Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Formalin-Fixed Paraffin-Embedded Tumoral Tissue
2.2. DNA Extraction
2.3. Microsatellite Instability (MSI) Analysis
2.4. Microsatellite Instability Interpretation
2.5. Statistical Analysis
3. Results
3.1. Gleason Score Distribution in Prostate Cancer Patients
3.2. Microsatellite Instability and Their Distribution According to Gleason Score in Mexican Prostate Cancer Patients
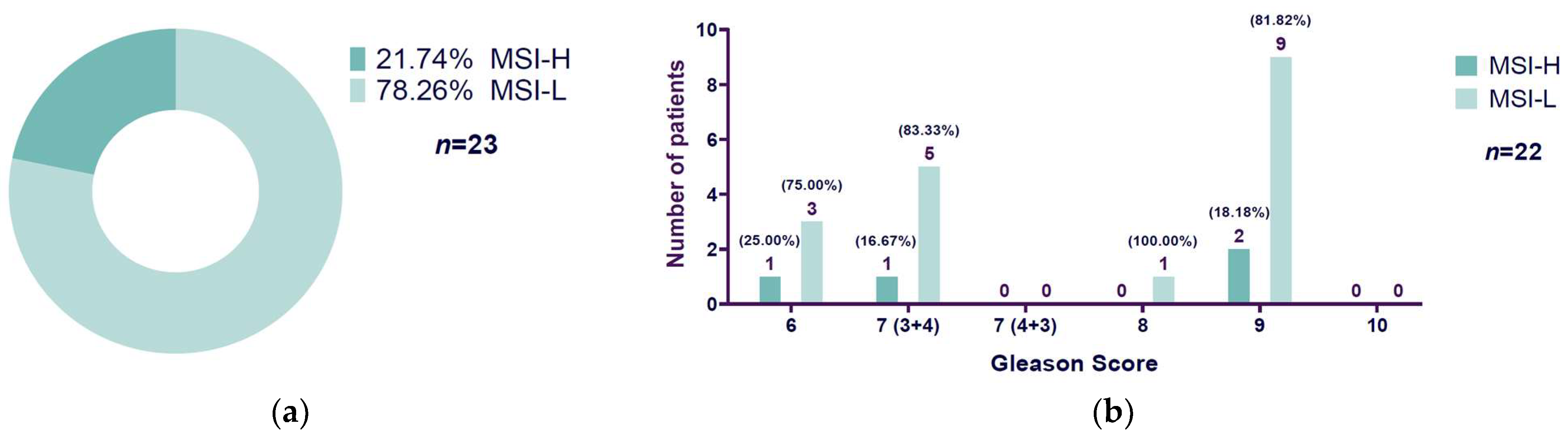
3.3. High Microsatellite Instability Presence Related to Gleason Score
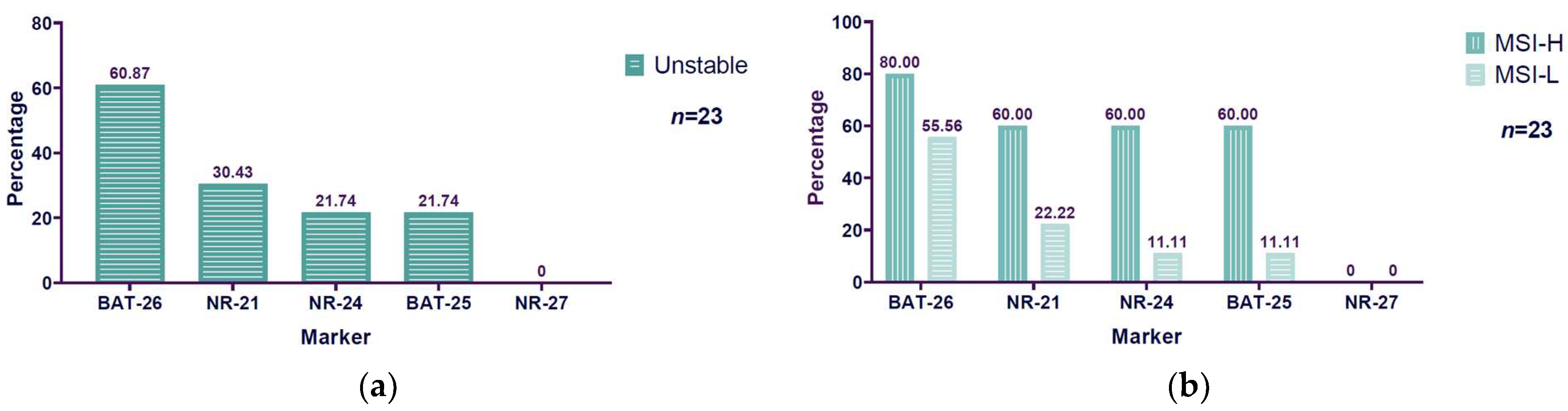
3.4. Unstable BAT-26 Marker Higher Among Microsatellite Instability Markers Evaluated
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortality, Males, in 2022, World. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&group_populations=1&sexes=1&populations=900&key=total&types=1 (accessed on 21 October 2024).
- Incidence, Males, in 2022, World. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&group_populations=1&sexes=1&populations=900&key=total (accessed on 21 October 2024).
- van Leenders, G.J.L.H.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Kavun, A.; Veselovsky, E.; Lebedeva, A.; Belova, E.; Kuznetsova, O.; Yakushina, V.; Grigoreva, T.; Mileyko, V.; Fedyanin, M.; Ivanov, M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Nádorvári, M.L.; Lotz, G.; Kulka, J.; Kiss, A.; Tímár, J. Microsatellite Instability and Mismatch Repair Protein Deficiency: Equal Predictive Markers? Pathol. Oncol. Res. 2024, 30, 1611719. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Morrissey, C.; Kumar, A.; Zhang, X.; Smith, C.; Coleman, I.; Salipante, S.J.; Milbank, J.; Yu, M.; Grady, W.M.; et al. Complex MSH2 and MSH6 Mutations in Hypermutated Microsatellite Unstable Advanced Prostate Cancer. Nat. Commun. 2014, 5, 4988. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Chung, J.H.; Dewal, N.; Sokol, E.; Mathew, P.; Whitehead, R.; Millis, S.Z.; Frampton, G.M.; Bratslavsky, G.; Pal, S.K.; Lee, R.J.; et al. Prospective Comprehensive Genomic Profiling of Primary and Metastatic Prostate Tumors. JCO Precis. Oncol. 2019, 3, PO.18.00283. [Google Scholar] [CrossRef]
- Fraune, C.; Simon, R.; Höflmayer, D.; Möller, K.; Dum, D.; Büscheck, F.; Hube-Magg, C.; Makrypidi-Fraune, G.; Kluth, M.; Hinsch, A.; et al. High Homogeneity of Mismatch Repair Deficiency in Advanced Prostate Cancer. Virchows Arch. 2020, 476, 745–752. [Google Scholar] [CrossRef]
- Zhang, N.; Hong, B.; Zhao, X.; Bai, Y. Genomic Profiling and Mutation Burden of Chinese Prostate Patients with Microsatellite Instability. J. Clin. Oncol. 2020, 38, e17531. [Google Scholar] [CrossRef]
- Hwang, C.; Henderson, N.C.; Chu, S.-C.; Holland, B.; Cackowski, F.C.; Pilling, A.; Jang, A.; Rothstein, S.; Labriola, M.; Park, J.J.; et al. Biomarker-Directed Therapy in Black and White Men With Metastatic Castration-Resistant Prostate Cancer. JAMA Netw. Open 2023, 6, e2334208. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Ravichandran, V.; Brown, S.; Alam, S.M.; Katims, A.; Truong, H.; Reisz, P.A.; Vasselman, S.; Nweji, B.; Autio, K.A.; et al. Microsatellite Instability, Tumor Mutational Burden, and Response to Immune Checkpoint Blockade in Patients with Prostate Cancer. Clin. Cancer Res. 2024, 30, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Angel, M.; Freile, B.; Rodriguez, A.; Cayol, F.; Manneh Kopp, R.; Rioja, P.; Soule, T.; Losco, F.; Bernal Vaca, L.; Penaloza, J.M.; et al. Genomic Landscape in Prostate Cancer in a Latin American Population. JCO Glob. Oncol. 2024, 10, e2400072. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, PO.17.00073. [Google Scholar] [CrossRef]
- Hause, R.J.; Pritchard, C.C.; Shendure, J.; Salipante, S.J. Classification and Characterization of Microsatellite Instability across 18 Cancer Types. Nat. Med. 2016, 22, 1342–1350. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Lee, S.; Park, W.-Y.; Kim, T.-M.; Park, P.J. A Molecular Portrait of Microsatellite Instability across Multiple Cancers. Nat. Commun. 2017, 8, 15180. [Google Scholar] [CrossRef]
- Diaz-Padilla, I.; Romero, N.; Amir, E.; Matias-Guiu, X.; Vilar, E.; Muggia, F.; Garcia-Donas, J. Mismatch Repair Status and Clinical Outcome in Endometrial Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol./Hematol. 2013, 88, 154–167. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, N.R.; Kim, A.; Lee, H.-J.; Park, W.-Y.; Kim, J.-Y.; Lee, C.-H.; Huh, G.-Y.; Park, D.Y. Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes. Korean J. Pathol. 2013, 47, 28–35. [Google Scholar] [CrossRef]
- Cheah, P.L.; Li, J.; Looi, L.M.; Koh, C.C.; Lau, T.P.; Chang, S.W.; Teoh, K.H.; Mun, K.S.; Nazarina, A.R. Screening for Microsatellite Instability in Colorectal Carcinoma: Practical Utility of Immunohistochemistry and PCR with Fragment Analysis in a Diagnostic Histopathology Setting. Malays. J. Pathol. 2019, 41, 91–100. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Guidelines Prostate Cancer Version 1.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Vuković Đerfi, K.; Salar, A.; Cacev, T.; Kapitanović, S. EMAST Type of Microsatellite Instability-A Distinct Entity or Blurred Overlap between Stable and MSI Tumors. Genes 2023, 14, 1474. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bigas, M.A.; Boland, C.R.; Hamilton, S.R.; Henson, D.E.; Srivastava, S.; Jass, J.R.; Khan, P.M.; Lynch, H.; Smyrk, T.; Perucho, M.; et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: Meeting Highlights and Bethesda Guidelines. JNCI J. Natl. Cancer Inst. 1997, 89, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of Tumor Microsatellite Instability Using Five Quasimonomorphic Mononucleotide Repeats and Pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, D.G.; Kim, K.-M. BAT26 Only Microsatellite Instability with High Tumor Mutation Burden-A Rare Entity Associated with PTEN Protein Loss and High PD-L1 Expression. Int. J. Mol. Sci. 2022, 23, 10730. [Google Scholar] [CrossRef]
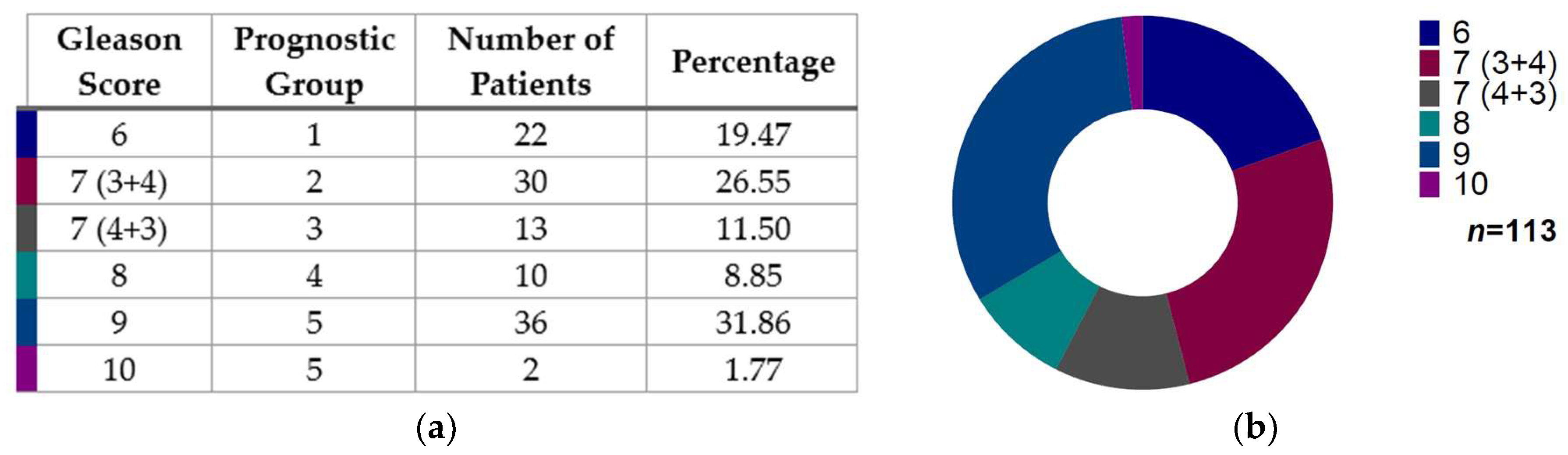
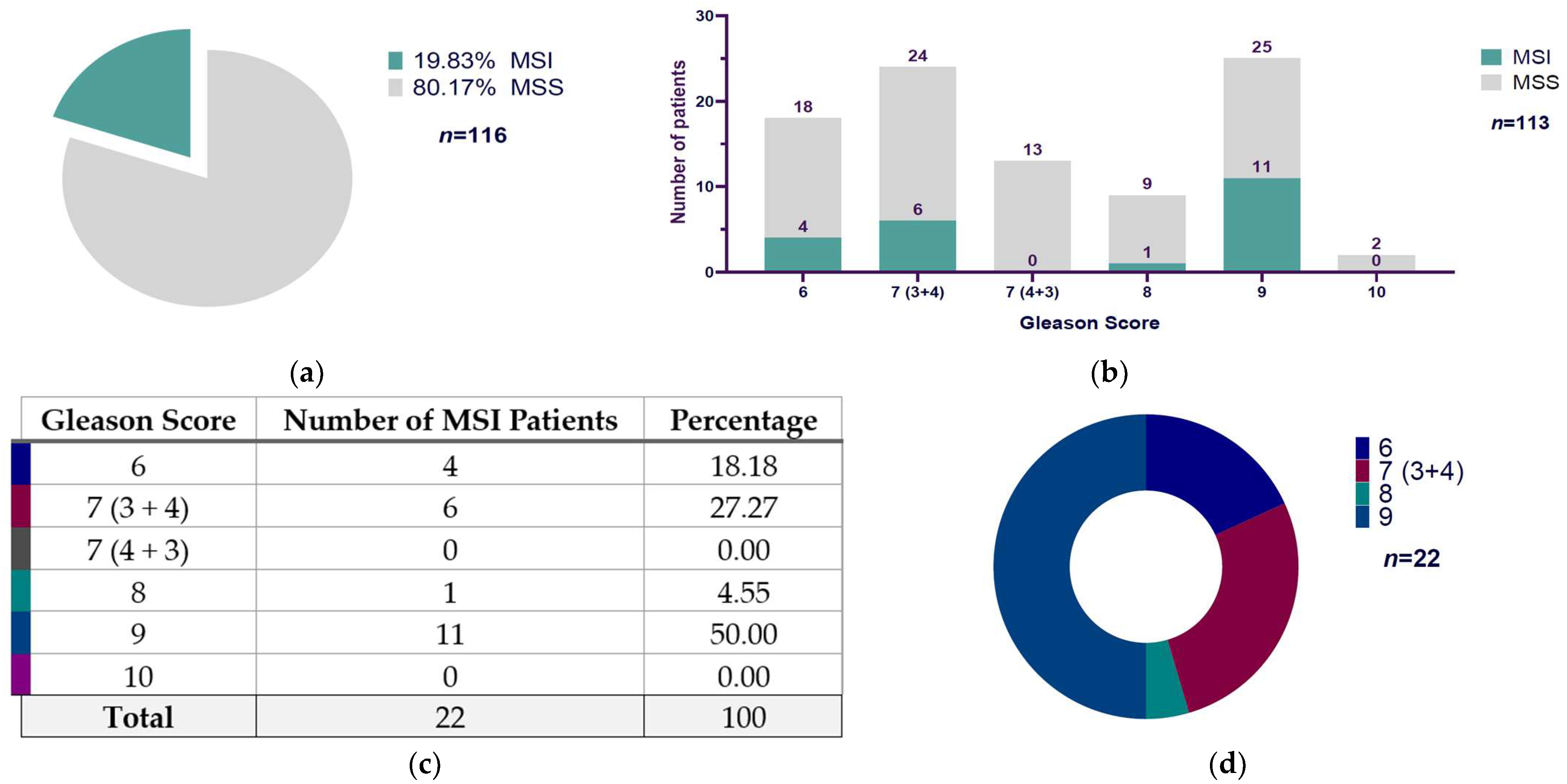
| Gleason Score | MSI | MSS | Total |
|---|---|---|---|
| 6 | 4 | 18 | 22 |
| 7 (3 + 4) | 6 | 24 | 30 |
| 7 (4 + 3) | 0 | 13 | 13 |
| 8 | 1 | 9 | 10 |
| 9 | 11 | 25 | 36 |
| 10 | 0 | 2 | 2 |
| Gleason Score | MSI-L | MSI-H | Total |
|---|---|---|---|
| 6 | 1 | 3 | 4 |
| 7 (3 + 4) | 1 | 5 | 6 |
| 7 (4 + 3) | 0 | 0 | 0 |
| 8 | 0 | 1 | 1 |
| 9 | 2 | 9 | 11 |
| 10 | 0 | 0 | 0 |
| BAT-26 | MSI-L | MSI-H | Total |
|---|---|---|---|
| Stable | 8 | 1 | 9 |
| Unstable | 10 | 4 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Islas, A.K.; Rico-Méndez, M.A.; Godínez-Rubí, M.; Villanueva-Pérez, M.A.; Sierra-Díaz, E.; Pereira-Suárez, A.L.; Beltrán-Ontiveros, S.A.; Gutiérrez-Arzapalo, P.Y.; Moreno-Ortiz, J.M.; Ramírez-de-Arellano, A. Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores. Diseases 2025, 13, 202. https://doi.org/10.3390/diseases13070202
Flores-Islas AK, Rico-Méndez MA, Godínez-Rubí M, Villanueva-Pérez MA, Sierra-Díaz E, Pereira-Suárez AL, Beltrán-Ontiveros SA, Gutiérrez-Arzapalo PY, Moreno-Ortiz JM, Ramírez-de-Arellano A. Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores. Diseases. 2025; 13(7):202. https://doi.org/10.3390/diseases13070202
Chicago/Turabian StyleFlores-Islas, Ana K., Manuel A. Rico-Méndez, Marisol Godínez-Rubí, Martha Arisbeth Villanueva-Pérez, Erick Sierra-Díaz, Ana Laura Pereira-Suárez, Saul A. Beltrán-Ontiveros, Perla Y. Gutiérrez-Arzapalo, José M. Moreno-Ortiz, and Adrián Ramírez-de-Arellano. 2025. "Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores" Diseases 13, no. 7: 202. https://doi.org/10.3390/diseases13070202
APA StyleFlores-Islas, A. K., Rico-Méndez, M. A., Godínez-Rubí, M., Villanueva-Pérez, M. A., Sierra-Díaz, E., Pereira-Suárez, A. L., Beltrán-Ontiveros, S. A., Gutiérrez-Arzapalo, P. Y., Moreno-Ortiz, J. M., & Ramírez-de-Arellano, A. (2025). Microsatellite Instability and BAT-26 Marker Expression in a Mexican Prostate Cancer Population with Different Gleason Scores. Diseases, 13(7), 202. https://doi.org/10.3390/diseases13070202







