Predicting Postoperative Lung Function in Patients with Lung Cancer Using Imaging Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. AI Algorithm
2.3. Chest CT Acquisition
2.4. Variables’ Explanation and Outcome Definition
3. Results
3.1. Overall Patient Characteristics
3.2. Comparison between FEV1-Non-Preserved Groups and -Preserved Groups
3.3. Factors Associated with Preserved Postoperative FEV1
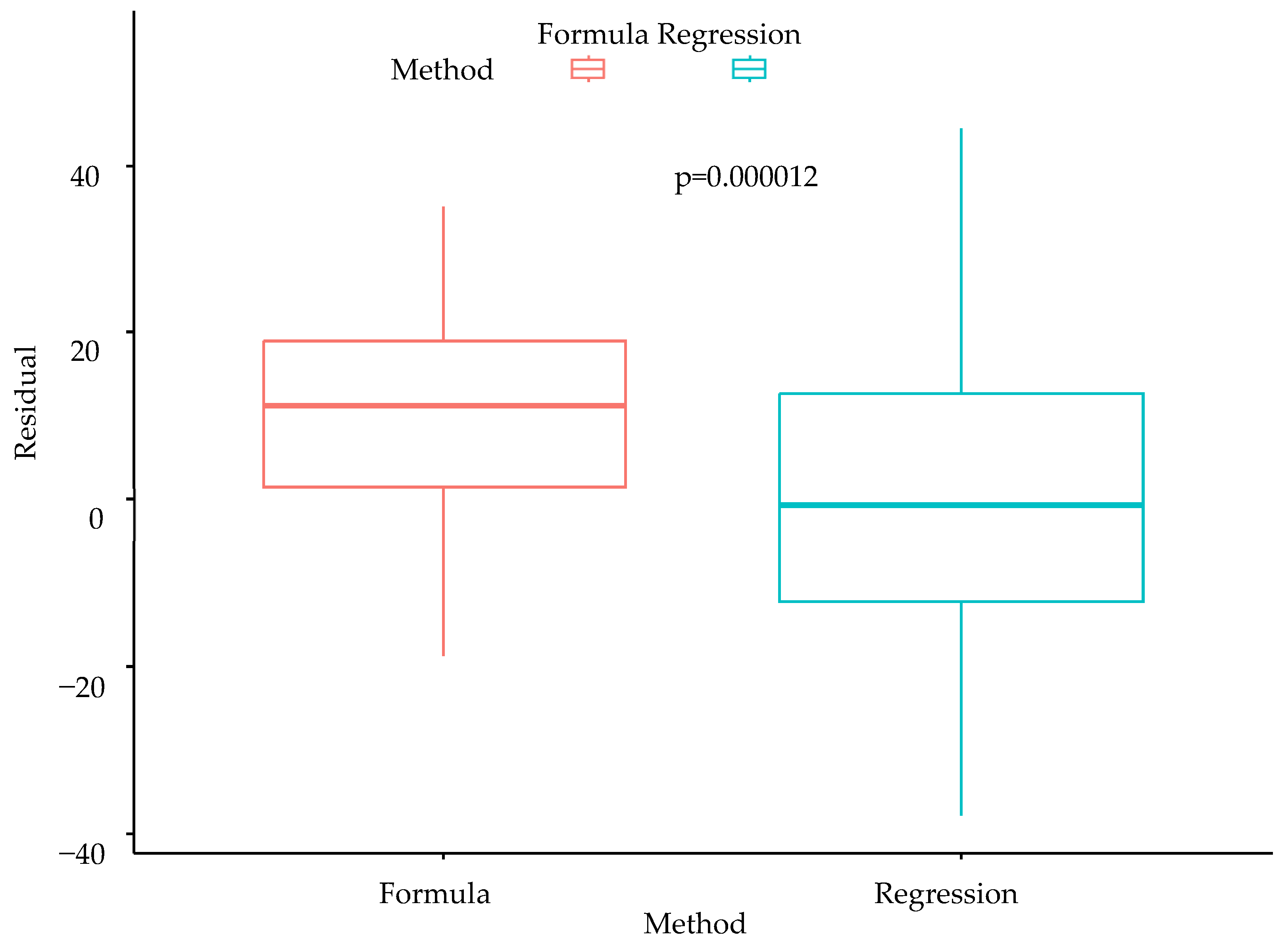
3.4. Comparison of Conventional Formula and Multiple Linear Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Lynch, D.A. Progress in Imaging COPD, 2004–2014. Chronic Obs. Pulm. Dis. 2014, 1, 73–82. [Google Scholar] [CrossRef][Green Version]
- Cukic, V. Preoperative prediction of lung function in pneumonectomy by spirometry and lung perfusion scintigraphy. Acta Inform. Med. 2012, 20, 221–225. [Google Scholar] [CrossRef]
- British Thoracic Society Society of Cardiothoracic Surgeons of Great Britain Ireland Working Party. BTS guidelines: Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef]
- Oswald, N.K.; Halle-Smith, J.; Mehdi, R.; Nightingale, P.; Naidu, B.; Turner, A.M. Predicting Postoperative Lung Function Following Lung Cancer Resection: A Systematic Review and Meta-analysis. EClinicalMedicine 2019, 15, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.B.; Yeo, C.D.; Lee, H.Y.; Kang, H.S.; Kim, S.K.; Kim, J.S.; Park, C.K.; Lee, S.H.; Kim, S.J.; Kim, J.W. The Value of Residual Volume/Total Lung Capacity as an Indicator for Predicting Postoperative Lung Function in Non-Small Lung Cancer. J. Clin. Med. 2021, 10, 4159. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Park, J.; Yu, D.; Yi, J.; Lee, M.; Park, H.J.; Lee, J.G.; Seo, J.B.; Kim, N. Improvement of fully automated airway segmentation on volumetric computed tomographic images using a 2.5 dimensional convolutional neural net. Med. Image Anal. 2019, 51, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lee, S.M.; Hwang, H.J.; Lee, S.M.; Yun, J.; Kim, N.; Seo, J.B. Artificial Intelligence in Lung Imaging. Semin. Respir. Crit. Care Med. 2022, 43, 946–960. [Google Scholar] [CrossRef] [PubMed]
- The Definition of Emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am. Rev. Respir. Dis. 1985, 132, 182–185. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.; Jin, K.N.; Jeong, Y.J.; Chae, K.J.; Lee, K.H.; Yong, H.S.; Gil, B.; Lee, H.J.; Lee, K.Y.; et al. A Challenge for Emphysema Quantification Using a Deep Learning Algorithm With Low-dose Chest Computed Tomography. J. Thorac. Imaging 2022, 37, 253–261. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; de Hoop, B.; Zanen, P.; Gietema, H.; Kruitwagen, C.L.; van Ginneken, B.; Isgum, I.; Mol, C.; van Klaveren, R.J.; Dijkstra, A.E.; et al. CT-quantified emphysema in male heavy smokers: Association with lung function decline. Thorax 2011, 66, 782–787. [Google Scholar] [CrossRef]
- Heussel, C.P.; Herth, F.J.; Kappes, J.; Hantusch, R.; Hartlieb, S.; Weinheimer, O.; Kauczor, H.U.; Eberhardt, R. Fully automatic quantitative assessment of emphysema in computed tomography: Comparison with pulmonary function testing and normal values. Eur. Radiol. 2009, 19, 2391–2402. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Nakano, Y.; Wong, J.C.; de Jong, P.A.; Buzatu, L.; Nagao, T.; Coxson, H.O.; Elliott, W.M.; Hogg, J.C.; Paré, P.D. The prediction of small airway dimensions using computed tomography. Am. J. Respir. Crit. Care Med. 2005, 171, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Seo, J.B.; Song, K.S.; Chae, E.J.; Kang, S.H. Semi-automatic measurement of the airway dimension by computed tomography using the full-width-half-maximum method: A study on the measurement accuracy according to the CT parameters and size of the airway. Korean J. Radiol. 2008, 9, 226–235. [Google Scholar] [CrossRef]
- Cho, Y.H.; Seo, J.B.; Lee, S.M.; Lee, S.M.; Choe, J.; Lee, D.; Kim, N. Quantitative CT Imaging in Chronic Obstructive Pulmonary Disease: Review of Current Status and Future Challenges. J. Korean Soc. Radiol. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tuder, R.M.; Cool, C.D.; Lynch, D.A.; Flores, S.C.; Voelkel, N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 2001, 163, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Grydeland, T.B.; Dirksen, A.; Coxson, H.O.; Pillai, S.G.; Sharma, S.; Eide, G.E.; Gulsvik, A.; Bakke, P.S. Quantitative computed tomography: Emphysema and airway wall thickness by sex, age and smoking. Eur. Respir. J. 2009, 34, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lim, M.-N.; Kim, W.J.; Bak, S.H. Quantitative assessment the longitudinal changes of pulmonary vascular counts in chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, C.; Chen, Z. The CT pulmonary vascular parameters and disease severity in COPD patients on acute exacerbation: A correlation analysis. BMC Pulm. Med. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A. Monitoring the progress of emphysema by repeat computed tomography scans with focus on noise reduction. Proc. Am. Thorac. Soc. 2008, 5, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Kazerooni, E.A.; Lynch, D.A.; Liu, L.X.; Murray, S.; Curtis, J.L.; Criner, G.J.; Kim, V.; Bowler, R.P.; Hanania, N.A.; et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: Associated radiologic phenotypes. Radiology 2011, 261, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Biselli, P.; Grossman, P.R.; Kirkness, J.P.; Patil, S.P.; Smith, P.L.; Schwartz, A.R.; Schneider, H. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J. Appl. Physiol. 2015, 119, 266–271. [Google Scholar] [CrossRef]
- Zeiher, B.G.; Gross, T.J.; Kern, J.A.; Lanza, L.A.; Peterson, M.W. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995, 108, 68–72. [Google Scholar] [CrossRef]
- Koo, H.J.; Lee, S.M.; Seo, J.B.; Lee, S.M.; Kim, N.; Oh, S.Y.; Lee, J.S.; Oh, Y.M. Prediction of Pulmonary Function in Patients with Chronic Obstructive Pulmonary Disease: Correlation with Quantitative CT Parameters. Korean J. Radiol. 2019, 20, 683–692. [Google Scholar] [CrossRef]
| Overall Patients (n = 79) | |
|---|---|
| Age (years) mean ± SD | 69.18 ± 7.89 |
| Sex, n (%) | |
| Male | 51 (64.56) |
| Female | 28 (35.44) |
| Histologic features, n (%) | |
| Adenocarcinoma | 55 (69.62) |
| Squamous cell carcinoma | 20 (25.32) |
| Others | 4 (5.06) |
| Smoking, n (%) | |
| Never smoker | 36 (45.57) |
| Ever smoker | 43 (54.43) |
| Operation, n(%) | |
| Open | 26 (32.91) |
| VATS | 53 (67.09) |
| Stage, n (%) | |
| I | 62 (78.48) |
| II | 8 (10.13) |
| III | 9 (11.39) |
| IV | 0 |
| Location, n (%) | |
| RUL | 27 (34.18) |
| RML | 4 (5.06) |
| RLL | 19 (24.05) |
| LUL | 23 (29.11) |
| LLL | 6 (7.59) |
| Therapy | |
| Adjuvant chemotherapy, n (%) | 18 (22.78) |
| Palliative chemotherapy, n (%) | 5 (6.33) |
| Adjuvant radiotherapy, n (%) | 5 (6.33) |
| Palliative radiotherapy, n (%) | 2 (2.53) |
| PFT | |
| FEV1 (pre) (%), mean ± SD | 101.99 ± 18.62 |
| FEV1/FVC (pre) (%), mean ± SD | 70.46 ± 8.57 |
| DLCO (pre) (%), mean ± SD | 98.23 ± 18.89 |
| RV/TLC (pre) (%), mean ± SD | 36.49 ± 8.99 |
| Radiological biomarkers | |
| Pi1 (HU) mean ± SD | −934.30 ± 33.36 |
| Pi15 (HU) mean ± SD | −890.30 ± 30.50 |
| Pi10fw (mm) mean ± SD | 5.00 ± 0.54 |
| Wafw (%) mean ± SD | 71.99 ± 2.99 |
| Waband (%) mean ± SD | 68.99 ± 14.12 |
| LAAsize (%) mean ± SD | 1.40 ± 2.77 |
| TAC (ea) mean ± SD | 141.41 ± 88.45 |
| VV (cc) mean ± SD | 108.95 ± 45.18 |
| TLV (cc) mean ± SD | 4216.28 ± 1098.24 |
| FEV1-Non-Preserved (n = 16, 20.25%) | FEV1-Preserved (n = 63, 79.75%) | p Value | |
|---|---|---|---|
| Age (years) mean ± SD | 67.31 ± 5.99 | 69.65 ± 8.28 | 0.209 |
| Sex, n (%) | 0.437 | ||
| Male | 9 (56.2) | 42 (66.7) | |
| Female | 7 (43.8) | 21 (33.3) | |
| Histologic features, n (%) | 0.187 | ||
| Sqcc. | 2 (12.5) | 18 (28.6) | |
| Non-Sqcc | 14 (87.5) | 45 (71.4) | |
| Smoking, n (%) | 0.690 | ||
| Never smoker | 8 (50.0) | 28 (44.4) | |
| Ever smoker | 8 (50.0) | 35 (55.6) | |
| Operation, n (%) | 0.451 | ||
| Open | 4 (25.0) | 22 (34.9) | |
| VATS | 12 (75.0) | 41(65.1) | |
| Stage, n (%) | 0.704 | ||
| I | 12 (75.0) | 50 (79.4) | |
| II–III | 4 (25.0) | 13 (20.6) | |
| Location, n (%) | 0.277 | ||
| BUL | 12 (75.0) | 38 (60.3) | |
| Other lobes | 4 (25.0) | 25 (39.7) | |
| PFT | |||
| FEV1 (pre) (%), mean ± SD | 100.94 ± 13.71 | 102.25 ± 19.76 | 0.758 |
| FEV1/FVC (pre (%), mean ± SD | 72.31 ± 7.02 | 69.98 ± 8.90 | 0.273 |
| DLCO (pre) (%), mean ± SD | 97.50 ± 11.80 | 98.42 ± 20.39 | 0.816 |
| RV/TLC (pre) (%), mean ± SD | 36.38 ± 9.19 | 36.52 ± 9.01 | 0954 |
| Radiological biomarkers | |||
| Pi1 (HU) mean ± SD | −934.56 ± 44.20 | −934.24 ± 30.46 | 0.978 |
| Pi15 (HU) mean ± SD | −886.44 ± 40.68 | −891.29 ± 27.66 | 0.657 |
| Pi10fw (cc) mean ± SD | 5.24 ± 1.07 | 4.94 ± 0.26 | 0.288 |
| Wafw (%) mean ± SD | 71.36 ± 3.04 | 72.15 ± 2.97 | 0.366 |
| Waband (%) mean ± SD | 60.75 ± 24.82 | 71.08 ± 8.94 | 0.121 |
| LAAsize (%) mean ± SD | 1.55 ± 2.64 | 1.36 ± 2.83 | 0.807 |
| TAC (ea) mean ± SD | 115.31 ± 61.56 | 148.03 ± 93.30 | 0.100 |
| VV (cc) mean ± SD | 89.99 ± 33.45 | 113.76 ± 46.70 | 0.027 |
| TLV (cc) mean ± SD | 4242.42 ± 1224.82 | 4209.64 ± 1074.34 | 0.922 |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Sex (Male vs. Female) | 1.556 | 0.509–4.758 | 0.439 | |||
| Age | 1.038 | 0.968–1.113 | 0.291 | |||
| Histology (Sqcc. vs. Non sqcc.) | 2.800 | 0.577–13.582 | 0.201 | |||
| Smoking (Ever vs. Never) | 1.250 | 0.417–3.750 | 0.691 | |||
| Stage (II–III vs. I) | 0.780 | 0.216–2.820 | 0.705 | |||
| Location (BUL vs. Other) | 0.507 | 0.147–1.745 | 0.282 | |||
| Operation (Open vs. VATS) | 1.610 | 0.464–5.588 | 0.453 | |||
| V0FEV1 | 1.004 | 0.975–1.034 | 0.799 | |||
| V0FVC | 1.009 | 0.970–1.048 | 0.663 | |||
| V0FEV1/FVC | 0.965 | 0.898–1.037 | 0.331 | |||
| V0DLco | 1.002 | 0.973–1.033 | 0.861 | |||
| RV/TLC (≥40% vs. <40%) | 0.496 | 0.153–1.608 | 0.243 | |||
| Adjuvant chemotherapy | 0.392 | 0.119–1.291 | 0.124 | 0.412 | 0.118–1.440 | 0.164 |
| Palliative chemotherapy | 0.350 | 0.053–2.297 | 0.274 | |||
| Adjuvant radiotherapy | 1.017 | 0.106–9.779 | 0.988 | |||
| Palliative radiotherapy | 0.242 | 0.014–4.094 | 0.325 | |||
| Pi1 | 1.000 | 0.984–1.017 | 0.972 | |||
| Pi15 | 0.995 | 0.978–1.012 | 0.569 | |||
| Pi10fw | 0.379 | 0.086–1.673 | 0.200 | 0.445 | 0.113–1.747 | 0.246 |
| Wafw | 1.087 | 0.912–1.294 | 0.351 | |||
| LAAsize | 0.977 | 0.808–1.181 | 0.811 | |||
| TAC | 1.006 | 0.997–1.016 | 0.167 | 0.999 | 0.989–1.009 | 0.865 |
| VV | 1.015 | 0.999–1.031 | 0.063 | 1.015 | 0.995–1.035 | 0.144 |
| TLV | 1.000 | 0.999–1.001 | 0.915 | |||
| Univariate Analysis | Multivariate Analysis (Adjusted R2 = 0.134), p Value 0.024 | ||||
|---|---|---|---|---|---|
| β ± SE | p Value | β ± SE | VIF | p Value | |
| Age | 0.253 ± 0.263 | 0.339 | 0.280 ± 0.259 | 1.070 | 0.283 |
| Pi1 | 0.094 ± 0.671 | 0.132 | 0.156 ± 0.075 | 1.619 | 0.042 |
| Pi15 | 0.084 ± 0.068 | 0.215 | |||
| Pi10fw | −2.06 ± 3.88 | 0.596 | − | ||
| Wafw | −1.219 ± 0.686 | 0.080 | −2.111 ± 0.737 | 1.237 | 0.005 |
| LAAsize | −0.914 ± 0.746 | 0.244 | |||
| TAC | 0.018 ± 0.024 | 0.446 | |||
| VV | −0.027 ± 0.046 | 0.563 | |||
| TLV | −0.003 ± 0.002 | 0.159 | −0.001 ± 0.002 | 1.440 | 0.656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.-B.; Lee, H.-U.; Park, H.-E.; Choi, J.-Y.; Kim, J.-W.; Lee, S.-H.; Yeo, C.-D. Predicting Postoperative Lung Function in Patients with Lung Cancer Using Imaging Biomarkers. Diseases 2024, 12, 65. https://doi.org/10.3390/diseases12040065
Kwon O-B, Lee H-U, Park H-E, Choi J-Y, Kim J-W, Lee S-H, Yeo C-D. Predicting Postoperative Lung Function in Patients with Lung Cancer Using Imaging Biomarkers. Diseases. 2024; 12(4):65. https://doi.org/10.3390/diseases12040065
Chicago/Turabian StyleKwon, Oh-Beom, Hae-Ung Lee, Ha-Eun Park, Joon-Young Choi, Jin-Woo Kim, Sang-Haak Lee, and Chang-Dong Yeo. 2024. "Predicting Postoperative Lung Function in Patients with Lung Cancer Using Imaging Biomarkers" Diseases 12, no. 4: 65. https://doi.org/10.3390/diseases12040065
APA StyleKwon, O.-B., Lee, H.-U., Park, H.-E., Choi, J.-Y., Kim, J.-W., Lee, S.-H., & Yeo, C.-D. (2024). Predicting Postoperative Lung Function in Patients with Lung Cancer Using Imaging Biomarkers. Diseases, 12(4), 65. https://doi.org/10.3390/diseases12040065





