Chest X-Ray Features in 130 Patients with Bronchiectasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions and Radiological Evaluation
2.3. Ethical Approval
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, M.P.; Hill, A.T. Non-cystic fibrosis bronchiectasis. Clin. Med. 2009, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Millett, E.R.; Joshi, M.; Navaratnam, V.; Thomas, S.L.; Hurst, J.R.; Smeeth, L.; Brown, J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016, 47, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ida, M.; Takeuchi, E.; Nakamura, Y.; Horiguchi, T.; Sato, A. Middle lobe syndrome--incidence and relationship to atypical mycobacterial pulmonary disease. Nihon Kyobu Shikkan Gakkai Zasshi 1996, 34, 57–62. [Google Scholar]
- Chalmers, J.D.; Polverino, E.; Crichton, M.L.; Ringshausen, F.C.; De Soyza, A.; Vendrell, M.; Burgel, P.R.; Haworth, C.S.; Loebinger, M.R.; Dimakou, K.; et al. Bronchiectasis in Europe: Data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir. Med. 2023, 11, 637–649. [Google Scholar] [CrossRef]
- Aksamit, T.R.; O’Donnell, A.E.; Barker, A.; Olivier, K.N.; Winthrop, K.L.; Daniels, M.L.A.; Johnson, M.; Eden, E.; Griffith, D.; Knowles, M.; et al. Adult Patients with Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017, 151, 982–992. [Google Scholar] [CrossRef]
- Asakura, T.; Morimoto, K.; Ito, A.; Suzuki, S.; Morino, E.; Oshitani, Y.; Nakagawa, T.; Yagi, K.; Kadowaki, T.; Saito, F.; et al. Etiology and health-related quality of life in non-cystic fibrosis bronchiectasis and nontuberculous mycobacterial pulmonary disease: The first analysis of the Japanese nontuberculous mycobacteriosis-bronchiectasis registry. In B106. Breakthroughs in NTM Diagnosis and Treatment; American Thoracic Society: New York, NY, USA, 2020; p. A4370. [Google Scholar]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society guideline for bronchiectasis in adults. BMJ Open Respir. Res. 2018, 5, e000348. [Google Scholar] [CrossRef]
- O’Donnell, A.E. Bronchiectasis—A Clinical Review. N. Engl. J. Med. 2022, 387, 533–545. [Google Scholar] [CrossRef]
- Simmonds, N.J.; Cullinan, P.; Hodson, M.E. Growing old with cystic fibrosis—The characteristics of long-term survivors of cystic fibrosis. Respir. Med. 2009, 103, 629–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cantin, L.; Bankier, A.A.; Eisenberg, R.L. Bronchiectasis. AJR Am. J. Roentgenol. 2009, 193, W158–W171. [Google Scholar] [CrossRef]
- Godet, C.; Brun, A.-L.; Couturaud, F.; Laurent, F.; Frat, J.-P.; Marchand-Adam, S.; Gagnadoux, F.; Blanchard, E.; Taillé, C.; Philippe, B.; et al. CT Imaging Assessment of Response to Treatment in Allergic Bronchopulmonary Aspergillosis in Adults With Bronchial Asthma. Chest 2024, 165, 1307–1318. [Google Scholar] [CrossRef]
- Kuhajda, I.; Zarogoulidis, K.; Tsirgogianni, K.; Tsavlis, D.; Kioumis, I.; Kosmidis, C.; Tsakiridis, K.; Mpakas, A.; Zarogoulidis, P.; Zissimopoulos, A.; et al. Lung abscess-etiology, diagnostic and treatment options. Ann. Transl. Med. 2015, 3, 183. [Google Scholar] [PubMed]
- Currie, D.C.; Cooke, J.C.; Morgan, A.D.; Kerr, I.H.; Delany, D.; Strickland, B.; Cole, P.J. Interpretation of bronchograms and chest radiographs in patients with chronic sputum production. Thorax 1987, 42, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Han, B.K.; Kim, T.S.; Hwang, J.H.; Yoon, J.H.; Paik, C.H.; Lee, K.S.; Cho, J.M.; Choi, S.H.; Yoon, H.K. Bronchiectasis: Diagnostic accuracy of chest computed radiography. J. Korean Radiol. Soc. 1999, 40, 871–877. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; De Gracia, J.; Vendrell Relat, M.; Girón, R.M.; Maiz Carro, L.; de la Rosa Carrillo, D.; Olveira, C. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 2014, 43, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yano, S.; Wakabayashi, K.; Kobayashi, K.; Ishikawa, S.; Kimura, M.; Ikeda, T. An analysis of etiology, causal pathogens, imaging patterns, and treatment of Japanese patients with bronchiectasis. Respir. Investig. 2015, 53, 37–44. [Google Scholar] [CrossRef]
- Ochiai, S.; Kido, Y.; Tanoue, S.; Kitahara, Y.; Harada, Y.; Harada, S.; Takamoto, M.; Ishibashi, T. Evaluation of CT appearance of Mycobacterium avium complex infection--comparison with bronchiectasia. Kekkaku 2000, 75, 341–347. [Google Scholar]
- Munchel, J.K.; Shea, B.S. Diagnosis and Management of Idiopathic Pulmonary Fibrosis. Rhode Isl. Med. J. 2021, 104, 26–29. [Google Scholar]
- Travis, W.D.; Hunninghake, G.; King, T.E., Jr.; Lynch, D.A.; Colby, T.V.; Galvin, J.R.; Brown, K.K.; Chung, M.P.; Cordier, J.F.; Du Bois, R.M.; et al. Idiopathic nonspecific interstitial pneumonia: Report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008, 177, 1338–1347. [Google Scholar] [CrossRef]
- Maguire, G. Bronchiectasis—A guide for primary care. Aust. Fam. Physician 2012, 41, 842–850. [Google Scholar]
- Magu, S.; Malhotra, R.; Gupta, K.B.; Mishra, D.S. Role of computed tomography in patients with hemoptysis and a normal chest skiagram. Indian J. Chest Dis. Allied Sci. 2000, 42, 101–104. [Google Scholar]
- Miyashita, N.; Sugiu, T.; Kawai, Y.; Yamaguchi, T.; Ouchi, K. Radiographic features of Mycoplasma pneumoniae pneumonia: Differential diagnosis and performance timing. BMC Med. Imaging 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
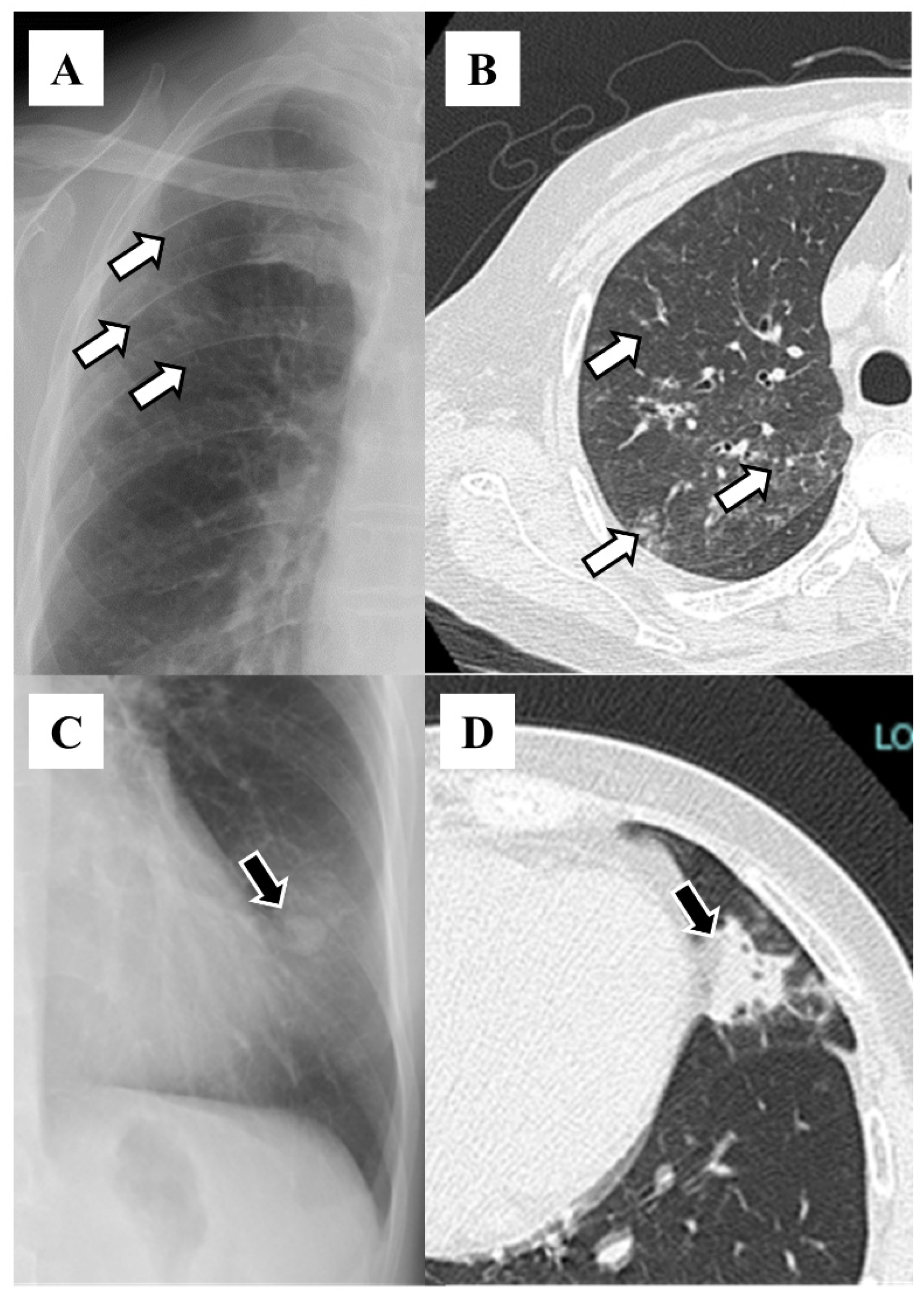
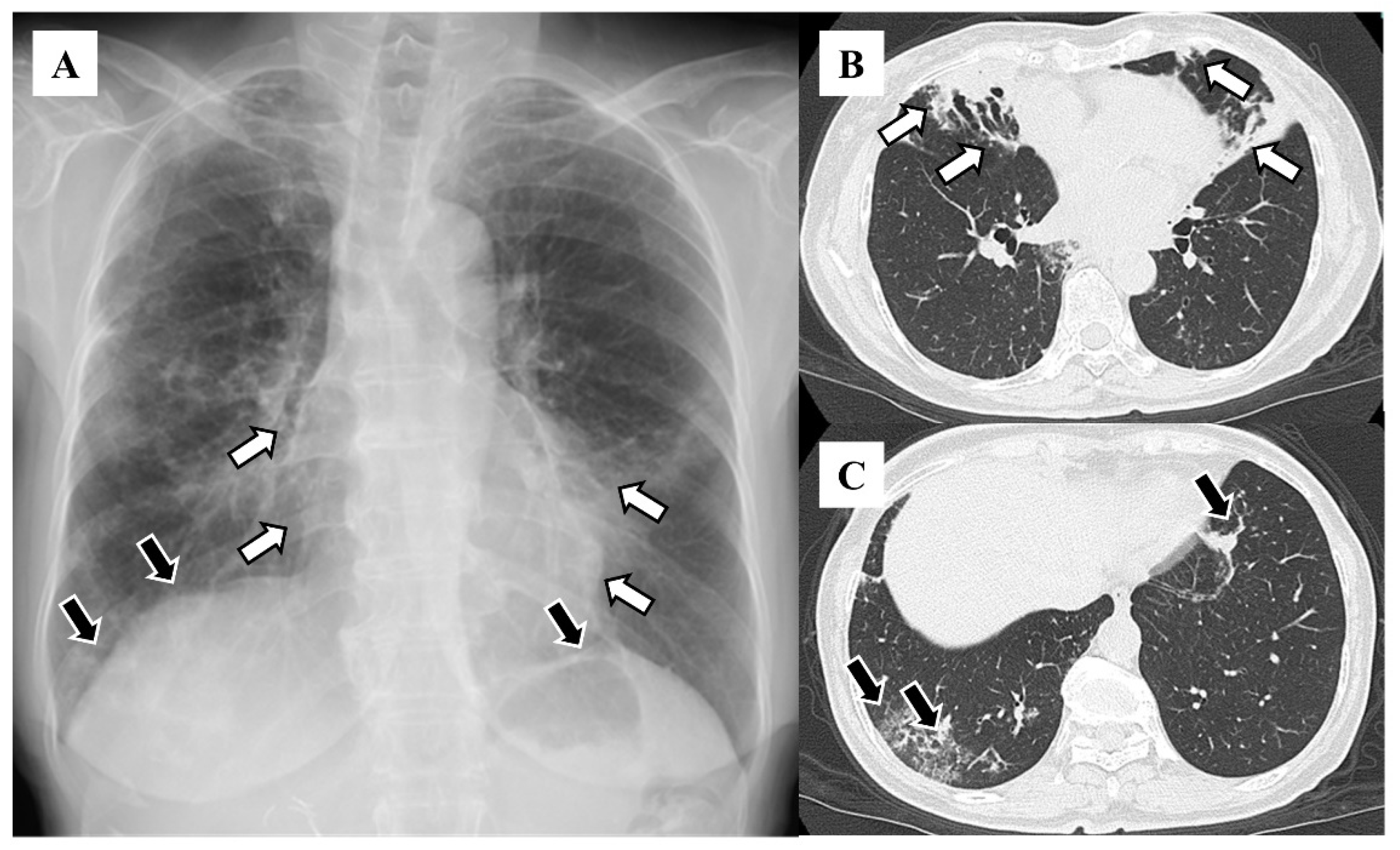
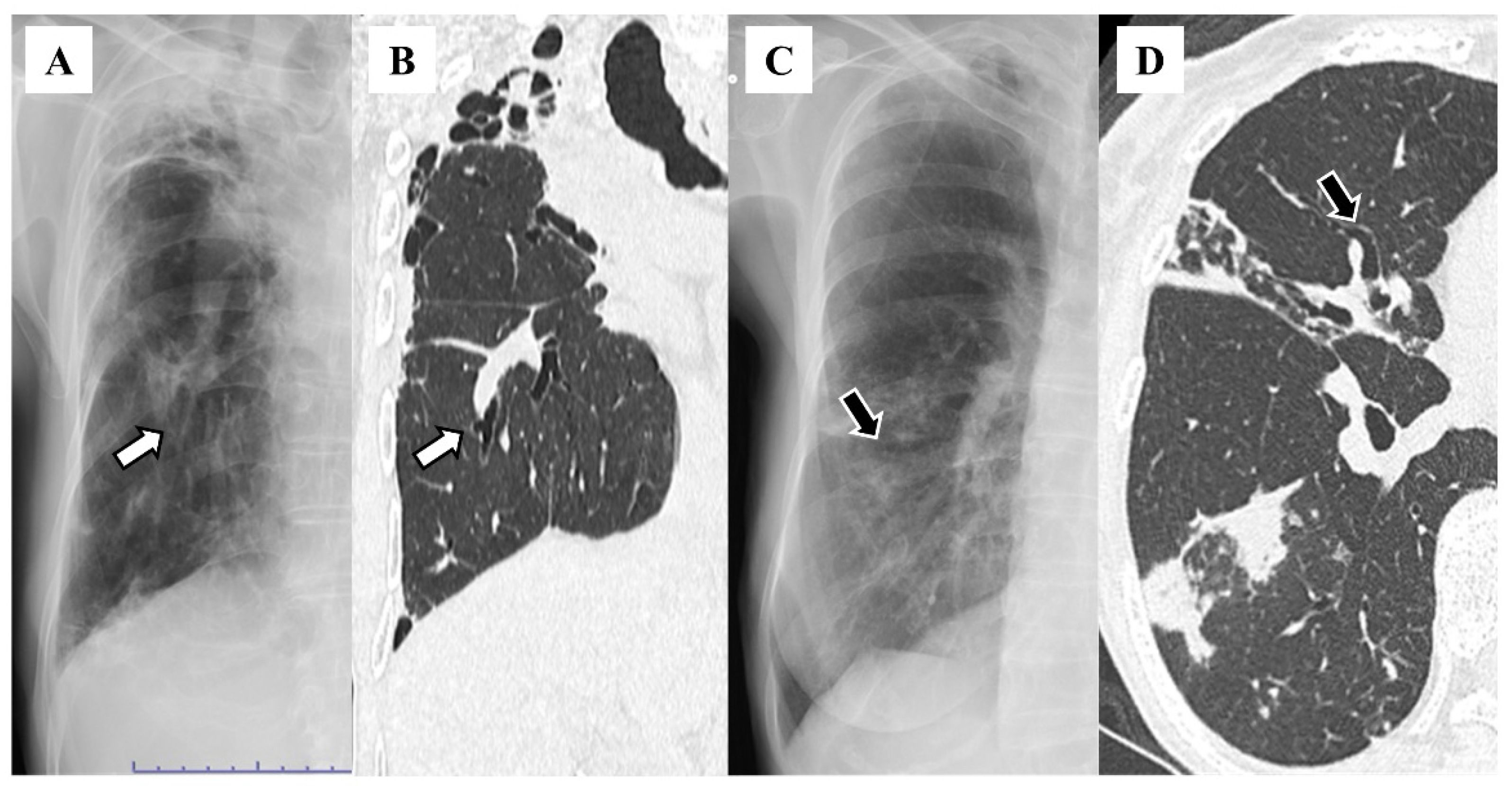
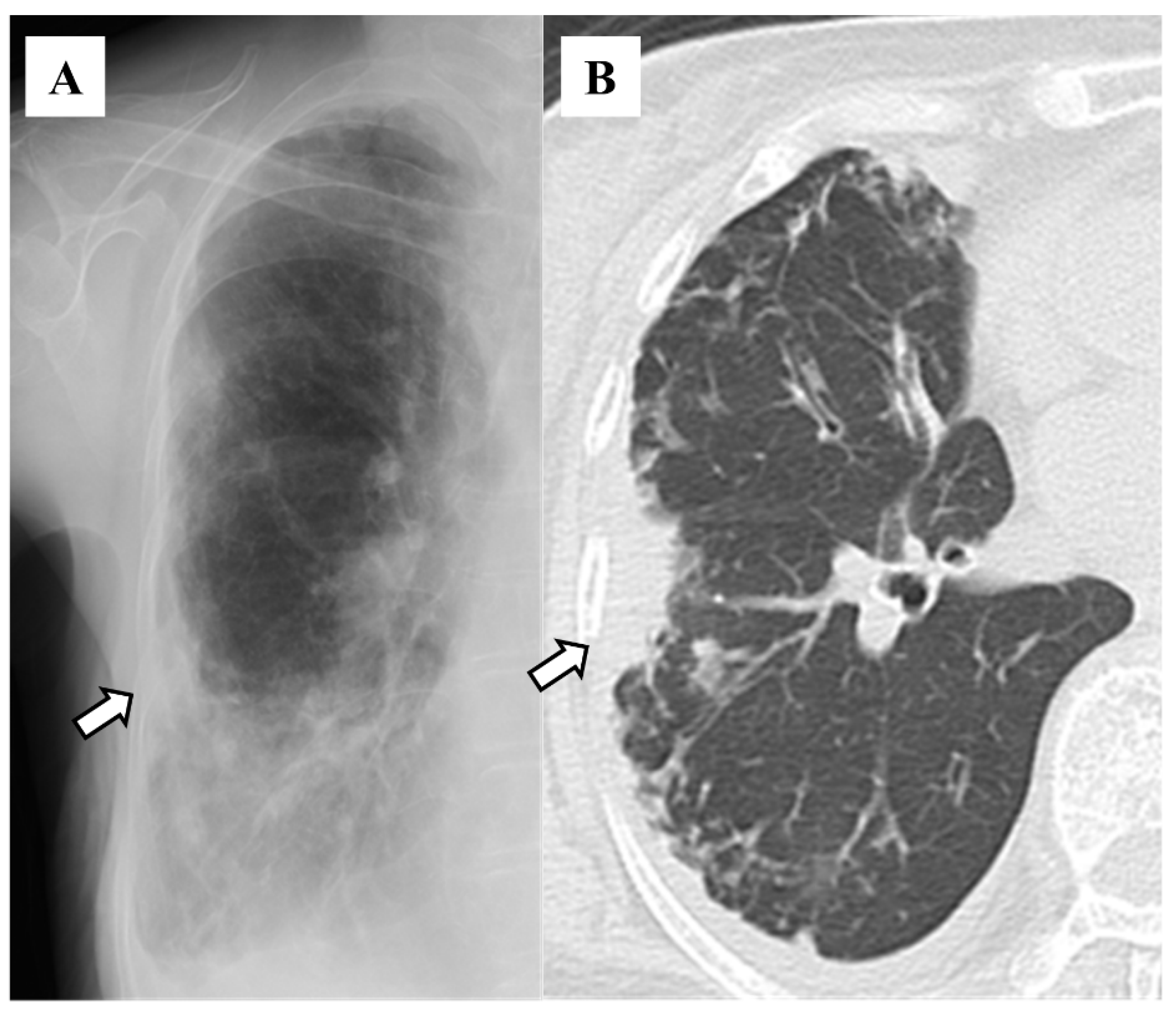
| Lobe | Number (%) |
|---|---|
| Right upper lobe | 56 (43.1) |
| Right middle lobe | 83 (63.8) |
| Right lower lobe | 58 (44.6) |
| Left upper segment | 22 (16.9) |
| Left lingular segment | 75 (57.7) |
| Left lower lobe | 45 (34.6) |
| Features | Number (%) |
|---|---|
| Granular shadows | 115 (88.5) |
| Vague cardiac silhouette | 63 (48.5) |
| Nodular shadows | 59 (45.4) |
| Tram-track appearance | 46 (35.4) |
| Pleural thickening | 35 (26.9) |
| Vague diaphragm | 33 (25.4) |
| Ring sign | 32 (24.6) |
| Lung volume loss | 15 (11.5) |
| Finger-in-glove sign | 12 (9.2) |
| Air–fluid level | 5 (3.8) |
| Atelectasis | 8 (6.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, H.; Kudoh, R.; Yokoyama, A.; Hagiwara, A.; Hiramatsu, K.; Kadota, J.-i.; Komiya, K. Chest X-Ray Features in 130 Patients with Bronchiectasis. Diseases 2024, 12, 323. https://doi.org/10.3390/diseases12120323
Sawada H, Kudoh R, Yokoyama A, Hagiwara A, Hiramatsu K, Kadota J-i, Komiya K. Chest X-Ray Features in 130 Patients with Bronchiectasis. Diseases. 2024; 12(12):323. https://doi.org/10.3390/diseases12120323
Chicago/Turabian StyleSawada, Hikaru, Ryohei Kudoh, Atsushi Yokoyama, Akihiko Hagiwara, Kazufumi Hiramatsu, Jun-ichi Kadota, and Kosaku Komiya. 2024. "Chest X-Ray Features in 130 Patients with Bronchiectasis" Diseases 12, no. 12: 323. https://doi.org/10.3390/diseases12120323
APA StyleSawada, H., Kudoh, R., Yokoyama, A., Hagiwara, A., Hiramatsu, K., Kadota, J.-i., & Komiya, K. (2024). Chest X-Ray Features in 130 Patients with Bronchiectasis. Diseases, 12(12), 323. https://doi.org/10.3390/diseases12120323






