Effect of Different Exercise Interventions on Grip Strength, Knee Extensor Strength, Appendicular Skeletal Muscle Index, and Skeletal Muscle Index Strength in Patients with Sarcopenia: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search
2.2. Eligibility Criteria
2.2.1. Inclusion
2.2.2. Exclusion
2.3. Data Extraction
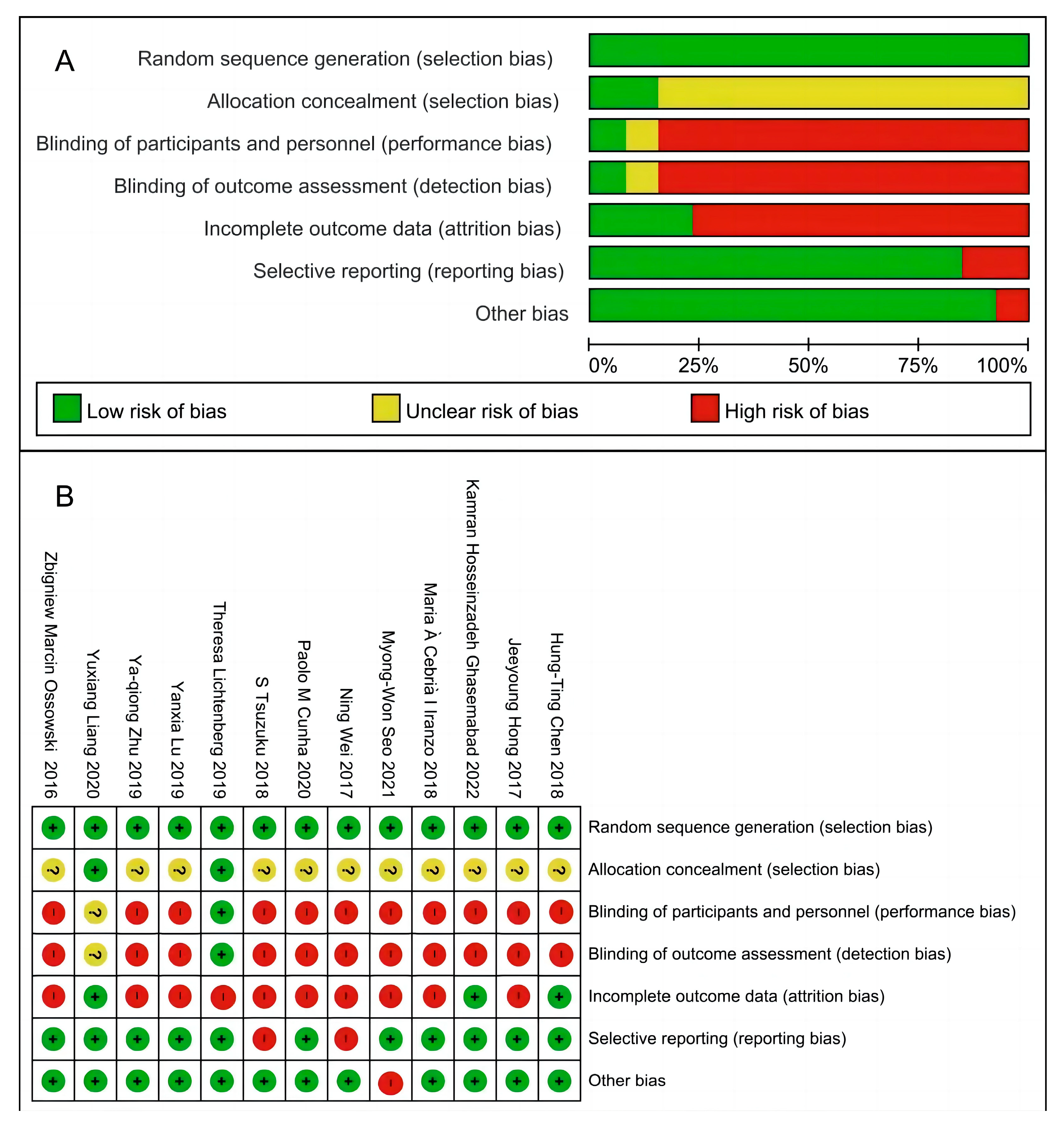
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
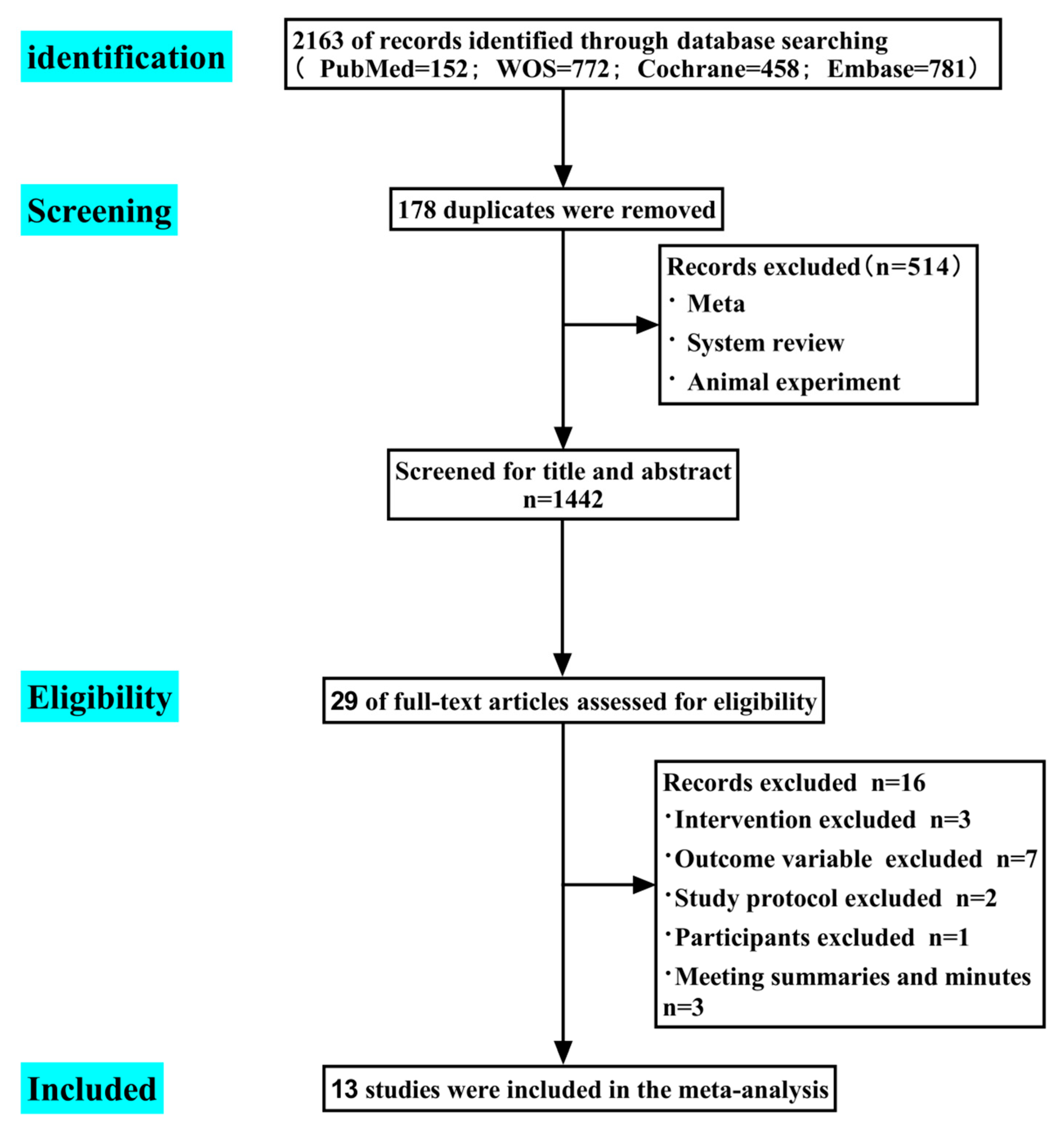
3. Results
3.1. Review Characteristics
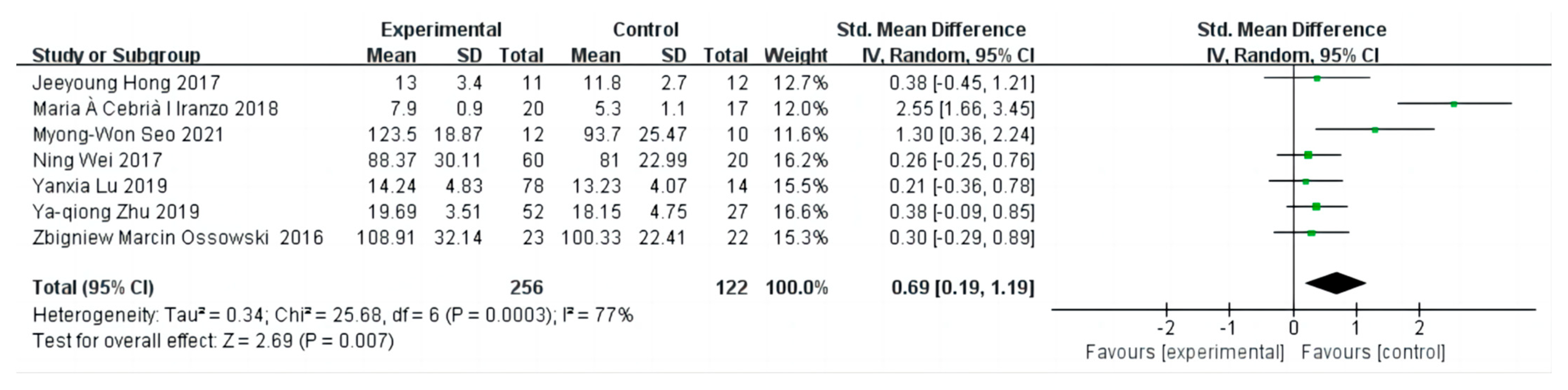
3.2. The Effect on Grip Strength
3.3. Sensitivity Analysis and Publication Bias
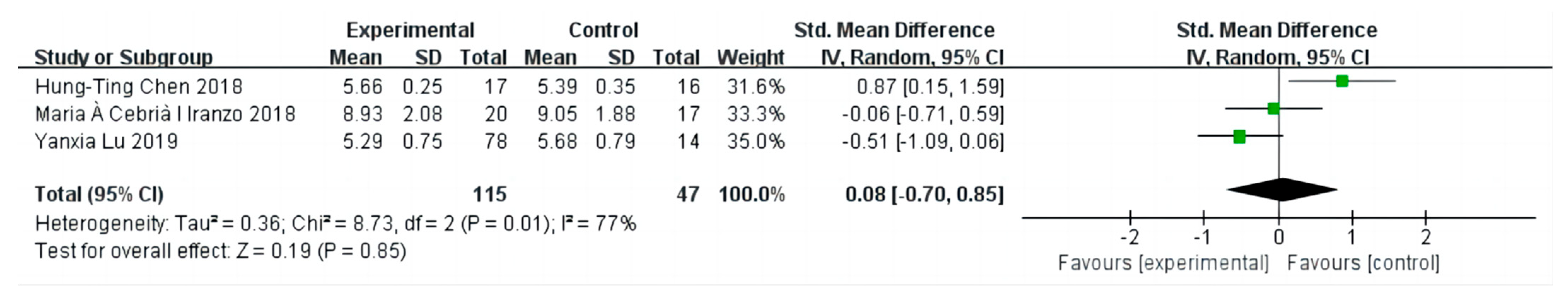
3.4. The Effect on Knee Extensor Strength
3.5. Sensitivity Analysis and Publication Bias
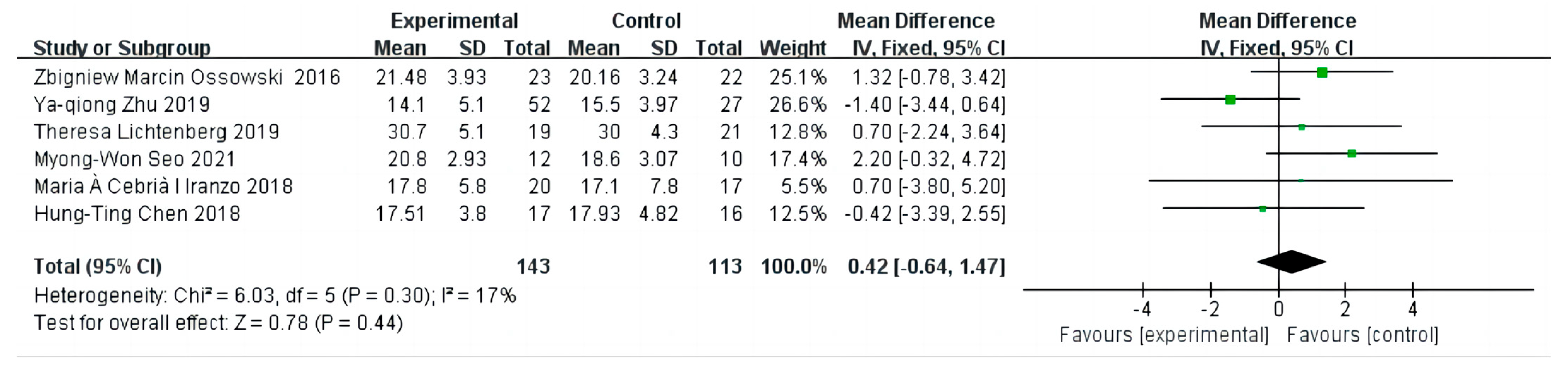
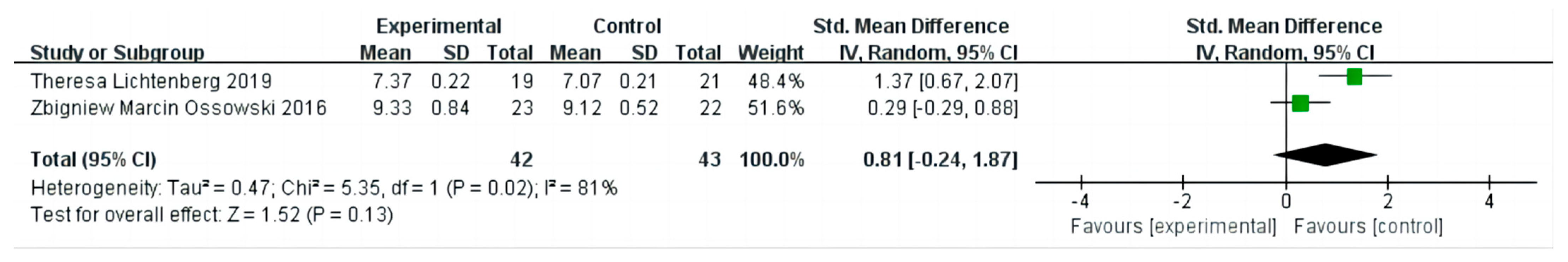
3.6. The Effect on Appendicular Skeletal Muscle Index
3.7. Sensitivity Analysis and Publication Bias
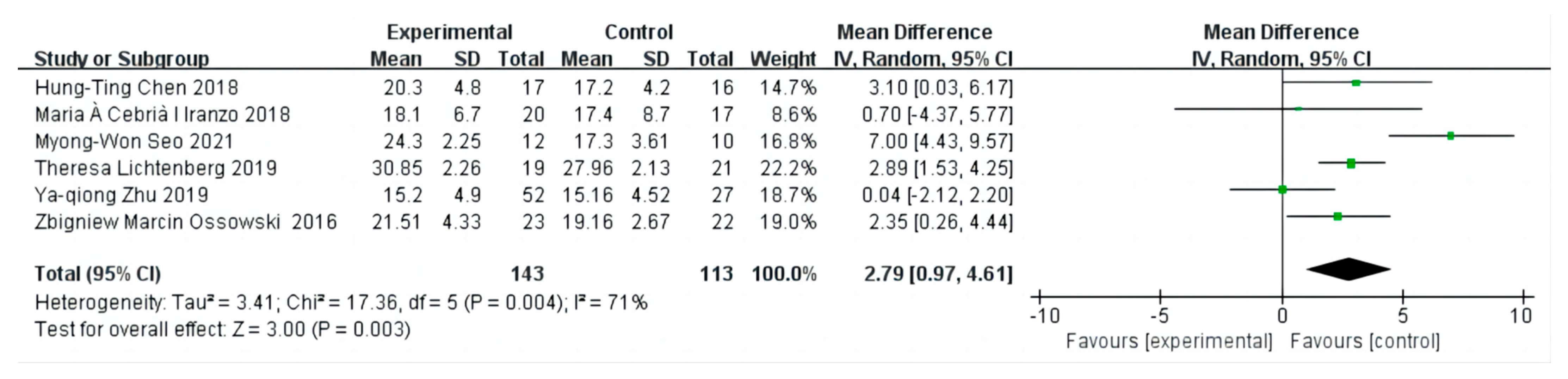
3.8. The Effect on Skeletal Muscle Index
3.9. Sensitivity Analysis and Publication Bias
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Nakakubo, S.; Doi, T.; Tsutsumimoto, K.; Kurita, S.; Ishii, H.; Shimada, H. Sleep duration and progression to sarcopenia in Japanese community-dwelling older adults: A 4 year longitudinal study. J. Cachexia Sarcopenia Muscle 2021, 12, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Woessner, M.N.; Sim, M.; Levinger, I. Sarcopenia definition: Does it really matter? Implications for resistance training. Ageing Res. Rev. 2022, 78, 101617. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, X.; He, Q.; Du, L.; Chen, K.; Chen, S.; Pan, Y. Prevalence of sarcopenia in Chinese community-dwelling elderly: A systematic review. BMC Public Health 2022, 22, 1702. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Treatment of sarcopenia: The road to the future. J. Cachexia Sarcopenia Muscle 2018, 9, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Sun, Y.; Zhang, T.; Zou, L.; Wu, X.; Wang, D.; Chen, Z. Exercise Programs for Muscle Mass, Muscle Strength and Physical Performance in Older Adults with Sarcopenia: A Systematic Review and Meta-Analysis. Aging Dis. 2020, 11, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.; Asakawa, Y.; Ishibashi, H.; Fujita, H.; Arai, T.; Yamaguchi, H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J. Phys. Ther. Sci. 2016, 28, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Wu, H.J.; Chen, Y.J.; Ho, S.Y.; Chung, Y.C. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Exp. Gerontol. 2018, 112, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Walker, S.; Van Driessche, S.; Baggen, R.; Coudyzer, W.; Bautmans, I.; Delecluse, C. Training load does not affect detraining’s effect on muscle volume, muscle strength and functional capacity among older adults. Exp. Gerontol. 2017, 98, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bårdstu, H.B.; Andersen, V.; Fimland, M.S.; Aasdahl, L.; Raastad, T.; Cumming, K.T.; Sæterbakken, A.H. Effectiveness of a resistance training program on physical function, muscle strength, and body composition in community-dwelling older adults receiving home care: A cluster-randomized controlled trial. Eur. Rev. Aging Phys. Act. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Martín Del Campo Cervantes, J.; Habacuc Macías Cervantes, M.; Monroy Torres, R. Monroy, Effect of a Resistance Training Program on Sarcopenia and Functionality of the Older Adults Living in a Nursing Home. J. Nutr. Health Aging 2019, 23, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals with Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar]
- Seo, M.W.; Jung, S.W.; Kim, S.W.; Lee, J.M.; Jung, H.C.; Song, J.K. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, Z.M.; Skrobot, W.; Aschenbrenner, P.; Cesnaitiene, V.J.; Smaruj, M. Effects of short-term Nordic walking training on sarcopenia-related parameters in women with low bone mass: A preliminary study. Clin. Interv. Aging 2016, 11, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Peng, N.; Zhou, M.; Liu, P.P.; Qi, X.L.; Wang, N.; Wang, G.; Wu, Z.P. Tai Chi and whole-body vibrating therapy in sarcopenic men in advanced old age: A clinical randomized controlled trial. Eur. J. Ageing 2019, 16, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, T.; von Stengel, S.; Sieber, C.; Kemmler, W. The Favorable Effects of a High-Intensity Resistance Training on Sarcopenia in Older Community-Dwelling Men with Osteosarcopenia: The Randomized Controlled FrOST Study. Clin. Interv. Aging 2019, 14, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Cebria I Iranzo, M.A.; Balasch-Bernat, M.; Tortosa-Chuliá, M.Á.; Balasch-Parisi, S. Effects of Resistance Training of Peripheral Muscles Versus Respiratory Muscles in Older Adults with Sarcopenia Who are Institutionalized: A Randomized Controlled Trial. J. Aging Phys. Act. 2018, 26, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kim, J.; Kim, S.W.; Kong, H.J. Effects of home-based tele-exercise on sarcopenia among community-dwelling elderly adults: Body composition and functional fitness. Exp. Gerontol. 2017, 87 Pt A, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Pang, M.Y.; Ng, S.S.; Ng, G.Y. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): A randomized controlled trial. Geriatr. Gerontol. Int. 2017, 17, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Niti, M.; Yap, K.B.; Tan, C.T.Y.; Zin Nyunt, M.S.; Feng, L.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.; et al. Assessment of Sarcopenia Among Community-Dwelling At-Risk Frail Adults Aged 65 Years and Older Who Received Multidomain Lifestyle Interventions: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1913346. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.; Yeo, A.; Yew, S.; Lim, J.P.; Tay, L.; Ding, Y.Y.; Lim, W.S. Muscle Strength Definitions Matter: Prevalence of Sarcopenia and Predictive Validity for Adverse Outcomes Using the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) Criteria. J. Nutr. Health Aging 2020, 24, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Van Ancum, J.M.; Alcazar, J.; Meskers, C.G.M.; Nielsen, B.R.; Suetta, C.; Maier, A.B. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 2020, 90, 104125. [Google Scholar] [CrossRef]
- Gerodimos, V.; Karatrantou, K.; Kakardaki, K.; Ioakimidis, P. Can maximal handgrip strength and endurance be improved by an 8-week specialized strength training program in older women? A randomized controlled study. Hand Surg. Rehabil. 2021, 40, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Santanasto, A.J.; Glynn, N.W.; Lovato, L.C.; Blair, S.N.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Hsu, F.C.; King, A.C.; Strotmeyer, E.S.; et al. Effect of Physical Activity versus Health Education on Physical Function, Grip Strength and Mobility. J. Am. Geriatr. Soc. 2017, 65, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Shiotsu, Y.; Watanabe, Y.; Tujii, S.; Yanagita, M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp. Gerontol. 2018, 111, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Yoo, Y.K.; Choi, C.H.; Kim, N.C. Effects of nordic walking on body composition, muscle strength, and lipid profile in elderly women. Asian Nurs. Res. (Korean Soc. Nurs. Sci.). 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Ning, H.T.; Xiao, S.M.; Hu, M.Y.; Wu, X.Y.; Deng, H.W.; Feng, H. Effects of vibration therapy on muscle mass, muscle strength and physical function in older adults with sarcopenia: A systematic review and meta-analysis. Eur. Rev. Aging Phys. Act. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Oguma, Y.; Ishii, N. The effects of whole-body vibration on muscle strength and power: A meta-analysis. J. Musculoskelet. Neuronal Interact. 2013, 13, 380–390. [Google Scholar] [PubMed]
- Lee, H.S.; Park, J.H. Effects of Nordic walking on physical functions and depression in frail people aged 70 years and above. J. Phys. Ther. Sci. 2015, 27, 2453–2456. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, R.; Jiang, J.; Tan, L.; Yang, M. A randomized controlled trial of resistance and balance exercise for sarcopenic patients aged 80–99 years. Sci. Rep. 2020, 10, 18756. [Google Scholar] [CrossRef] [PubMed]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shivappa, N.; Dong, X.; Ming, J.; Zhao, Q.; Xu, H.; Liang, P.; Cheng, M.; Liu, J.; Sun, P.; et al. Association between appendicular skeletal muscle index and leukocyte telomere length in adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Clin. Nutr. 2021, 40, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.A.; Madan, K.; Prabhudesai, A.A.; Agarwal, A.R.; Rastogi, R.; Bhargava, R.; Kriplani, P.; Pampaniya, H.; Gupta, S.; Indrayan, A. Normative cutoffs of muscle mass, muscle strength, and frailty for healthy Indian population. Indian J. Gastroenterol. 2022, 41, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.H.; Hewitt, J.; Keogh, J.W.; Bermeo, S.; Duque, G.; Henwood, T.R. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatr. Nurs. 2016, 37, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ribeiro, A.S.; Schoenfeld, B.J.; Nascimento, M.A.; Tomeleri, C.M.; Souza, M.F.; Pina, F.L.; Cyrino, E.S. The improvement in walking speed induced by resistance training is associated with increased muscular strength but not skeletal muscle mass in older women. Eur. J. Sport Sci. 2017, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Rieping, T.; Furtado, G.E.; Letieri, R.V.; Chupel, M.U.; Colado, J.C.; Hogervorst, E.; Filaire, E.; Teixeira, A.M.M.B.; Ferreira, J.P. Effects of Different Chair-Based Exercises on Salivary Biomarkers and Functional Autonomy in Institutionalized Older Women. Res. Q. Exerc. Sport 2019, 90, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia-The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, S.; Lim, C.; McKendry, J.; Mcleod, J.C.; Stokes, T.; Phillips, S.M. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Research 2020, 9, F1000 Faculty Rev-141. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, N.; Choi, Y.J.; Lee, Y.; Yun, J.; Park, S.J.; Park, H.S.; Chung, Y.S.; Park, Y.K. Leucine-Enriched Protein Supplementation Increases Lean Body Mass in Healthy Korean Adults Aged 50 Years and Older: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, L.; Wu, Y.; Cai, M.; Wang, L. Effect of Different Exercise Interventions on Grip Strength, Knee Extensor Strength, Appendicular Skeletal Muscle Index, and Skeletal Muscle Index Strength in Patients with Sarcopenia: A Meta-Analysis of Randomized Controlled Trials. Diseases 2024, 12, 71. https://doi.org/10.3390/diseases12040071
Wang X, Wang L, Wu Y, Cai M, Wang L. Effect of Different Exercise Interventions on Grip Strength, Knee Extensor Strength, Appendicular Skeletal Muscle Index, and Skeletal Muscle Index Strength in Patients with Sarcopenia: A Meta-Analysis of Randomized Controlled Trials. Diseases. 2024; 12(4):71. https://doi.org/10.3390/diseases12040071
Chicago/Turabian StyleWang, Xinxiang, Lijuan Wang, Yu Wu, Ming Cai, and Liyan Wang. 2024. "Effect of Different Exercise Interventions on Grip Strength, Knee Extensor Strength, Appendicular Skeletal Muscle Index, and Skeletal Muscle Index Strength in Patients with Sarcopenia: A Meta-Analysis of Randomized Controlled Trials" Diseases 12, no. 4: 71. https://doi.org/10.3390/diseases12040071
APA StyleWang, X., Wang, L., Wu, Y., Cai, M., & Wang, L. (2024). Effect of Different Exercise Interventions on Grip Strength, Knee Extensor Strength, Appendicular Skeletal Muscle Index, and Skeletal Muscle Index Strength in Patients with Sarcopenia: A Meta-Analysis of Randomized Controlled Trials. Diseases, 12(4), 71. https://doi.org/10.3390/diseases12040071





