Abstract
Autoimmune gastritis (AIG) is an uncommon and often underestimated condition in children, characterized by chronic stomach inflammation leading to the destruction of oxyntic glands with subsequent atrophic and metaplastic changes. This condition is associated with hypo-/achlorhydria, impairing iron and vitamin B12 absorption. The pathogenesis involves the activation of helper type 1 CD4+/CD25-T-cells against parietal cells. Clinical manifestations in children are not specific and include abdominal pain, bloating, nausea, vomiting, and iron deficiency anemia (IDA). The disease is also linked to an increased risk of pernicious anemia, intestinal-type gastric cancer, and type I neuroendocrine tumors. AIG is often diagnosed through the presence of autoantibodies in the serum, such as parietal cell (APCA) and intrinsic factor (IF) antibodies. However, therapeutic recommendations for pediatric AIG are currently lacking. We aim to present two clinical cases of pediatric-onset AIG, highlighting the heterogeneous clinical manifestations and the challenges in diagnosis with the support of an updated literature review. A 9-year-old girl presented with refractory IDA, initial hypogammaglobulinemia, and a 12-year-old boy was initially diagnosed with eosinophilic esophagitis. Both cases underline the importance of considering AIG in children with chronic gastrointestinal symptoms and gastric atrophy. Diagnostic workup, including endoscopy and serological tests, is crucial for accurate identification. A better understanding of this condition is imperative for timely intervention and regular monitoring, given the potential long-term complications, including the risk of malignancy. These cases contribute to expanding the clinical spectrum of pediatric AIG and highlight the necessity for comprehensive evaluation and management in affected children.
1. Introduction
Autoimmune gastritis (AIG) is a rare and often underrecognized condition in children [1]. AIG is a chronic inflammatory disorder of the stomach, which is typically restricted to the corpus, characterized by the destruction of oxyntic glands and their replacement by atrophic and metaplastic mucosa, and usually accompanied by lymphoplasmacytic infiltration of the lamina propria and the gradual destruction of parietal cells [2]. The pathophysiological consequence of this phenomenon is hypochlorhydria or achlorhydria, which interferes with intestinal iron absorption and loss of intrinsic factor (IF), which compromises vitamin B12 intestinal absorption [3]. The exact pathogenetic mechanism is not fully understood [4]. Still, it is characterized, though inconstantly, by the presence in the serum of autoantibodies against the proton pump H+/K+ adenosine triphosphatase of gastric parietal cells (anti-parietal-cells antibodies [APCA]) and IF [5,6,7,8]. The etiopathogenetic process involves the activation of T helper type 1 CD4+/CD25− T-cells directed against parietal cells [9,10]. Macroscopically, there is thinning or flattening of the gastric mucosa rugae. Histologically, there is a loss of gastric glandular structures in the oxyntic mucosa, inappropriately replaced by inflammatory cells and metaplastic epithelium or fibrous tissue [9,10]. All these features result in achlorhydria and hypergastrinemia, with the consequent proliferation of enterochromaffin-like cells (ECL) [9,10,11].
AIG is a well-known cause of pernicious anemia ([PA] megaloblastic anemia and vitamin B12 deficiency) in adult patients. Children generally show nonspecific and heterogeneous symptoms, including abdominal pain, nausea, vomiting, and loss of appetite, that are not responsive to treatments [2,3,4,5]. However, the most common clinical manifestation in children is iron deficiency anemia (IDA), consistent with the more rapid depletion of iron storage compared to vitamin B12 [2,9,12]. Diagnosis often involves a combination of medical history, physical examination, blood tests to check for anemia and vitamin deficiencies, and endoscopy with biopsy to examine the stomach lining. Treatment generally involves addressing nutrient deficiencies, mainly iron deficiency in children, and, where needed, vitamin B12 supplements. AIG may require ongoing monitoring and management, as it can affect a child’s growth and development [13]. Intestinal-type gastric cancer and type I neuroendocrine tumors are two possible oncological consequences of AIG [14]. Considering children’s long-life expectancy and the cancer risk, endoscopic surveillance of AIG every five years has been suggested [14].
Although a variety of therapeutic approaches have been suggested, including netazepide, somatostatin, or antrectomy to reduce the amount of circulating gastrin, to date, no treatment recommendations exist for pediatric patients with AIG, regardless of the absence or presence of metaplastic change [15].
Considering the rarity of the disease in children and the lack of age-specific guidelines, we aim to present two cases of pediatric-onset AIG, discuss their features and diagnostic-therapeutic work-up, and compare our experience with the currently available literature. A better understanding of AIG is mandatory, particularly regarding the nonspecific and heterogeneous clinical and histopathological features in children and adolescents. An early and precise diagnosis is crucial to better manage these patients for two reasons: the first is that a correct nutritional state is fundamental for proper development; the second is that the principal and worst complication is the risk of gastric neoplastic diseases. Thus, regular endoscopic follow-up could impact the prognosis of these patients.
2. Methods
We retrospectively enrolled two children diagnosed with AIG who have been followed at our Pediatric Clinic in Pavia, Italy. Diagnosis of AIG was made according to current Italian guidelines and is based on clinical, serological, and histological features [15]. Clinical, endoscopic, and histological data were collected during diagnosis and follow-up visits. Findings from radiological examinations were also reported when performed. Patients were screened for several autoimmune diseases, including autoimmune thyroiditis (AITD), celiac disease (CeD), type 1 diabetes mellitus (T1DM), and inflammatory bowel diseases (IBDs). We also ruled out immunodeficiencies. We included patients whose parents signed written informed consent. The Ethical Committee of Fondazione IRCCS San Matteo di Pavia, Italy, approved this study (protocol number: 0003241/22).
The literature review was performed using the online database PubMed and the MeSH terms “autoimmune gastritis” OR “atrophic gastritis” AND “children.” The research was conducted in November 2024. We included retrospective studies, cross-sectional and cohort studies, case series, and case reports published in English and peer-reviewed journals in which participants were children and adolescents (0–18 years) with a diagnosis of histologically confirmed AIG, without limits on the year of publication. Two authors manually screened and reviewed potentially eligible publications and excluded non-relevant publications (e.g., narrative/systematic review, languages other than English, adult population, and other pathological conditions).
3. Clinical Case Description
3.1. Case 1
A 9-year-old girl was referred to our Pediatric Clinic for unexplained asthenia and weight loss during the previous year. Her family history was negative for chronic or infectious diseases. Her grandfather died because of an unspecified gastric neoplasia. The first clinical observation and physical examination were negative. The initial diagnostic assessment showed severe iron deficiency anemia (IDA) (hemoglobin 7 g/dL), treated with oral iron therapy, and IgG deficiency with mild IgG1 and IgG2 subclasses deficiency (Table 1). Lymphocyte subsets showed a mild reduction in B-lymphocytes. Vaccine immune responses (hepatitis B, measles, tetanus, and diphtheria) were conserved. An in vitro proliferation test (response to mitogens and IgG production) resulted within the normal range. Comprehensive autoimmune screening was negative. Helicobacter pylori (H. pylori) fecal antigen was negative. The fecal occult blood test was highly positive, and fecal calprotectin was slightly positive. Upper and lower gastrointestinal (GI) endoscopy showed diffuse atrophic gastric mucosa without the normal folds. Histological examination showed antral gastric mucosa with mild chronic inflammation, whereas the oxyntic mucosa featured severe mucosal atrophy with sclerosis, diffuse pseudo-pyloric metaplasia, and linear hyperplasia of enterochromaffin-like (ECL) cells (Figure 1A). Microbiological investigations for H pylori were negative. Moreover, the histological examination showed lamina propria chronic inflammation with eosinophilic infiltration (99 intramucosal eosinophilic granulocytes (eos)/high power field (hpf) in the antrum, 98 eos/hpf in the upper gastric body, and 89 eos/hpf in the lower gastric body) and presence of numerous plasma cells (CD138+, MUM1+) (Figure 1A(D)). No pathognomonic signs of IBD were observed, but only nodular lymphoid hypertrophy of the last tract of the ileum was found. No parietal cell antibodies (APCA) or anti-IF were found. Vitamin B12 serum levels were normal. Allergy tests were negative. The patient underwent a video capsule endoscopy to complete the diagnostic workup, revealing diffuse epithelial erosion of the duodenum, multiple vascular ectasias, blood vessel fragility, and nodular lymphoid hypertrophy of the terminal ileum. The abdominal ultrasound and entero-MRI findings were negative for bowel thickening and inflammation.

Table 1.
Main clinical and laboratory findings of the cases presented.
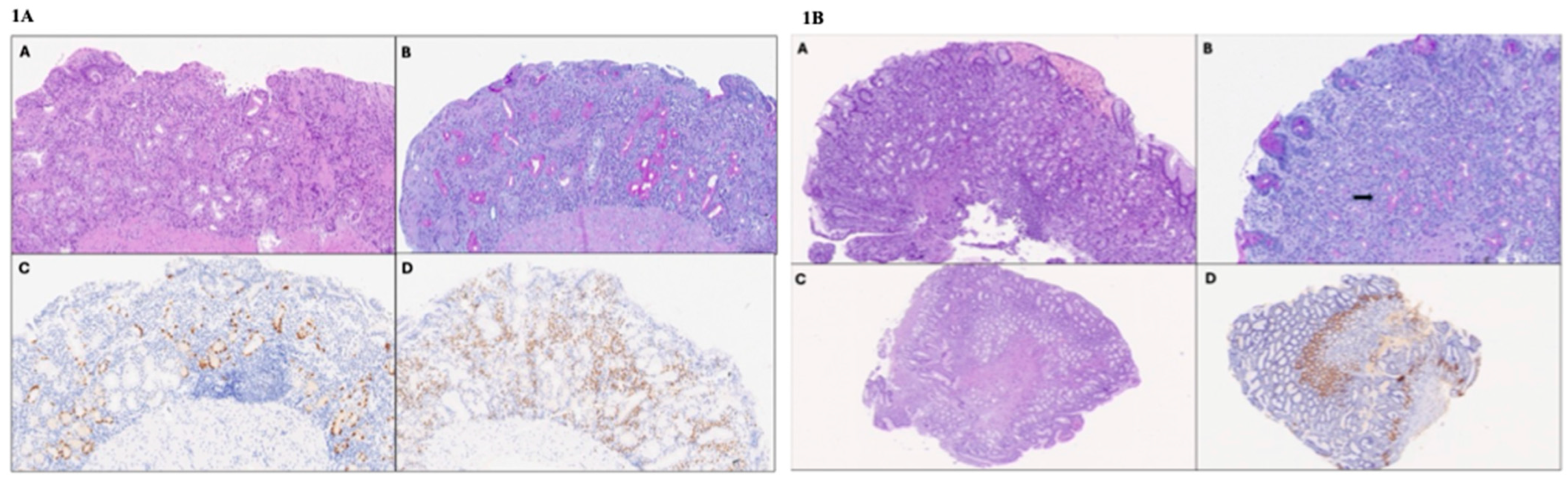
Figure 1.
(1A) Histologic images of corpus biopsies of Case 1 (high-power field 40×). (A) The oxyntic mucosa shows moderate chronic inflammation, including eosinophils and severe atrophy, with a reduction in the number of appropriate glandular units, fibrosclerosis, and metaplastic changes in the epithelium (hematoxylin and eosin). (B) Diffuse pseudopyloric (neutral mucin-producing) metaplasia of the oxyntic glands (Periodic Acid Schiff–Alcian Blue staining). (C) Linear ECL cell hyperplasia (chromogranin A immunostaining). (D) Presence of numerous MUM1-positive plasma cells in the lamina propria (MUM1 immunostaining). (1B) Histologic images of gastric biopsies of Case 2 (high-power field 40×). (A) The fundic mucosa features moderate chronic inflammation and mild atrophy (hematoxylin and eosin). (B) Focal pseudopyloric metaplasia (arrow; Periodic Acid Schiff–Alcian Blue staining). The antral mucosa appears normotrophic (C) (hematoxylin and eosin), with gastrin cell hyperplasia (D) (gastrin immunohistochemistry).
Genetic tests were performed to rule out inherited causes of vascular ectasia and blood vessel fragility. After performing karyotype analysis, which produced normal results, the multi-gene next-generation sequencing revealed that the father inherited a frame-shift mutation in the heterozygous state of the ABCC6 gene: c.960delC (p.ser321ValfsTer35), compatible with a heterozygous form of pseudoxanthoma elasticum.
After the endoscopy, to treat the abnormal eosinophilic inflammation, we decided to start a topical steroid treatment (budesonide 9 mg daily) with progressive decalage in six months. Hereafter, the fecal occult blood test was always negative, and fecal calprotectin normalized, but the previously reported gastric atrophy persisted. The upper and lower GI endoscopies, repeated through the follow-up, confirmed the past histological features with the resolution of the eosinophilic inflammation. IDA was resolved with chronic oral iron supplementation, but iron body stocks were never wholly restored. Therefore, she underwent intravenous supplementation with complete iron status normalization. Considering immunological findings, IgA and IgG serum levels normalized over the years. To date, she is under strict clinical and endoscopic follow-up for a total period of 5 years. The last upper GI endoscopy performed 4 years after the diagnosis revealed the following findings: (i) diffused mild active chronic inflammation and atrophy (severe in the corpus) with sclerosis and antrum gastrin-cell hyperplasia; (ii) corpus lymphoid cells infiltration, pseudo-pyloric metaplasia, and linear ECL-cells hyperplasia; and (iii) fundus simple ECL-cells hyperplasia. Moreover, she is currently taking intramuscular vitamin B12 supplementation.
3.2. Case 2
A 12-year-old boy was referred to our Pediatric Clinic for eosinophilic esophagitis (EoE) and suspected AIG (Table 1). He was previously referred to another hospital for nausea, dyspepsia, heartburn, and food impaction episodes not responsive to a proton pump inhibitor (PPI) therapy. His past medical history revealed an IgE-mediated hazelnut allergy. His family history was positive for gastric neoplasia (father and grandfather) and for multiple autoimmune diseases. His father was affected by Basedow–Graves’ disease and presented auto-antibodies against pancreatic and testicular tissues.
The upper GI endoscopy performed at the other center showed a normal esophageal mucosa aspect and hyperemic gastric antral mucosa. The EoE was histologically confirmed (>100 eos/hpf in esophageal biopsies). A gastric mucosa examination revealed mild chronic antral mucosa inflammation, mild atrophy, and linear ECL-cell hyperplasia. A H pylori search was negative after Giemsa-stained coloration and immunohistochemical staining with anti-H. pylori-specific antibody. He started a treatment with PPI and topical corticosteroid (swallowed fluticasone 875 μg/die). After four months, the upper GI endoscopy was repeated, showing the histological remission of EoE. Gastric biopsies revealed a non-atrophic antral mucosa with gastrin cell hyperplasia, chronic inflammation, and mild atrophy of the fundic mucosa, with focal pseudopyloric metaplasia and linear ECL cell hyperplasia (Figure 1B). The initial diagnostic assessment showed positivity for APCA (1:160). Anemia was not found as an iron status, and vitamin B12 serum levels were normal.
When the child came to our attention, we stopped the PPI treatment and reduced the dose of swallowed steroid (to 500 μg/die) because of EoE remission. Moreover, to exclude the presence of an inborn error of immunity (IEI), we assessed the immunological status, checking immunoglobulins and IgG sub-classes levels that fell within the normal age-matched range.
The esophagogastroduodenoscopy was repeated in our pediatric Center one year after the onset of the symptoms. It confirmed the histological remission of EoE, so we further decreased the amount of swallowed fluticasone (to 250 μg/die). Gastric mucosa was still characterized by moderate atrophy and mild chronic active inflammation of the corpus and the antrum with pseudo-pyloric metaplasia, linear ECL-cell hyperplasia, and corpus lymphoid infiltration.
4. Evidence from the Literature Review
We found thirty-nine (39) papers, thirteen of which were selected for the review. Six papers were manually added to the search query due to their relevance, and finally, nineteen papers were analyzed for the review. Pediatric AIG has been diagnosed in 134 children (min 8.5, max 15.7 years).
Among the retrieved articles, pediatric-onset AIG mainly affected females and generally occurred during late childhood and adolescence (Table 2) [2,12,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Almost 60% of enrolled patients also presented concomitant autoimmune diseases, particularly AITD and T1DM (20% of cases), followed by CeD (~5%). Addison disease, autoimmune hepatitis, and cytopenia were comorbidities, affecting about 2% of all children [12,13,14,16,17,20,21,23,25,26,27,28,29]. Although family history often did not reveal relevant information, in some cases, there was a positivity for autoimmune diseases [12,20,22]. In the case report by Greenwood et al., family history was positive for autoimmune polyendocrine syndrome type 2 [20].

Table 2.
Summary of the pediatric autoimmune gastritis reports.
More than half of the patients presented IDA, which was considered the most common clinical complication of AIG in pediatric patients. Otherwise, megaloblastic anemia was an uncommon presentation (in less than 10% of the patients) [2,12,13,14,16,17,18,19,20,21,22,23,24,25].
APCAs are found in more than two-thirds of the patients [2,12,13,14,16,17,18,20,21,23,24,25,26,27,28,29], while anti-IF antibodies are not always detected (<10% of patients, but not all the patients have been investigated with anti-IF) [2,12,13,20,21,23,25,27,28].
Seronegative AIG (negative APCA screening) was an even rarer clinical entity in children that has been described in eleven patients. Three of these patients had an IEI. One patient had a common variable immunodeficiency (CVID), one CVID plus immune-dysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome, and the last was a T-cell primary immunodeficiency [22,23,27]. Moreover, an IEI was identified in four out of twenty-three patients enrolled in a multicenter cohort [28]. Two children had a genetic mutation in the tumor necrosis factor alpha-induced protein 3 (TNFAIP3) and cytotoxic T lymphocyte antigen-4 (CTLA4) genes. In contrast, the other two had clinical and laboratory features of an IEI without a specific mutation. Interestingly, all of them had concomitant autoimmune comorbidities (i.e., autoimmune enteropathy, autoimmune hepatitis, thyroiditis, Evans’ syndrome, Addison’s disease, CeD, and lichen sclerosis) [16].
Endoscopic features were non-specific (e.g., mucosal erythema, softening of the mucosa, polyps/nodules) and often normal. The histological examination mainly revealed corpus-predominant chronic atrophic gastritis (detected in almost 60% of the patients reported). Still, different reports described more diffuse gastritis/pangastritis (detected in nearly 60% of the patients) [2,12,13,16,17,18,19,20,21,22,23,24]. Pseudopyloric and/or intestinal metaplasia was reported in about half of the patients [2,12,13,16,17,18,19,20,21,22,23,24,27]. The development of hypergastrinemia and achlorhydria was frequently reported during the follow-up, but only a minority (about 40%) of children with AIG presented ECL cell hyperplasia [13,14,16,17,18,19,20,21,23,24]. Moreover, about 25% of patients had a concurrent H. pylori infection [2,12,16,20,21,22,23,24]. Gastric adenocarcinomas and type I neuroendocrine tumors were rarely reported [2,12,13,18,19,21,22,24,27,29]. None of the enrolled children was treated with a specific therapy. However, they were often managed using iron and vitamin B12 supplementation [13,16,18,19,20].
5. Discussion
We reported two cases of pediatric-onset AIG showing different clinical, serological, and histological presentations.
The first case presented severe and refractory IDA, the most frequent manifestation of AIG in the pediatric age, as confirmed by the literature review [2,12]. IDA is much more common in females, implying that monthly menstrual blood loss may have a further role in its development, aggravated by the inability to increase food-iron absorption due to hypochlorhydria [2]. Our patient tested negative for APCA. Seronegative AIG (APCA negativity) occurs infrequently in pediatric patients with AIG, while it is more common in elderly patients [15,30]. Some patients could be seronegative, especially those with CVID and other IEIs [15,22,23,27,29]. AIG can rarely be associated with selective IgA deficiency [13,14,18]. PA has also been described in patients with other immunodeficiency syndromes, such as chromosome 18q deletion syndrome, X-linked hypogammaglobulinemia, Good syndrome, and CVID [3]. Daniels et al. reported that CVID could affect the GI tract with a broad spectrum of histologic patterns that can mimic lymphocytic gastritis [31]. Nonetheless, AIG could manifest in the context of other IEIs, like TNFAIP3 and CTLA4 deficiency, especially with concomitant other autoimmune manifestations [28]. Sometimes, AIG can be incidentally diagnosed during the screening of children with other autoimmune diseases, including T1DM and AITD [12,14,20,21,32]. Especially in patients with T1DM, there is a significant long-term risk of developing AIG [32]. Overall, the most sensitive serum test for AIG is the detection of APCA, but their absence does not exclude a diagnosis of AIG [30]. Even though detecting anti-IF, low pepsinogen, or high gastrinemia levels assists in diagnosing, their sensitivity and specificity are low and need to be integrated with endoscopic and histopathological findings [33].
In case 2, the diagnosis of AIG was incidental and concomitant to the EoE diagnosis. Familiar history is crucial to tailor a patient’s follow-up, particularly regarding neoplastic complications. Patient 2 did not show signs and symptoms of anemia or vitamin B12 deficiency. Pediatric-onset AIG should be considered in the case of refractory IDA and non-specific gastrointestinal disorders non-responsive to conventional treatments. A structured approach is mandatory to ensure comprehensive assessment and management (Figure 2).
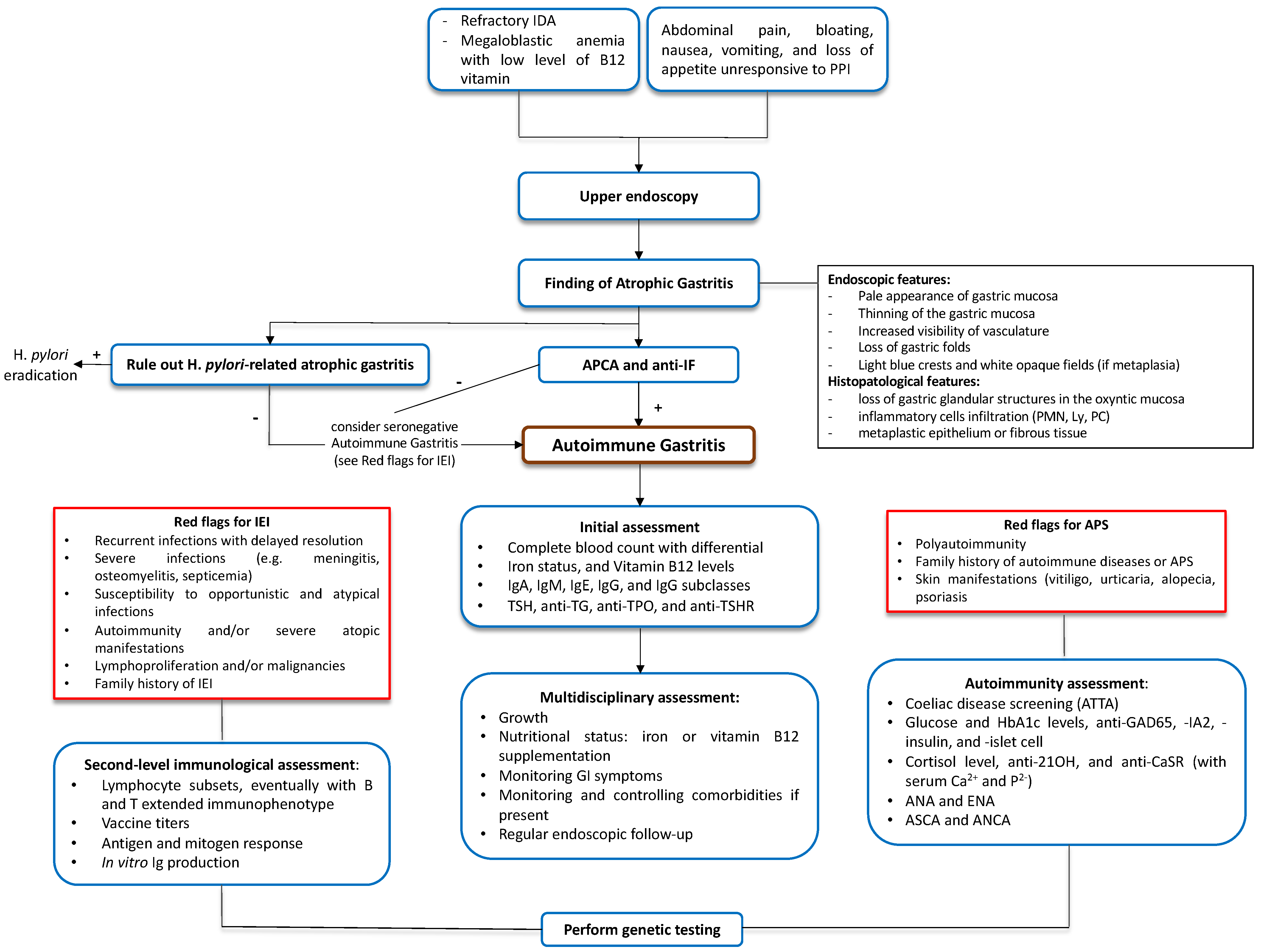
Figure 2.
Suggested diagnostic flowchart for pediatric autoimmune gastritis. ANA, anti-nuclear antibodies. ANCA, anti-neutrophil cytoplasmic antibodies. Anti-21OH, anti-21-hydroxylase antibodies. Anti-CaSR, anti-Calcium Sensing Receptor antibodies. Anti-GAD65, anti-glutamic acid decarboxylase 65-kilodalton isoform antibodies. Anti-IA2, anti-tyrosine phosphatase-related islet antigen two antibodies. Anti-IF, anti-intrinsic factor antibodies. Anti-TG, anti-thyroglobulin antibodies. Anti-TPO, anti-thyreoperoxidase antibodies. anti-TSHR, anti-thyroid-stimulating hormone receptor. APCA, anti-parietal cell antibodies. APS, autoimmune polyglandular syndrome. ASCA, anti-Saccharomyces cerevisiae antibodies. ATTA, Anti-Tissue Transglutaminase Antibodies. ENA, anti-extractable nuclear antigen antibodies. H. pylori, Helycobacter pylori. HbA1c, hemoglobin A1c. IDA, iron-deficiency anemia. IEI, inborn error of immunity. Ig, immunoglobulin. Ly, lymphocyte. PC, plasma cells. PMN, neutrophils. PPI, proton pump inhibitor. TSH, thyroid-stimulating hormone.
A correct serological and endoscopic investigation is needed to differentiate between H. pylori-related and autoimmune atrophic gastritis. A comprehensive laboratory assessment is required to evaluate potential AIG-associated conditions and specific warning signs for IEI and APS [2,12,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28]. Finally, a multidisciplinary approach is advised to ensure the optimal management of these patients.
Both our patients showed pathological intestinal eosinophilic inflammation and overlap with eosinophilic gastrointestinal disorder, presenting with non-specific symptoms. Ayaki et al. described a case of a 67-year-old patient with autoimmune polyendocrine syndrome type 2 (APS-2), with both AIG and EoE [34]. No pediatric cases of eosinophilic gastrointestinal diseases and AIG have been reported. The association between AIG and primary eosinophilic gastrointestinal disorders should be further investigated in adult and pediatric populations.
None of our patients had H. pylori infection, which should be ruled out when atrophic gastritis is found. Nevertheless, a relatively high prevalence of H. pylori is reported in young patients with AIG and microcytic anemia. In the adult model, the causative role of H. pylori through a molecular mimicry mechanism still needs to be demonstrated; therefore, no definitive correlations between AIG and H. pylori have been found to date [2,17]. Examples of atrophic pangastritis in H. pylori-negative patients, frequently associated with other autoimmune disorders, have been recognized. APCA and anti-IF antibodies have been reported in these cases. However, this type of gastritis is usually seen in autoimmune enteropathy and suggests a generalized autoimmune disorder of the gastrointestinal tract or other immunodeficiency disorders (congenital or acquired) [35].
From the genetic point of view, two regions on distal chromosome 4 conferred susceptibility to autoimmune gastritis (Gasa1 and Gasa2). Two minor autoimmune gastritis susceptibility loci have also been identified on chromosome 6 (Gasa3 and Gasa4). AIRE deficiency is also associated with AIG in mouse models and clinical studies on APECED patients (APS1). Of note, the etiology of the AIG that occurs in AIRE-deficient mice is different from that of other mouse models, as the autoantigen targeted is not the H+/K+ ATPase but rather mucin 6 [10]. CTLA4 and PTPN22 (APS-2), ATP4A, IL1B, IFNGR1, LRBA (IEI due to LRBA deficiency), RIPK1 (RIPK1-Induced Autoinflammatory syndrome), and several human leukocyte antigens (HLA) genes (B-8, B-18, Bw-15, DR-2, DR-4, DR-5, DRB1*03, DRB1*04) increase the risk of developing AIG [10].
The risk of gastric cancer is generally low in pediatric AIG [36]. Several risk factors have been proposed, including the entity of diagnostic delay and disease duration, severity of atrophy and metaplasia, type of metaplasia (complete or incomplete, intestinal or pseudopyloric), presence of epithelial dysplasia, concurrent or previous H. pylori infection, concurrent antrum atrophy (atrophic pangastritis), gastric microbiota alterations, and previous gastric surgery [15]. Segni et al. suggested performing upper GI endoscopy with multiple biopsies in patients with AITD, APCA positivity, and hypergastrinemia and endoscopically monitoring patients with AIG every five years, as indicated in the adult population. Moreover, since gastric autoimmunity may occur at any age, they suggested determining APCA every two years in APCA-negative patients with AITD [14]. Despite these findings, recent evidence shows the risk of developing gastric adenocarcinoma without H. pylori infection seems not to exceed those of the general population. Any previous or current H. pylori infection promotes pangastritis atrophy and is a cancer-promoting cofactor [37]. The same experts also agree that endoscopic surveillance should be recommended at 3–5 years intervals, which is more tailored for the early detection of NETs rather than for gastric cancer secondary prevention. However, only limited data are available [32].
Finally, although increasing knowledge about AIG is available, no specific treatment exists. Therefore, iron and vitamin B12 supplementation are the only therapeutic strategies to avoid nutritional and metabolic complications [15].
6. Conclusions
Pediatric onset AIG is a rare disease with a proteiform spectrum of clinical manifestations that range from incidental endoscopic findings to severe IDA. Diagnosis can be challenging and often delayed. Many comorbidities can be associated with pediatric AIG, including autoimmune diseases, IEI, or rarely, eosinophilic gastrointestinal disorders. The diagnostic workup of pediatric AIG needs a multistep approach and a multidisciplinary team that involves a gastroenterologist, endoscopist, pathologist, nutritionist, immunologist, and family pediatrician. A periodic follow-up and the early identification of potential complications (gastric cancer, autoimmune diseases, and immunodeficiencies) are pivotal for managing these children accurately.
Author Contributions
Methodology, I.T.; writing—original draft, I.T. and M.V.; visualization, I.T.; conceptualization, M.V.; writing—reviewing and editing, R.C., M.D.F., O.L., A.V., M.B. and A.R.; supervision, A.D.S., M.V.L., G.L.M. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
No financial or non-financial benefits have been received or will be obtained from any party related directly or indirectly to the subject of this article.
Institutional Review Board Statement
This study was conducted according to the Helsinki Declaration. All patients provided written informed consent. Due to the retrospective character of the case report and literature review, ethical review and approval were waived for this study. The Ethical Committee of Fondazione IRCCS Policlinico San Matteo approved this study (protocol number 3241/22, 15 December 2021).
Informed Consent Statement
Written informed consent was obtained from the legal guardians (patient’s parents) and the subject involved in the study and from his family. Moreover, the patient’s legal guardian(s) were involved in the manuscript’s development, and a draft was provided to them for review. Their feedback and suggestions have been incorporated into the final manuscript.
Data Availability Statement
The dataset generated and analyzed during the current study is available from the corresponding author upon reasonable request (ivan.taietti@gmail.com).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adamu, M.A.; Weck, M.N.; Gao, L.; Brenner, H. Incidence of chronic atrophic gastritis: Systematic review and meta-analysis of follow-up studies. Eur. J. Epidemiol. 2010, 25, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Saglietti, C.; Sciarra, A.; Abdelrahman, K.; Schneider, V.; Karpate, A.; Nydegger, A.; Sempoux, C. Autoimmune Gastritis in the Pediatric Age: An Underestimated Condition Report of Two Cases and Review. Front. Pediatr. 2018, 6, 123. [Google Scholar] [CrossRef]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis—Pathogenesis, pathology, and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Facciotti, F.; Miceli, E.; Vanoli, A.; Fornasa, G.; Lahner, E.; Spadoni, I.; Giuffrida, P.; Arpa, G.; Pasini, A.; et al. Mucosal Overexpression of Thymic Stromal Lymphopoietin and Proinflammatory Cytokines in Patients with Autoimmune Atrophic Gastritis. Clin. Transl. Gastroenterol. 2022, 13, e00510. [Google Scholar] [CrossRef]
- Lenti, M.V.; Rossi, C.M.; Melazzini, F.; Gastaldi, M.; Bugatti, S.; Rotondi, M.; Bianchi, P.I.; Gentile, A.; Chiovato, L.; Montecucco, C.; et al. Seronegative autoimmune diseases: A challenging diagnosis. Autoimmun. Rev. 2022, 21, 103143. [Google Scholar] [CrossRef]
- Toh, B.H.; van Driel, I.R.; Gleeson, P.A. Pernicious Anemia. N. Engl. J. Med. 1997, 337, 1441–1448. [Google Scholar] [CrossRef]
- Taylor, K.B.; Roitt, I.M.; Doniach, D.; Couchman, K.G.; Shapland, C. Autoimmune Phenomena in Pernicious Anaemia: Gastric Antibodies. Br. Med. J. 1962, 2, 1347–1352. [Google Scholar] [CrossRef]
- Taylor, K.B. Inhibition of intrinsic factor by pernicious anæmia sera. Lancet 1959, 2, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Faller, G.; Kirchner, T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005, 446, 1–9. [Google Scholar] [CrossRef]
- Hogan, T.V.; Ang, D.K.Y.; Gleeson, P.A.; van Driel, I.R. Extrathymic mechanisms of T cell tolerance: Lessons from autoimmune gastritis. J. Autoimmun. 2008, 31, 268–273. [Google Scholar] [CrossRef]
- Lehy, T.; Roucayrol, A.M.; Mignon, M. Histomorphological characteristics of gastric mucosa in patients with Zollinger-Ellison syndrome or autoimmune gastric atrophy: Role of gastrin and atrophying gastritis. Microsc. Res. Tech. 2000, 48, 327–338. [Google Scholar] [CrossRef]
- Gonçalves, C.; Oliveira, M.E.; Palha, A.M.; Ferrão, A.; Morais, A.; Lopes, A.I. Autoimmune gastritis presenting as iron deficiency anemia in childhood. World J. Gastroenterol. 2014, 20, 15780–15786. [Google Scholar] [CrossRef] [PubMed]
- Mitsinikos, T.; Shillingford, N.; Cynamon, H.; Bhardwaj, V. Autoimmune Gastritis in Pediatrics: A Review of 3 Cases. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 252–257. [Google Scholar] [CrossRef]
- Segni, M.; Borrelli, O.; Pucarelli, I.; Delle Fave, G.; Pasquino, A.M.; Annibale, B. Early manifestations of gastric autoimmunity in patients with juvenile autoimmune thyroid diseases. J. Clin. Endocrinol. Metab. 2004, 89, 4944–4948. [Google Scholar] [CrossRef]
- Lahner, E.; Zagari, R.M.; Zullo, A.; Di Sabatino, A.; Meggio, A.; Cesaro, P.; Lenti, M.V.; Annibale, B.; Corazza, G.R. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig. Liver Dis. 2019, 51, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Miguel, N.; Costa, E.; Santalha, M., Jr.; Lima, R.; Vizcaino, J.R.; Pereira, F.; Barbot, J. Refractory iron-deficiency anemia and autoimmune atrophic gastritis in pediatric age group: Analysis of 8 clinical cases. J. Pediatr. Hematol. Oncol. 2014, 36, 134–139. [Google Scholar] [CrossRef]
- Moreira-Silva, H.; Silva, G.; Costa, E.; Guerra, I.; Santos-Silva, E.; Tavares, M.; Cleto, E.; Lima, R. Insights into Pediatric Autoimmune Gastritis: Is There a Role for Helicobacter pylori Infection? J. Pediatr. Gastroenterol. Nutr. 2019, 68, e99–e104. [Google Scholar] [CrossRef]
- Kirsaclioglu, C.T.; Kuloglu, Z.; Kansu, A.; Ensari, A.; Siklar, Z.; Berberoğlu, M.; Ocal, G. Gastric carcinoid tumor in a 14-year-old girl. Scand. J. Gastroenterol. 2014, 49, 1391–1393. [Google Scholar] [CrossRef]
- Katz, S.; Berernheim, J.; Kaufman, Z.; Lazar, L.; Erez, I.; Wolach, B. Pernicious anemia and adenocarcinoma of the stomach in an adolescent: Clinical presentation and histopathology. J. Pediatr. Surg. 1997, 32, 1384–1385. [Google Scholar] [CrossRef]
- Greenwood, D.L.; Crock, P.; Braye, S.; Davidson, P.; Sentry, J.W. Autoimmune gastritis and parietal cell reactivity in two children with abnormal intestinal permeability. Eur. J. Pediatr. 2008, 167, 917–925. [Google Scholar] [CrossRef]
- Fröhlich-Reiterer, E.E.; Huber, J.; Katz, H.; Suppan, E.; Obermayer-Pietsch, B.; Deutschmann, A.; Demel, U.; Acham-Roschitz, B.; Weinhandl, G.; Ambros-Rudolph, C.M.; et al. Do children and adolescents with type 1 diabetes mellitus have a higher frequency of parietal cell antibodies than healthy controls? J. Pediatr. Gastroenterol. Nutr. 2011, 52, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.C.; Black, J.O.; Schwartz, D.A.; Correa, H.; Rosen, M.J. 15-year-old Girl with Metaplastic Atrophic Gastritis and Enterochromaffin-like Cell Hyperplasia. J. Pediatr. Gastroenterol. Nutr. 2012, 55, e148–e151. [Google Scholar] [CrossRef]
- Pogoriler, J.; Kamin, D.; Goldsmith, J.D. Pediatric non-Helicobacter pylori atrophic gastritis: A case series. Am. J. Surg. Pathol. 2015, 39, 786–792. [Google Scholar] [CrossRef]
- Koca, T.; Dereci, S.; Karahan, N.; Akcam, M. Gastrointestinal Neuroendocrine Tumors in Two Children. Indian. Pediatr. 2016, 53, 70–72. [Google Scholar]
- Besançon, A.; Michaud, B.; Beltrand, J.; Goncalves, T.; Jais, J.P.; Polak, M.; Chatenoud, L.; Robert, J.J. Revisiting autoimmune gastritis in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2017, 18, 772–776. [Google Scholar] [CrossRef]
- Calcaterra, V.; Montalbano, C.; Miceli, E.; Luinetti, O.; Albertini, R.; Vinci, F.; Regalbuto, C.; Larizza, D. Anti-gastric parietal cell antibodies for autoimmune gastritis screening in juvenile autoimmune thyroid disease. J. Endocrinol. Investig. 2020, 43, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kulak, O.; Gurram, B.; Montgomery, E.A.; Park, J.Y. Pediatric autoimmune gastritis: Clinical correlates and histologic features. Hum. Pathol. 2021, 116, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Granot, M.; Beinvogl, B.C.; Schvimer, M.; Goldsmith, J.D.; Matar, M.; Ben Tov, A.; Feler, A.Y.; Nachum, N.; Morgenstern, S.; Mayer, C.; et al. Clinical characteristics and outcomes of pediatric patients with autoimmune gastritis. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.M.; Berberoğlu Ateş, B.; Hızal, G.; Yaman, A.; Tuna Kırsaçlıoğlu, C.; Oğuz, A.S.; Karakuş, E.; Yaralı, N.; Özbek, N.Y. Autoimmune atrophic gastritis: The role of Helicobacter pylori infection in children. Helicobacter 2020, 25, e12716. [Google Scholar] [CrossRef]
- Conti, L.; Lenti, M.V.; Di Sabatino, A.; Miceli, E.; Galli, G.; Cazzato, M.; Falangone, F.; Annibale, B.; Lahner, E. Seronegative autoimmune atrophic gastritis is more common in elderly patients. Dig. Liver Dis. 2020, 52, 1310–1314. [Google Scholar] [CrossRef]
- Daniels, J.A.; Lederman, H.M.; Maitra, A.; Montgomery, E.A. Gastrointestinal Tract Pathology in Patients with Common Variable Immunodeficiency (CVID): A Clinicopathologic Study and Review. Am. J. Surg. Pathol. 2007, 31, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Genta, R.M.; Malfertheiner, P.; Dinis-Ribeiro, M.; El-Serag, H.; Graham, D.Y.; Kuipers, E.J.; Leung, W.K.; Park, J.Y.; Rokkas, T.; et al. RE.GA.IN.: The Real-world Gastritis Initiative-updating the updates. Gut 2024, 73, 407–441. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, J.; Bertilsson, R.; Bülow, E.; Carlsson, S.; Åkesson, S.; Eliasson, B.; Hanas, R.; Åkesson, K. Autoimmune comorbidity in type 1 diabetes and its association with metabolic control and mortality risk in young people: A population-based study. Diabetologia 2024, 67, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Manabe, N.; Fujita, M.; Nakamura, J.; Sunago, A.; Kamada, T.; Haruma, K. A case of eosinophilic esophagitis with autoimmune polyendocrine syndrome type 2, including autoimmune gastritis. Clin. J. Gastroenterol. 2021, 14, 460–465. [Google Scholar] [CrossRef]
- Srivastava, A.; Lauwers, G.Y. Pathology of non-infective gastritis. Histopathology 2007, 50, 15–29. [Google Scholar] [CrossRef]
- Miceli, E.; Lenti, M.V.; Gentile, A.; Gambini, G.; Petrucci, C.; Pitotti, L.; Mengoli, C.; Di Stefano, M.; Vanoli, A.; Luinetti, O.; et al. Long-Term Natural History of Autoimmune Gastritis: Results from a Prospective Monocentric Series. Am. J. Gastroenterol. 2024, 119, 837–845. [Google Scholar] [CrossRef]
- Rugge, M.; Fassan, M.; Pizzi, M.; Graham, D.Y. Letter: Gastric cancer and pernicious anaemia--often Helicobacter pylori in disguise. Aliment. Pharmacol. Ther. 2013, 37, 764–765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).