Dramatic Deterioration of Subclinical Hyperparathyroidism in Children and Adolescents During the Post-COVID-19 Period
Abstract
1. Introduction
2. Materials and Methods
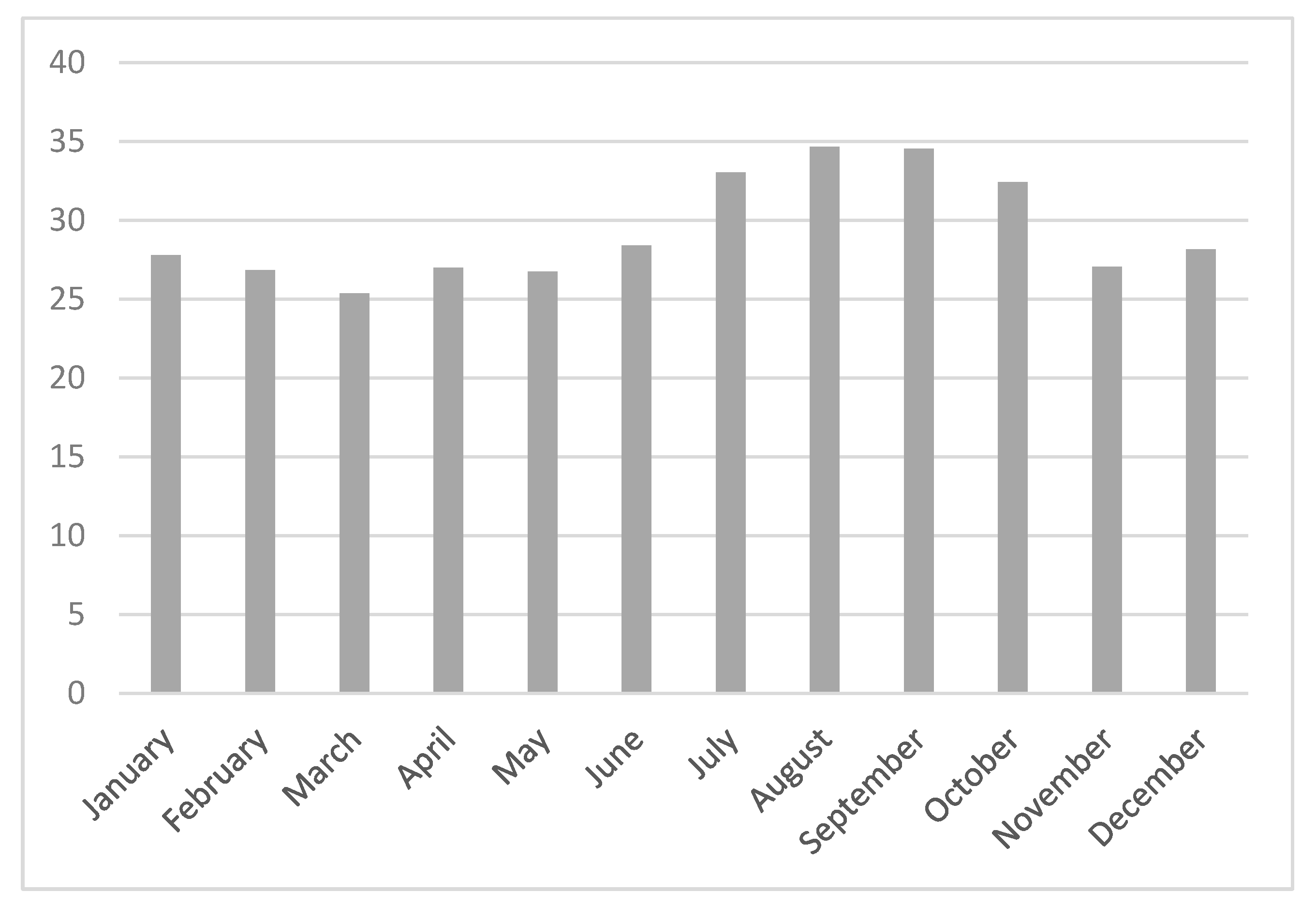
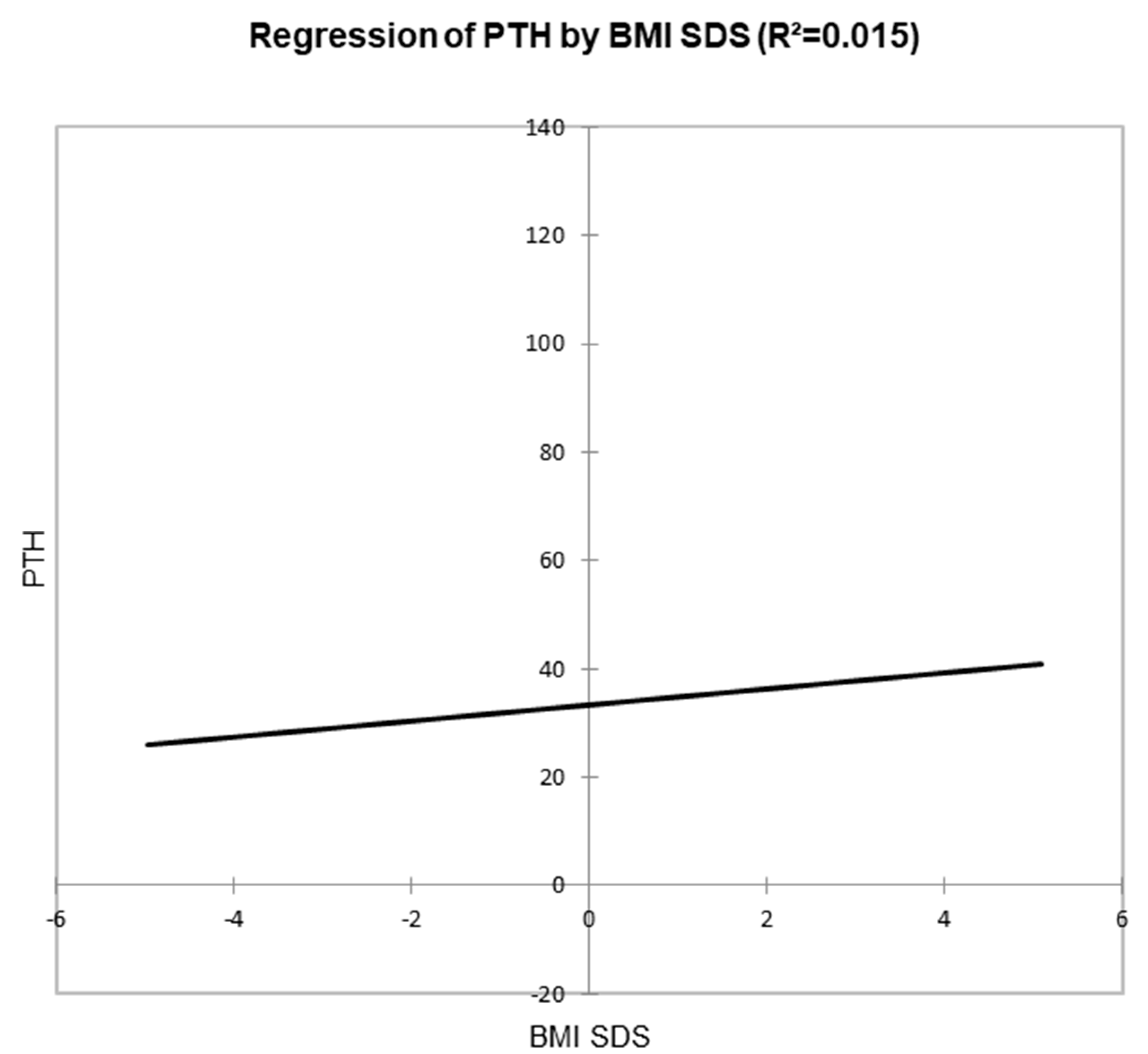
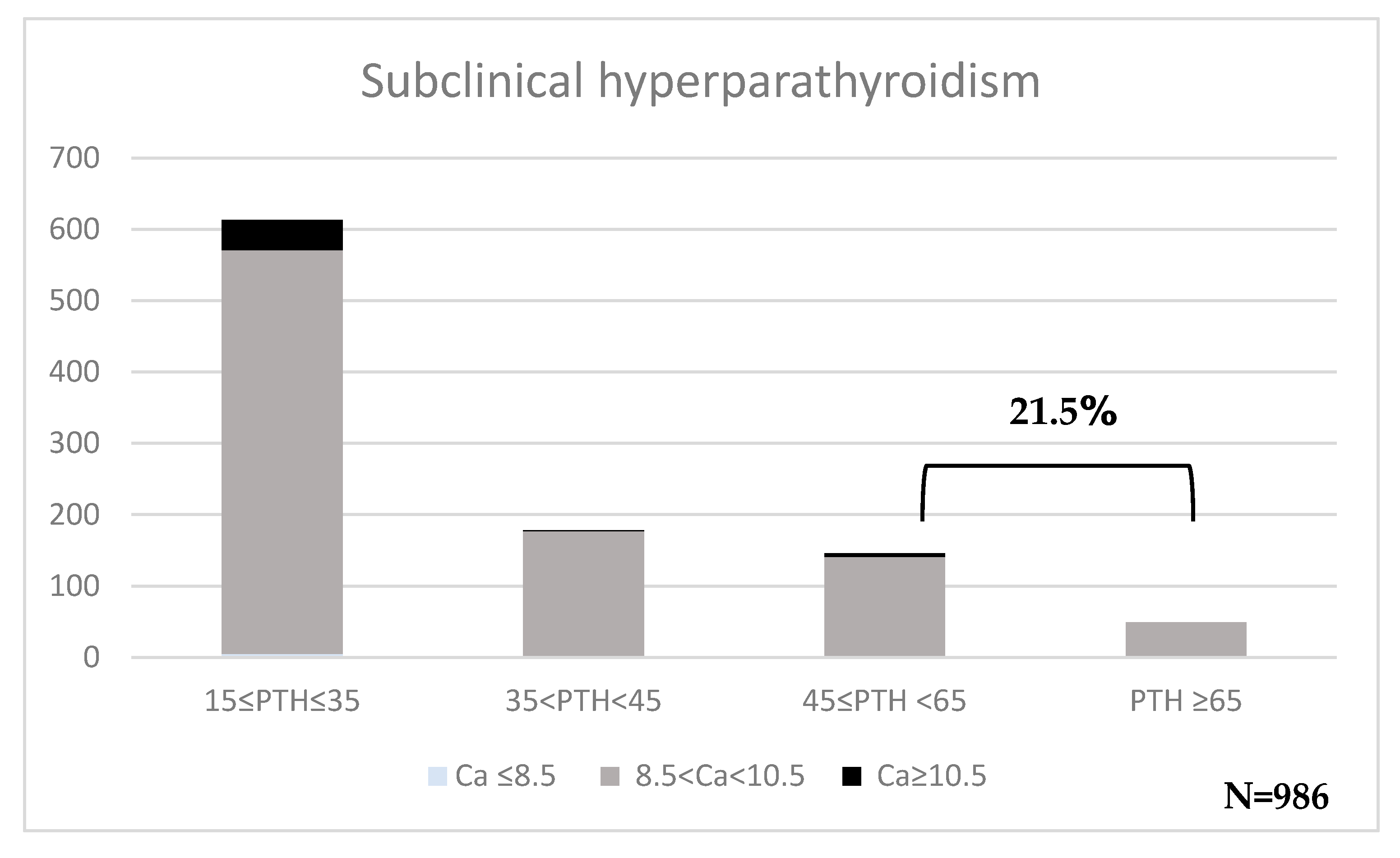
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | Calcitriol (active form of vitamin D) |
| 25(OH)D | 25-hydroxyvitamin D |
| ALP | Alkaline Phosphatase |
| BMI | Body Mass Index |
| BMI SDS | Body Mass Index Standard Deviation Score |
| Ca | Calcium |
| COVID-19 | Coronavirus Disease 2019 |
| DBP | Vitamin D Binding Protein |
| FT4 | Free Thyroxine |
| P | Phosphorus |
| PHPT | Primary Hyperparathyroidism |
| PTH | Parathyroid Hormone |
| RANK | Receptor Activator of Nuclear Factor Kappa-B |
| VDBP | Vitamin D-Binding Protein |
| VDR | Vitamin D Receptor |
| WHO | World Health Organization |
References
- Zhang, R.H.; He, D.H.; Zhou, B.; Zhu, Y.B.; Zhao, D.; Huang, L.C.; Ding, G.Q. Analysis of Vitamin D Status in Men Highly Exposed to Sunlight. Biomed. Environ. Sci. 2015, 28, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Ogata, H.; Koiwa, F. Secondary Hyperparathyroidism: Pathogenesis and Latest Treatment. Ther. Apher. Dial. 2018, 22, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.T.; Vassaras, A.K.; Holick, M.F. Association between Population Vitamin D Status and SARS-CoV-2 Related Serious-Critical Illness and Deaths: An Ecological Integrative Approach. World J. Virol. 2021, 10, 111–129. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Bouillon, R. Calcifediol Cornerstone of the Vitamin D Endocrine System. Nutrients 2023, 15, 2290. [Google Scholar] [CrossRef]
- Lifschitz, C. Vitamin D. Ann. Nutr. Metab. 2020, 76 (Suppl. S2), 1–4. [Google Scholar] [CrossRef]
- Shinchuk, L.M.; Holick, M.F. Vitamin D and Rehabilitation: Improving Functional Outcomes. Nutr. Clin. Pract. 2007, 22, 297–304. [Google Scholar] [CrossRef]
- Cuomo, A.; Giordano, N.; Goracci, A.; Fagiolini, A. Depression and Vitamin D Deficiency: Causality, Assessment, and Clinical Practice Implications. Neuropsychiatry 2017, 7, 606–614. [Google Scholar] [CrossRef]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-Analysis of All-Cause Mortality According to Serum 25-Hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulos, I.; Michalopoulou, E.; Mitsopoulou, A.V.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Association of Serum Vitamin D Status with Dietary Intake and Sun Exposure in Adults. Clin. Nutr. ESPEN 2019, 34, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Singhellakis, P.N.; Malandrinou, F.C.; Psarrou, C.J.; Danelli, A.M.; Tsalavoutas, S.D.; Constandellou, E.S. Vitamin D Deficiency in White, Apparently Healthy, Free-Living Adults in a Temperate Region. Hormones 2011, 10, 131–143. [Google Scholar] [CrossRef]
- Kyriakaki, A.; Fragkoulis, E. The Vitamin D Paradox: High Prevalence of Deficiency in Sunny Athens (Greece). Ann. Res. Hosp. 2019, 3, 13. [Google Scholar] [CrossRef]
- Paparodis, R.D.; Bantouna, D.; Karvounis, E.; Zoupas, I.; Livadas, S.; Angelopoulos, N.; Imam, S.; Papadimitriou, D.T.; Jaume, J.C. Intense Testing and Use of Vitamin D Supplements Leads to Slow Improvement in Vitamin D Adequacy Rates: A Cross-Sectional Analysis of Real-World Data. Nutrients 2024, 16, 111. [Google Scholar] [CrossRef]
- Papadimitriou, D.T. The Big Vitamin D Mistake. J. Prev. Med. Public Health 2017, 50, 278–281. [Google Scholar] [CrossRef]
- Papapetrou, P.D.; Triantaphyllopoulou, M.; Karga, H.; Zagarelos, P.; Aloumanis, K.; Kostakioti, E.; Vaiopoulos, G. Vitamin D Deficiency in the Elderly in Athens, Greece. J. Bone Miner. Metab. 2007, 25, 198–203. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Hulshof, T.; Bourhis, A.S.; Hull, G.L.J.; Dowling, K.G.; Kiely, M.E.; Cashman, K.D. Prevalence of Vitamin D Deficiency and Insufficiency among Schoolchildren in Greece: The Role of Sex, Degree of Urbanisation and Seasonality. Br. J. Nutr. 2017, 118, 550–558. [Google Scholar] [CrossRef][Green Version]
- Sinopoli, A.; Sciurti, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The Efficacy of Multivitamin, Vitamin A, Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention and Management of COVID-19 and Long-COVID: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 1345. [Google Scholar] [CrossRef]
- Pavlidou, E.; Poulios, E.; Papadopoulou, S.K.; Fasoulas, A.; Dakanalis, A.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity. Med. Sci. 2024, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, D.P.; Yu, N.; Leese, G.P. Subclinical and asymptomatic parathyroid disease: Implications of emerging data. Lancet Diabetes Endocrinol. 2013, 1, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Silverberg, S.J.; Bilezikian, J.P. Normocalcemic primary hyperparathyroidism. J. Clin. Densitom. 2013, 16, 33–39. [Google Scholar] [CrossRef]
- Jamal, S.A.; Miller, P.D. Secondary and tertiary hyperparathyroidism. J. Clin. Densitom. 2013, 16, 64–68. [Google Scholar] [CrossRef]
- Rao, D.S.; Wilson, R.J.; Kleerekoper, M.; Parfitt, A.M. Lack of Biochemical Progression or Continuation of Accelerated Bone Loss in Mild Asymptomatic Primary Hyperparathyroidism: Evidence for Biphasic Disease Course. J. Clin. Endocrinol. Metab. 1988, 67, 1294–1298. [Google Scholar] [CrossRef]
- Papadimitriou, D.T.; Dermitzaki, E.; Kleanthous, K.; Papadimitriou, A.; Mastorakos, G. MON-541 Successful Treatment of Normocalcemic Hyperparathyroidism in Children. J. Endocr. Soc. 2019, 3 (Suppl. S1), MON-541. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Smit, M.A.; van Kinschot, C.M.J.; van der Linden, J.; van Noord, C.; Kos, S. Clinical Guidelines and PTH Measurement: Does Assay Generation Matter? Endocr. Rev. 2019, 40, 1468–1480. [Google Scholar] [CrossRef]
- Stagi, S.; Cavalli, L.; Ricci, S.; Mola, M.; Marchi, C.; Seminara, S.; Brandi, M.L.; de Martino, M. Parathyroid Hormone Levels in Healthy Children and Adolescents. Horm. Res. Paediatr. 2015, 84, 124–129. [Google Scholar] [CrossRef]
- Minisola, S.; Pepe, J.; Piemonte, S.; Cipriani, C. The Diagnosis and Management of Hypercalcaemia. BMJ 2015, 350, h2723. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; Al-Shaikh, D.A.; Al-Turki, H.A. Parathyroid Glands Response to Low Vitamin D Levels in Healthy Adults: A Cross-Sectional Study. Ulst. Med. J. 2015, 84, 26–29. [Google Scholar] [PubMed Central]
- Vissing Landgrebe, A.; Asp Vonsild Lund, M.; Lausten-Thomsen, U.; Frithioff-Bøjsøe, C.; Esmann Fonvig, C.; Lind Plesner, J.; Aas Holm, L.; Jespersen, T.; Hansen, T.; Christian Holm, J. Population-Based Pediatric Reference Values for Serum Parathyroid Hormone, Vitamin D, Calcium, and Phosphate in Danish/North-European White Children and Adolescents. Clin. Chim. Acta 2021, 523, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Hu, M.; Gupta, M.; Butler, R.; Mitchell, J.; Berber, E.; Siperstein, A.; Milas, M. A New, Vitamin D-Based, Multidimensional Nomogram for the Diagnosis of Primary Hyperparathyroidism. Endocr. Pract. 2012, 18, 124–131. [Google Scholar] [CrossRef]
- Levine, M.A. Assessing Bone Health in Children and Adolescents. Indian J. Endocrinol. Metab. 2012, 16, S205–S212. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Lawson-Body, E.; Hammadi, B.; Sarfati, É.; Kahan, A.; Cormier, C. The Use in Clinical Practice of Parathyroid Hormone Normative Values Established in Vitamin D-Sufficient Subjects. J. Clin. Endocrinol. Metab. 2003, 88, 3501–3504. [Google Scholar] [CrossRef]
- Marrero, A.J.R.; Astete, C.A.G.; Román, M.M.; Coronado, M.R.; Sánchez, J.M.D.R.; Lozano, A.G.; Tinedo, M.A.T.; Díaz, M.V.; Gómez, I.A. Prevalence of Vitamin D Deficiency and Association with Parathyroid Hormone. Adv. Lab. Med. 2022, 3, 51–58. [Google Scholar]
- Younes, N.A.; Shafagoj, Y.; Khatib, F.; Ababneh, M. Laboratory Screening for Hyperparathyroidism. Clin. Chim. Acta 2005, 353, 1–12. [Google Scholar] [CrossRef]
- Lowe, H.; McMahon, D.J.; Rubin, M.R.; Bilezikian, J.P.; Silverberg, S.J. Normocalcemic Primary Hyperparathyroidism: Further Characterization of a New Clinical Phenotype. J. Clin. Endocrinol. Metab. 2007, 92, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Siperstein, A.E.; Shen, W.; Chan, A.K.; Duh, Q.-Y.; Clark, O.H. Normocalcemic Hyperparathyroidism: Biochemical and Symptom Profiles Before and After Surgery. Arch. Surg. 1992, 127, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Preece, M.A.; Tomlinson, S.; Ribot, C.A.; Pietrek, J.; Korn, H.T.; Davies, D.M.; Ford, J.A.; Dunnigan, M.G.; O’Riordan, J.L.H. Studies of vitamin D deficiency in man. Q. J. Med. 1975, 44, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Maruani, G.; Hertig, A.; Paillard, M.; Houillier, P. Normocalcemic Primary Hyperparathyroidism: Evidence for a Generalized Target-Tissue Resistance to Parathyroid Hormone. J. Clin. Endocrinol. Metab. 2003, 88, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Maalouf, N.M.; Wang, P.Y.; Zhang, C.; Cremers, S.C.; Haney, E.M.; Bauer, D.C.; Orwoll, E.S.; Bilezikian, J.P. Normocalcemic Hyperparathyroidism and Hypoparathyroidism in Two Community-Based Nonreferral Populations. J. Clin. Endocrinol. Metab. 2013, 98, 2734–2741. [Google Scholar] [CrossRef]
- Schini, M.; Jacques, R.M.; Oakes, E.; Peel, N.F.A.; Walsh, J.S.; Eastell, R. Normocalcemic Hyperparathyroidism: Study of its Prevalence and Natural History. J. Clin. Endocrinol. Metab. 2020, 105, e1171–e1186. [Google Scholar] [CrossRef]
- Šiprová, H.; Fryšák, Z.; Souček, M. Primary Hyperparathyroidism, With a Focus on Management of the Normocalcemic Form: To Treat or Not to Treat? Endocr. Pract. 2016, 22, 294–301. [Google Scholar] [CrossRef]
- Tordjman, K.M.; Greenman, Y.; Osher, E.; Shenkerman, G.; Stern, N. Characterization of Normocalcemic Primary Hyperparathyroidism. Am. J. Med. 2004, 117, 861–863. [Google Scholar] [CrossRef]
- Lu, H.-K.; Zhang, Z.; Ke, Y.-H.; He, J.-W.; Fu, W.-Z.; Zhang, C.-Q.; Zhang, Z.-L. High Prevalence of Vitamin D Insufficiency in China: Relationship with the Levels of Parathyroid Hormone and Markers of Bone Turnover. PLoS ONE 2012, 7, e47264. [Google Scholar] [CrossRef]
- Wagner, C.L.; Greer, F.R.; Section on Breastfeeding; Committee on Nutrition. Prevention of Rickets and Vitamin D Deficiency in Infants, Children, and Adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef]
- Saneifard, H.; Shakiba, M.; Sheikhy, A.; Baniadam, L.; Abdollah Gorji, F.; Fallahzadeh, A. Vitamin D Deficiency in Children and Adolescents: Role of Puberty and Obesity on Vitamin D Status. Nutr. Metab. Insights 2021, 14, 11786388211018726. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Cediel, G.; Corvalán, C.; López de Romaña, D.; Mericq, V.; Uauy, R. Prepubertal Adiposity, Vitamin D Status, and Insulin Resistance. Pediatrics 2016, 138, e20160076. [Google Scholar] [CrossRef] [PubMed]
- Somjen, D.; Weisman, Y.; Kohen, F.; Gayer, B.; Limor, R.; Sharon, O.; Jaccard, N.; Knoll, E.; Stern, N. 25-Hydroxyvitamin D3-1α-Hydroxylase Is Expressed in Human Vascular Smooth Muscle Cells and Is Upregulated by Parathyroid Hormone and Estrogenic Compounds. Circulation 2005, 111, 1666–1671. [Google Scholar] [CrossRef]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef]
- Reis, J.P.; von Mühlen, D.; Miller, E.R.; Michos, E.D.; Appel, L.J. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 2009, 124, e371–e379. [Google Scholar] [CrossRef]
- Queiroz, N.N.M.; de Melo, F.T.C.; de Souza Resende, F.; Janaú, L.C.; de Souza Neto, N.J.K.; de Lemos, M.N.; Virgolino, A.C.L.; de Oliveira, M.C.N.I.; de Alcântara, A.L.; de Moraes, L.V.; et al. Vitamin D and PTH: Data from a Cross-Sectional Study in an Equatorial Population. Endocr. Connect. 2020, 9, 784–794. [Google Scholar] [CrossRef]
- Vanlint, S. Vitamin D and Obesity. Nutrients 2013, 5, 949–956. [Google Scholar] [CrossRef]
- Compston, J.E.; Vedi, S.; Ledger, J.E.; Webb, A.; Gazet, J.C.; Pilkington, T.R. Vitamin D status and bone histomorphometry in gross obesity. Am. J. Clin. Nutr. 1981, 34, 2359–2363. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Pellegrino, M.; Marra, M.; Scali, E.; Sinicropi, M.S.; Aquaro, S. The Ongoing Impact of COVID-19 on Pediatric Obesity. Pediatr. Rep. 2024, 16, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Beyazgül, G.; Bağ, Ö.; Yurtseven, İ.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G.; Çelik, F.; Nalbantoğlu, Ö.; Özkan, B. How Vitamin D Levels of Children Changed During COVID-19 Pandemic: A Comparison of Pre-pandemic and Pandemic Periods. J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials. Clin. Nutr. 2023, 42, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Mosca, C.; Colucci, A.; Savoia, F.; Calì, C.; Del Bene, M.; Ranucci, G.; Maglione, A.; Pepe, A.; Morelli, A.; Vajro, P.; et al. Vitamin D Levels in the Pre- and Post-COVID-19 Pandemic Periods and Related Confinement at Pediatric Age. Nutrients 2023, 15, 2089. [Google Scholar] [CrossRef]
- Wong, R.S.; Tung, K.T.S.; So, H.-K.; Wong, W.H.S.; Wong, S.Y.; Tsang, H.W.; Tung, J.Y.L.; Chua, G.T.; Ho, M.H.K.; Wong, I.C.K.; et al. Impact of COVID-19 Pandemic on Serum Vitamin D Level among Infants and Toddlers: An Interrupted Time Series Analysis and before-and-after Comparison. Nutrients 2021, 13, 1270. [Google Scholar] [CrossRef]
- Chandankere, R. Impact of COVID lockdown: Increased prevalence of symptomatic Vitamin D deficiency in adolescents. J. Clin. Orthop. Trauma 2023, 47, 102316. [Google Scholar] [CrossRef]
- Bretz, G.P.M.; Campos, J.R.; Veloso, A.A.; Gomes, K.B.; Fernandes, A.B. Impact of COVID-19 pandemic on serum 25-hydroxyvitamin D levels in Brazilian patients. J. Lab. Precis. Med. 2023, 8, 31. [Google Scholar] [CrossRef]
- Mazziotti, G.; Lavezzi, E.; Brunetti, A.; Mirani, M.; Favacchio, G.; Pizzocaro, A.; Sandri, M.T.; Di Pasquale, A.; Voza, A.; Ciccarelli, M.; et al. Vitamin D deficiency, secondary hyperparathyroidism and respiratory insufficiency in hospitalized patients with COVID-19. J. Endocrinol. Investig. 2021, 44, 2285–2293. [Google Scholar] [CrossRef]
- Mutnuri, S.; Fernandez, I.; Kochar, T. Suppression of Parathyroid Hormone in a Patient with Severe Magnesium Depletion. Case Rep. Nephrol. 2016, 2016, 2608538. [Google Scholar] [CrossRef]




| Age Groups | n | Mean ± SD 25(OH)D | Sufficiency | Insufficiency | Deficiency |
|---|---|---|---|---|---|
| <1 year | 33 | 38.70 ± 19.30 | 60.0% | 27.3% | 12.1% |
| 1–5 years | 166 | 31.10 ± 13.60 | 46.9% | 36.7% | 16.2% |
| 6–12 years | 663 | 29.50 ± 11.50 | 37.5% | 45.5% | 16.9% |
| 13–16 years | 276 | 28.70 ± 12.16 | 35.5% | 40.5% | 23.9% |
| p-value (between all age groups) | p < 0.01 | p < 0.005 | |||
| Preadolescence n = 269 | Adolescence n = 869 | |
|---|---|---|
| 25(OH)D | ||
| Sufficiency | 13.0% | 19.9% |
| Insufficiency | 36.8% | 44.3% |
| Deficiency | 49.8% | 35.7% |
| p-Value | p < 0.001 | |
| PTH | ||
| PTH ≤ 15 pg/mL | 11.8% | 3.3% |
| 15 < PTH < 35 pg/mL | 63.9% | 52.3% |
| 35 ≤ PTH < 45 pg/mL | 13.7% | 19.2% |
| 45 ≤ PTH < 65 pg/mL | 8.5% | 18.7% |
| PTH ≥ 65 pg/mL | 1.8% | 6.3% |
| p-Value | p < 0.001 | |
| Preadolescence n = 269 | ||
| 25(OH)D Mean ± SD [Range] | Mean ± SD PTH [Range] | |
| Girls | 33.42 ± 12.68 [9.46–90.60] | 28.92 ± 13.68 [6.10–88.10] |
| Boys | 31.26 ± 15.02 [8.34–107.00] | 27.20 ± 12.64 [5.40–71.60] |
| p-Value | p > 0.05 | p > 0.05 |
| Adolescence n = 869 | ||
| 25(OH)D Mean ± SD [Range] | Mean ± SD PTH [Range] | |
| Girls | 27.90 ± 12.34 [0.30–107.00] | 37.62 ± 17.74 [9.80–113.80] |
| Boys | 29.52 ± 11.24 [5.90–85.00] | 36.20 ± 18.84 [9.30–137.80] |
| p-Value | p < 0.05 | p > 0.05 |
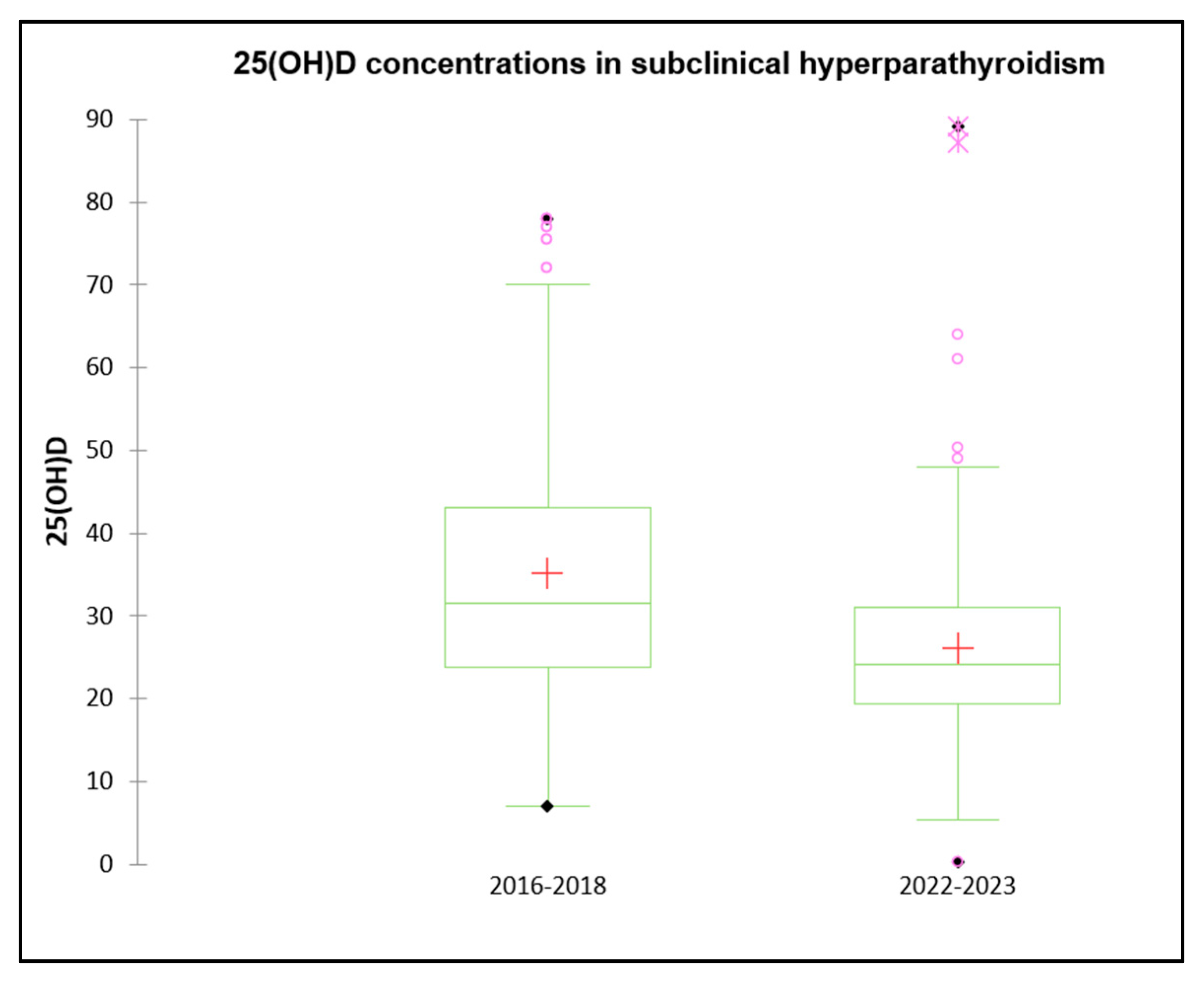
| 2016–2018 (n = 157) | 2022–2023 (n = 246) | p-Value | |
|---|---|---|---|
| Sex (M/F) | 64/93 (40.8% M) | 115/131 (53.3% M) | <0.001 |
| Age (years) | 11.56 ± 3.15 [0.94–16.00] | 10.84 ± 3.38 [0.10–16.00] | 0.06 |
| 25(OH)D (ng/mL) | 35.20 ± 16.81 [7.00–78.00] | 26.10 ± 11.21 [5.0–89.10] | <0.001 |
| Calcium (mg/dL) | 9.58 ± 0.55 [7.90–10.89] | 9.73 ± 0.75 [8.10–9.73] | 0.05 |
| Vitamin D Status | |||
| Sufficiency | 52.0% | 29.5% | <0.001 |
| Insufficiency | 29.0% | 47.3% | <0.05 |
| Deficiency | 17.8% | 24.1% | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loutsou, M.; Dermitzaki, E.; Paparodis, R.D.; Michoula, A.N.; Angelopoulos, N.; Christopoulos, P.; Diamantopoulos, S.; Mastorakos, G.; Grivea, I.N.; Papadimitriou, D.T. Dramatic Deterioration of Subclinical Hyperparathyroidism in Children and Adolescents During the Post-COVID-19 Period. Diseases 2025, 13, 198. https://doi.org/10.3390/diseases13070198
Loutsou M, Dermitzaki E, Paparodis RD, Michoula AN, Angelopoulos N, Christopoulos P, Diamantopoulos S, Mastorakos G, Grivea IN, Papadimitriou DT. Dramatic Deterioration of Subclinical Hyperparathyroidism in Children and Adolescents During the Post-COVID-19 Period. Diseases. 2025; 13(7):198. https://doi.org/10.3390/diseases13070198
Chicago/Turabian StyleLoutsou, Maria, Eleni Dermitzaki, Rodis D. Paparodis, Aspasia N. Michoula, Nicholas Angelopoulos, Panagiotis Christopoulos, Stavros Diamantopoulos, George Mastorakos, Ioanna N. Grivea, and Dimitrios T. Papadimitriou. 2025. "Dramatic Deterioration of Subclinical Hyperparathyroidism in Children and Adolescents During the Post-COVID-19 Period" Diseases 13, no. 7: 198. https://doi.org/10.3390/diseases13070198
APA StyleLoutsou, M., Dermitzaki, E., Paparodis, R. D., Michoula, A. N., Angelopoulos, N., Christopoulos, P., Diamantopoulos, S., Mastorakos, G., Grivea, I. N., & Papadimitriou, D. T. (2025). Dramatic Deterioration of Subclinical Hyperparathyroidism in Children and Adolescents During the Post-COVID-19 Period. Diseases, 13(7), 198. https://doi.org/10.3390/diseases13070198









