Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in the Community of Pathumthani, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Outcomes
2.3. Statistical Analysis
3. Results
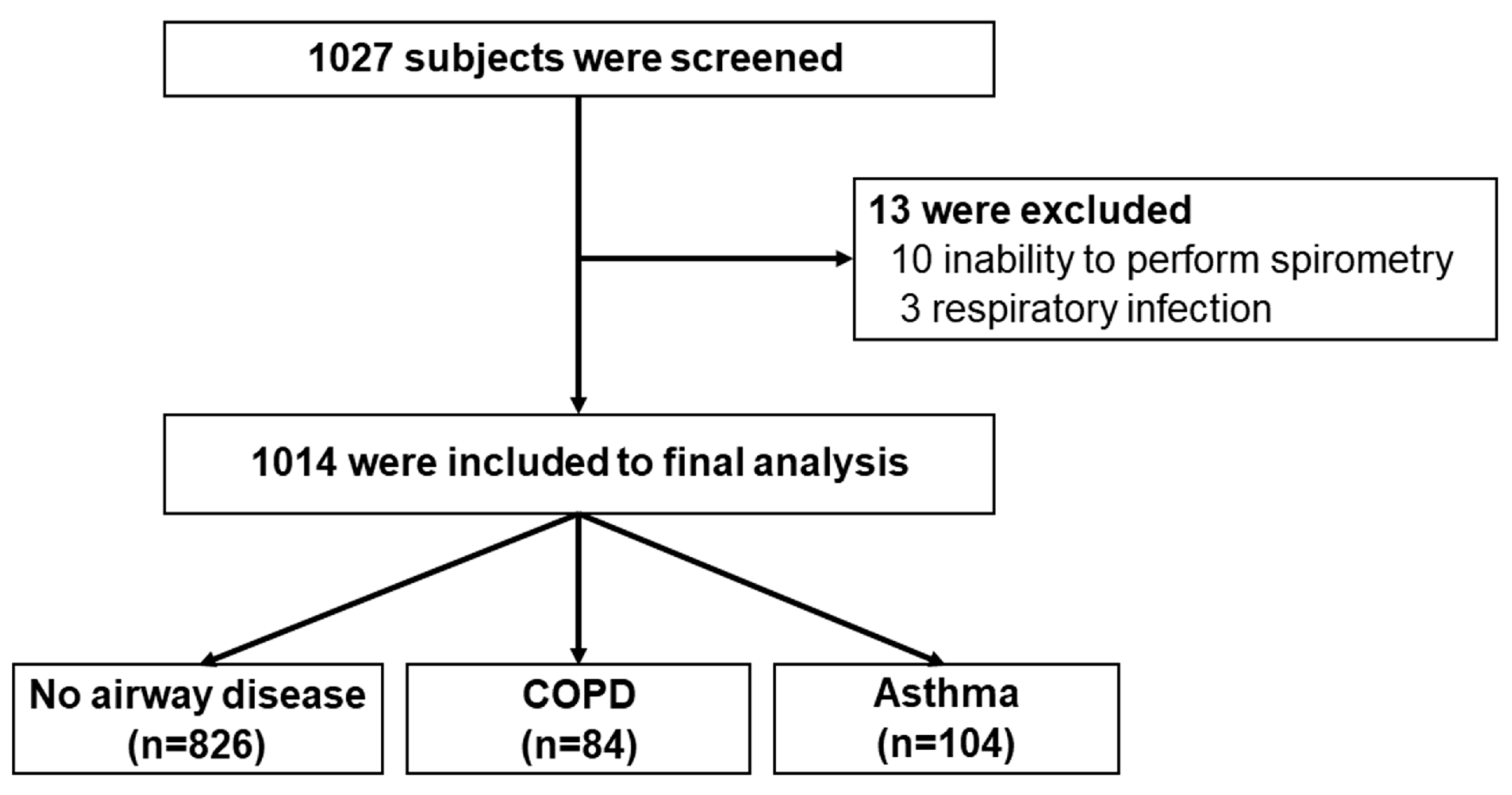
3.1. Participants
3.2. Prevalence of COPD and Asthma
3.3. Factors Associated with COPD and Asthma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cukic, V.; Lovre, V.; Dragisic, D.; Ustamujic, A. Asthma and chronic obstructive pulmonary disease (COPD)—Differences and similarities. Mater. Sociomed. 2012, 24, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Surit, P.; Wongtanasarasin, W.; Boonnag, C.; Wittayachamnankul, B. Association between air quality index and effects on emergency department visits for acute respiratory and cardiovascular diseases. PLoS ONE 2023, 18, e0294107. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 exposure and asthma development: The key role of oxidative stress. Oxid. Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J.; Chojenta, C.; Byles, J.E. Air pollution exposures from multiple point sources and risk of incident chronic obstructive pulmonary disease (COPD) and asthma. Environ. Res. 2019, 179, 108783. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease 2024 Report. Available online: http://goldcopd.org (accessed on 2 January 2024).
- Mannino, D.M.; Buist, A.S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef]
- Al Wachami, N.; Guennouni, M.; Iderdar, Y.; Boumendil, K.; Arraji, M.; Mourajid, Y.; Bouchachi, F.Z.; Barkaoui, M.; Louerdi, M.L.; Hilali, A.; et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. BMC Public Health 2024, 24, 297. [Google Scholar] [CrossRef]
- Saiphoklang, N.; Tirakitpanich, K. Correlation between handgrip strength and air trapping in patients with stable chronic obstructive pulmonary disease. J. Thorac. Dis. 2024, 16, 5634–5642. [Google Scholar] [CrossRef]
- Mannino, D.M.; Higuchi, K.; Yu, T.C.; Zhou, H.; Li, Y.; Tian, H.; Suh, K. Economic burden of COPD in the presence of comorbidities. Chest 2015, 148, 138–150. [Google Scholar] [CrossRef]
- Lopez-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Song, P.; Adeloye, D.; Salim, H.; Dos Santos, J.P.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of asthma in 2019: A systematic analysis and modelling study. J. Glob. Health 2022, 12, 04052. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2023 Update. Available online: https://ginasthma.org/ (accessed on 2 January 2024).
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Maranetra, K.N.; Chuaychoo, B.; Dejsomritrutai, W.; Chierakul, N.; Nana, A.; Lertakyamanee, J.; Naruman, C.; Suthamsmai, T.; Sangkaew, S.; Sreelum, W.; et al. The prevalence and incidence of COPD among urban older persons of Bangkok Metropolis. J. Med. Assoc. Thai 2002, 85, 1147–1155. [Google Scholar] [PubMed]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Mullerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Baan, E.J.; de Roos, E.W.; Engelkes, M.; de Ridder, M.; Pedersen, L.; Berencsi, K.; Prieto-Alhambra, D.; Lapi, F.; Van Dyke, M.K.; Rijnbeek, P.; et al. Characterization of asthma by age of onset: A multi-database cohort study. J. Allergy Clin. Immunol. Pract. 2022, 10, 1825–1834.e1828. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Phetsuk, N.; Pisalthanapuna, S.; Chetsadaphan, N.; Inchai, J. A comparative study of COPD burden between urban vs rural communities in northern Thailand. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Zhang, B.; Ni, M.; Kumari, S.; Bauermeister, S.; Gallacher, J.; Webster, C. Environmental correlates of chronic obstructive pulmonary disease in 96 779 participants from the UK Biobank: A cross-sectional, observational study. Lancet Planet. Health 2019, 3, e478–e490. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.J.; Lee, C.H.; Lee, C.H.; Lee, H.W. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Environ. Res. 2021, 194, 110703. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Wu, C.D.; Chung, M.C.; Shen, T.C.; Lai, T.J.; Chen, C.Y.; Wang, R.Y.; Chung, C.J. The effects of traffic-related air pollutants on chronic obstructive pulmonary disease in the community-based general population. Respir. Res. 2021, 22, 217. [Google Scholar] [CrossRef]
- Doiron, D.; de Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Aaron, C.P.; Madrigano, J.; Hoffman, E.A.; Angelini, E.; Yang, J.; Laine, A.; Vetterli, T.M.; Kinney, P.L.; Sampson, P.D.; et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA 2019, 322, 546–556. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, T.; Zhang, Y.; Chen, H.; Sang, S. Global burden of COPD attributable to ambient PM2.5 in 204 countries and territories, 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Sci. Total Environ. 2021, 796, 148819. [Google Scholar] [CrossRef]
- Aungkulanon, S.; Pitayarangsarit, S.; Bundhamcharoen, K.; Akaleephan, C.; Chongsuvivatwong, V.; Phoncharoen, R.; Tangcharoensathien, V. Smoking prevalence and attributable deaths in Thailand: Predicting outcomes of different tobacco control interventions. BMC Public Health 2019, 19, 984. [Google Scholar] [CrossRef]
- Boonsawat, W.; Charoenphan, P.; Kiatboonsri, S.; Wongtim, S.; Viriyachaiyo, V.; Pothirat, C.; Thanomsieng, N. Survey of asthma control in Thailand. Respirology 2004, 9, 373–378. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of air pollution on asthma outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Ratanachina, J.; Amaral, A.; De Matteis, S.; Cullinan, P.; Burney, P. Farming, pesticide exposure and respiratory health: A cross-sectional study in Thailand. Occup. Environ. Med. 2022, 79, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Skloot, G.S. The effects of aging on lung structure and function. Clin. Geriatr. Med. 2017, 33, 447–457. [Google Scholar] [CrossRef]
- Buist, A.S.; Vollmer, W.M.; McBurnie, M.A. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int. J. Tuberc. Lung Dis. 2008, 12, 703–708. [Google Scholar]
- Kotlyarov, S. The role of smoking in the mechanisms of development of chronic obstructive pulmonary disease and atherosclerosis. Int. J. Mol. Sci. 2023, 24, 8725. [Google Scholar] [CrossRef]
- Aisanov, Z.; Khaltaev, N. Management of cardiovascular comorbidities in chronic obstructive pulmonary disease patients. J. Thorac. Dis. 2020, 12, 2791–2802. [Google Scholar] [CrossRef]
- Cui, Y.; Zhan, Z.; Ma, Y.; Huang, K.; Liang, C.; Mao, X.; Zhang, Y.; Ren, X.; Lei, J.; Chen, Y.; et al. Clinical and economic burden of comorbid coronary artery disease in patients with acute exacerbation of chronic obstructive pulmonary disease: Sex differences in a nationwide cohort study. Respir. Res. 2022, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Bollmeier, S.G.; Hartmann, A.P. Management of chronic obstructive pulmonary disease: A review focusing on exacerbations. Am. J. Health Syst. Pharm. 2020, 77, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular comorbidities in chronic obstructive pulmonary disease (COPD)-current considerations for clinical practice. J. Clin. Med. 2019, 8, 69. [Google Scholar] [CrossRef]
- Tochino, Y.; Asai, K.; Shuto, T.; Hirata, K. Asthma-COPD overlap syndrome-Coexistence of chronic obstructive pulmonary disease and asthma in elderly patients and parameters for their differentiation. J. Gen. Fam. Med. 2017, 18, 5–11. [Google Scholar] [CrossRef]
- Alrasheedi, S.M.; Alkhalifah, K.M.; Alnasyan, S.; Alwattban, R.R.; Alsubhi, R.A.; Alsamani, R.I.; Alfouzan, Y.A. The prevalence and impact of allergic rhinitis on asthma exacerbations in asthmatic adult patients in the Qassim region of Saudi Arabia: A cross-sectional study. Cureus 2023, 15, e44997. [Google Scholar] [CrossRef] [PubMed]
- Sriprasart, T.; Saiphoklang, N.; Kawamatawong, T.; Boonsawat, W.; Mitthamsiri, W.; Chirakalwasan, N.; Chiewchalermsri, C.; Athipongarporn, A.; Kamalaporn, H.; Kornthatchapong, K.; et al. Allergic rhinitis and other comorbidities associated with asthma control in Thailand. Front. Med. 2023, 10, 1308390. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 1014) | No Airway Disease (n = 826) | Airway Disease (n = 188) | p-Value |
|---|---|---|---|---|
| Age, years | 56.6 ± 13.3 | 55.6 ± 12.8 | 61.0 ± 14.7 | <0.001 |
| Female | 727 (71.7) | 607 (73.5) | 120 (63.8) | 0.008 |
| Male | 287 (28.3) | 219 (26.5) | 68 (36.2) | 0.008 |
| Body mass index, kg/m2 | 25.1 ± 4.6 | 25.2 ± 4.5 | 24.5 ± 4.8 | 0.049 |
| Smoking (current or former) | 105 (10.4) | 79 (9.6) | 26 (13.8) | <0.001 |
| Amount of smoking, pack-years | 13.4 ± 16.4 | 11.7 ± 13.5 | 17.3 ± 21.1 | 0.128 |
| Fuel, hours per year | 9982.7 ± 10,345.2 | 9959.3 ± 10,217.3 | 10,090.6 ± 10,948.3 | 0.887 |
| Occupations | ||||
| Government officer | 120 (11.8) | 104 (12.6) | 16 (8.5) | 0.118 |
| Farmer | 89 (8.8) | 73 (8.8) | 16 (8.5) | 0.886 |
| Merchant | 227 (22.4) | 201 (24.3) | 26 (13.8) | 0.002 |
| General worker | 150 (14.8) | 130 (15.7) | 20 (10.6) | 0.075 |
| Others | 81 (8.0) | 67 (8.1) | 14 (7.4) | 0.823 |
| Unemployed | 347 (34.2) | 251 (30.4) | 96 (51.1) | <0.001 |
| Preexisting comorbidities | ||||
| Hypertension | 330 (32.5) | 254 (30.4) | 76 (40.4) | 0.011 |
| Hyperlipidemia | 267 (26.3) | 207 (25.1) | 60 (31.9) | 0.054 |
| Diabetes | 152 (15.0) | 119 (14.4) | 33 (17.6) | 0.275 |
| Coronary heart disease | 35 (3.5) | 18 (2.2) | 17 (9.0) | <0.001 |
| Cerebrovascular disease | 10 (1.0) | 7 (0.8) | 3 (1.6) | 0.405 |
| Obesity | 23 (2.3) | 15 (1.8) | 8 (4.3) | 0.055 |
| Allergic rhinitis | 120 (11.8) | 96 (11.6) | 24 (12.8) | 0.661 |
| Asthma | 27 (2.7) | 0 (0) | 27 (14.7) | <0.001 |
| COPD | 9 (0.9) | 0 (0) | 9 (4.8) | <0.001 |
| Respiratory symptoms | 379 (37.4) | 280 (33.9) | 99 (52.7) | <0.001 |
| Cough | 187 (18.4) | 133 (16.1) | 54 (28.7) | <0.001 |
| Sputum production | 147 (14.5) | 110 (13.3) | 37 (19.7) | 0.025 |
| Breathlessness | 101 (10.0) | 64 (7.7) | 37 (19.7) | <0.001 |
| Wheezes | 21 (2.1) | 8 (1.0) | 13 (6.9) | <0.001 |
| Chest tightness | 39 (3.8) | 31 (3.8) | 8 (4.3) | 0.747 |
| Runny nose | 69 (6.8) | 44 (5.3) | 25 (13.3) | <0.001 |
| Nasal obstruction | 68 (6.7) | 53 (6.4) | 15 (8.0) | 0.440 |
| Sore throat | 34 (3.4) | 26 (3.1) | 8 (4.3) | 0.446 |
| Dyspnea on exertion | 42 (4.1) | 32 (3.9) | 10 (5.3) | 0.369 |
| History of respiratory treatment and cost | ||||
| Previous treatment of dyspnea | 59 (5.8) | 22 (2.7) | 37 (19.7) | <0.001 |
| Prior ED visit in the past year | 31 (3.1) | 19 (2.3) | 12 (6.4) | 0.003 |
| Treatment cost, USD in the past year (n = 5) | 822 ± 947 | 680 ± 822 | 1388 | 0.582 |
| Parameters | Total | Healthy | COPD | Asthma | p-Value |
|---|---|---|---|---|---|
| Number of subjects, n (%) | 1014 (100) | 826 (81.5) | 84 (8.3) | 104 (10.3) | NA |
| FVC, L | 2.60 ± 0.71 | 2.63 ± 0.68 | 2.86 ± 0.88 | 2.15 ± 0.67 | <0.001 |
| FVC, %predicted | 94.2 ± 15.7 | 94.7 ± 14.3 | 100.4 ± 19.0 | 85.7 ± 19.8 | <0.001 |
| FVC improvement after BDR test, % | 1.2 ± 6.9 | −0.1 ± 4.5 | −0.2 ± 6.9 | 12.8 ± 12.1 | <0.001 |
| FEV1, L | 2.11 ± 0.58 | 2.19 ± 0.55 | 1.95 ± 0.63 | 1.65 ± 0.51 | <0.001 |
| FEV1, %predicted | 93.6 ± 16.0 | 96.0 ± 14.1 | 84.7 ± 17.1 | 81.7 ± 20.8 | <0.001 |
| FEV1 improvement after BDR test, % | 3.5 ± 6.1 | 2.1 ± 3.6 | 4.3 ± 4.8 | 15.0 ± 10.9 | <0.001 |
| FEV1/FVC, % | 81.7 ± 7.8 | 83.5 ± 5.5 | 68.2 ± 6.3 | 77.9 ± 11.4 | <0.001 |
| FEF25-75, L/s | 2.11 ± 0.92 | 2.30 ± 0.86 | 1.10 ± 0.54 | 1.46 ± 0.89 | <0.001 |
| FEF25-75, %predicted | 85.2 ± 33.6 | 92.0 ± 30.6 | 44.1 ± 15.2 | 65.0 ± 35.2 | <0.001 |
| BDR | 90 (8.9) | 0 (0) | 5 (6.0) | 85 (81.7) | <0.001 |
| Variables | Odds Ratio (95% CI) | p-Value |
| Age for every 1-year increase | 1.023 (1.007–1.039) | 0.004 |
| Smoking | 2.247 (1.068–4.728) | 0.033 |
| Coronary heart disease | 2.709 (1.250–5.873) | 0.012 |
| Wheezing | 3.128 (1.109–8.824) | 0.031 |
| Runny nose | 1.911 (1.050–3.477) | 0.034 |
| Previous treatment of dyspnea | 6.749 (3.670–12.409) | <0.001 |
| Variables | COPD (n = 84) | Asthma (n = 104) | p-Value |
| Age, years | 60.9 ± 13.8 | 61.2 ± 15.4 | 0.880 |
| Female | 42 (50.0) | 78 (75.0) | <0.001 |
| Male | 42 (50.0) | 26 (25.0) | <0.001 |
| Body mass index, kg/m2 | 24.6 ± 4.6 | 24.4 ± 4.9 | 0.803 |
| Smoking (current or former) | 19 (22.6) | 7 (6.7) | <0.001 |
| Amount of smoking, pack-years | 18.3 ± 19.3 | 14.6 ± 26.8 | 0.635 |
| Fuel, hours per year | 9666.7 ± 13,625.2 | 10,434.7 ± 8232.5 | 0.667 |
| Occupations | |||
| Government officer | 8 (9.5) | 8 (7.7) | 0.655 |
| Farmer | 12 (11.5) | 4 (4.8) | 0.098 |
| Others | 30 (35.7) | 29 (27.9) | 0.250 |
| Unemployed | 41 (48.8) | 55 (52.9) | 0.480 |
| Preexisting comorbidities | |||
| Hypertension | 31 (36.9) | 45 (43.3) | 0.377 |
| Hyperlipidemia | 22 (26.2) | 38 (36.5) | 0.130 |
| Diabetes | 15 (17.9) | 18 (17.3) | 0.922 |
| Coronary heart disease | 8 (9.5) | 9 (8.7) | 0.836 |
| Cerebrovascular disease | 0 (0) | 3 (2.9) | 0.167 |
| Obesity | 1 (1.2) | 7 (6.7) | 0.061 |
| Allergic rhinitis | 12 (14.3) | 12 (11.5) | 0.575 |
| Asthma | 2 (2.4) | 25 (24.0) | <0.001 |
| COPD | 8 (9.5) | 1 (1.0) | 0.006 |
| Respiratory symptoms | 41 (48.8) | 58 (55.8) | 0.342 |
| Cough | 21 (25.0) | 33 (31.7) | 0.311 |
| Sputum production | 17 (20.2) | 20 (19.2) | 0.863 |
| Breathlessness | 11 (13.1) | 26 (25.0) | 0.041 |
| Wheezes | 5 (6.0) | 8 (7.7) | 0.640 |
| Chest tightness | 3 (3.6) | 5 (4.8) | 0.733 |
| Runny nose | 9 (10.7) | 16 (15.4) | 0.348 |
| Nasal obstruction | 7 (8.3) | 8 (7.7) | 0.872 |
| Sore throat | 4 (4.8) | 4 (3.8) | 0.757 |
| Dyspnea on exertion | 3 (3.6) | 7 (6.7) | 0.516 |
| History of respiratory treatment and cost | |||
| Previous treatment of dyspnea | 14 (16.7) | 23 (22.1) | 0.350 |
| Prior ED visit in the past year | 4 (4.8) | 8 (7.7) | 0.414 |
| Spirometry data | |||
| FVC, L | 2.86 ± 0.88 | 2.15 ± 0.67 | <0.001 |
| FVC, %predicted | 100.4 ± 19.0 | 85.7 ± 19.8 | <0.001 |
| FVC improvement after BDR test, % | −0.1 ± 6.9 | 12.8 ± 12.1 | <0.001 |
| FEV1, L | 1.95 ± 0.63 | 1.65 ± 0.51 | 0.001 |
| FEV1, %predicted | 84.7 ± 17.1 | 81.7 ± 20.8 | 0.027 |
| FEV1 improvement after BDR test, % | 4.3 ± 4.8 | 15.0 ± 10.9 | <0.001 |
| FEV1/FVC, % | 68.2 ± 6.3 | 77.9 ± 11.4 | <0.001 |
| FEF25-75, L/s | 1.10 ± 0.54 | 1.46 ± 0.89 | 0.001 |
| FEF25-75, %predicted | 44.1 ± 15.2 | 65.0 ± 35.2 | <0.001 |
| BDR | 5 (6.0) | 85 (81.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiphoklang, N.; Ruchiwit, P.; Kanitsap, A.; Tantiyavarong, P.; Vatcharavongvan, P.; Palungrit, S.; Leelasittikul, K.; Pugongchai, A.; Poachanukoon, O. Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in the Community of Pathumthani, Thailand. Diseases 2025, 13, 130. https://doi.org/10.3390/diseases13050130
Saiphoklang N, Ruchiwit P, Kanitsap A, Tantiyavarong P, Vatcharavongvan P, Palungrit S, Leelasittikul K, Pugongchai A, Poachanukoon O. Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in the Community of Pathumthani, Thailand. Diseases. 2025; 13(5):130. https://doi.org/10.3390/diseases13050130
Chicago/Turabian StyleSaiphoklang, Narongkorn, Pitchayapa Ruchiwit, Apichart Kanitsap, Pichaya Tantiyavarong, Pasitpon Vatcharavongvan, Srimuang Palungrit, Kanyada Leelasittikul, Apiwat Pugongchai, and Orapan Poachanukoon. 2025. "Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in the Community of Pathumthani, Thailand" Diseases 13, no. 5: 130. https://doi.org/10.3390/diseases13050130
APA StyleSaiphoklang, N., Ruchiwit, P., Kanitsap, A., Tantiyavarong, P., Vatcharavongvan, P., Palungrit, S., Leelasittikul, K., Pugongchai, A., & Poachanukoon, O. (2025). Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in the Community of Pathumthani, Thailand. Diseases, 13(5), 130. https://doi.org/10.3390/diseases13050130







