77, 439–441. [Google Scholar] [CrossRef]
- Francis, A.; Bais, G.; Caddy, J.; Kirby, A.; Butt, S.; Hobson, E.; Siddiqi, H.; Wang, J.; Patel, D. Myelin-oligodendrocyte glycoprotein antibody–associated disease following ChAdOx1 nCoV-19 vaccination. Mult. Scler. Relat. Disord. 2022, 60, 103739. [Google Scholar] [CrossRef]
- Sah, P.; Patel, S.N.; Desai, S.D.; Shah, J.; Amin, P.; Chaudhari, R.; Mehta, A.; Bhatt, P.; Singh, D.; Kumar, V.; et al. Guillain-Barré syndrome after adenoviral-vector COVID-19 vaccination: Case–control analysis and mechanistic considerations. Neurology 2024, 102, e612–e620. [Google Scholar] [CrossRef]
- Cipriani, A.; Patone, M.; Hackett, K.; Vouga, M.; Malhotra, A.; Walker, A.J.; Smeeth, L.; Katsoularis, I.; Thomas, S.L.; Hippisley-Cox, J.; et al. Myopericarditis after NVX-CoV2373 protein subunit COVID-19 vaccination: A global pharmacovigilance signal. Vaccine 2023, 41, 6302–6308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Barrios, J. Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects. Diseases 2025, 13, 204. https://doi.org/10.3390/diseases13070204
Crespo-Barrios J. Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects. Diseases. 2025; 13(7):204. https://doi.org/10.3390/diseases13070204
Chicago/Turabian StyleCrespo-Barrios, Jose. 2025. "Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects" Diseases 13, no. 7: 204. https://doi.org/10.3390/diseases13070204
APA StyleCrespo-Barrios, J. (2025). Vaxtherapy, a Multiphase Therapeutic Protocol Approach for Longvax, the COVID-19 Vaccine-Induced Disease: Spike Persistence as the Core Culprit and Its Downstream Effects. Diseases, 13(7), 204. https://doi.org/10.3390/diseases13070204





