Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks
Abstract
1. Introduction
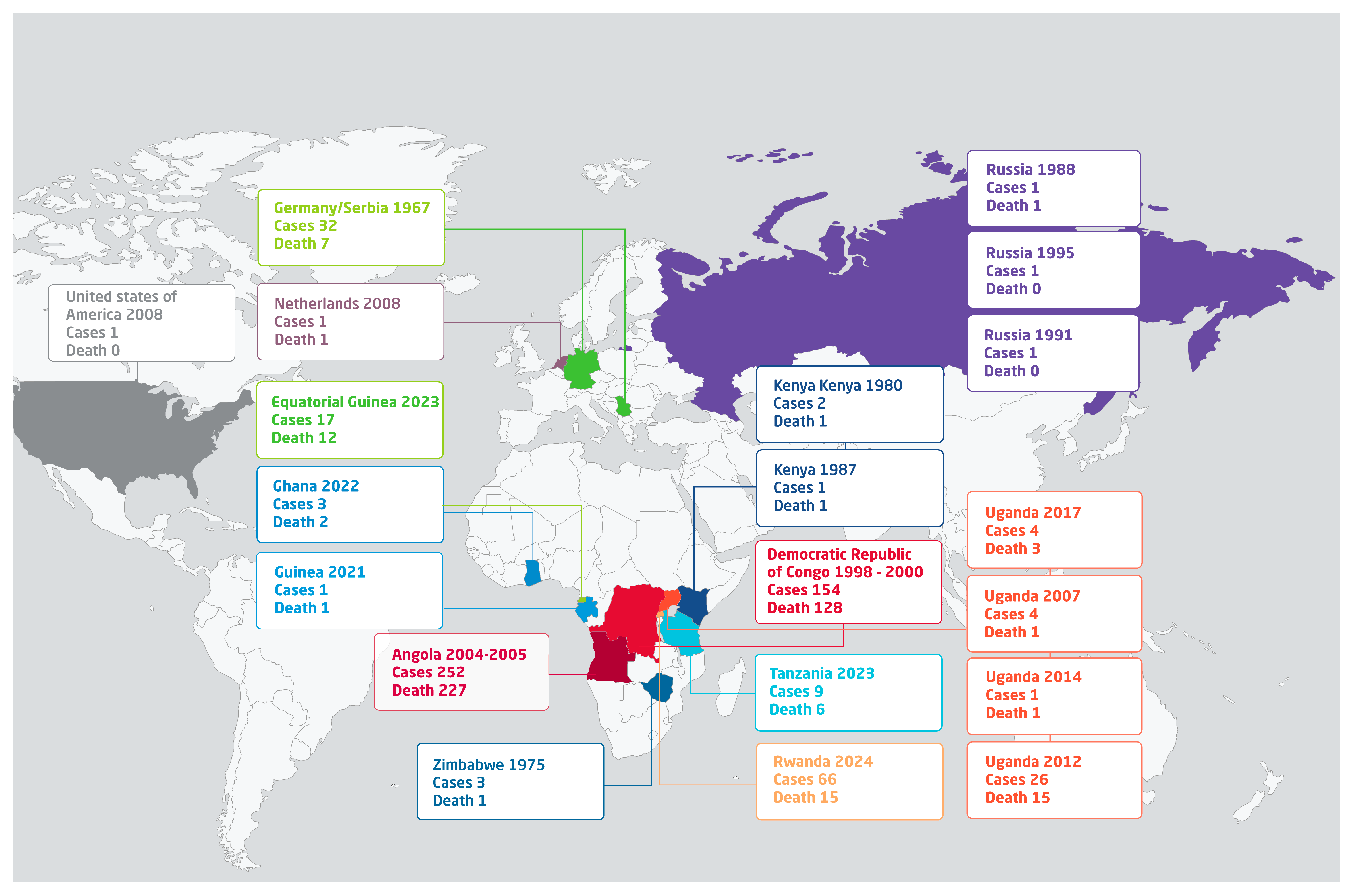
2. Historical Epidemiology
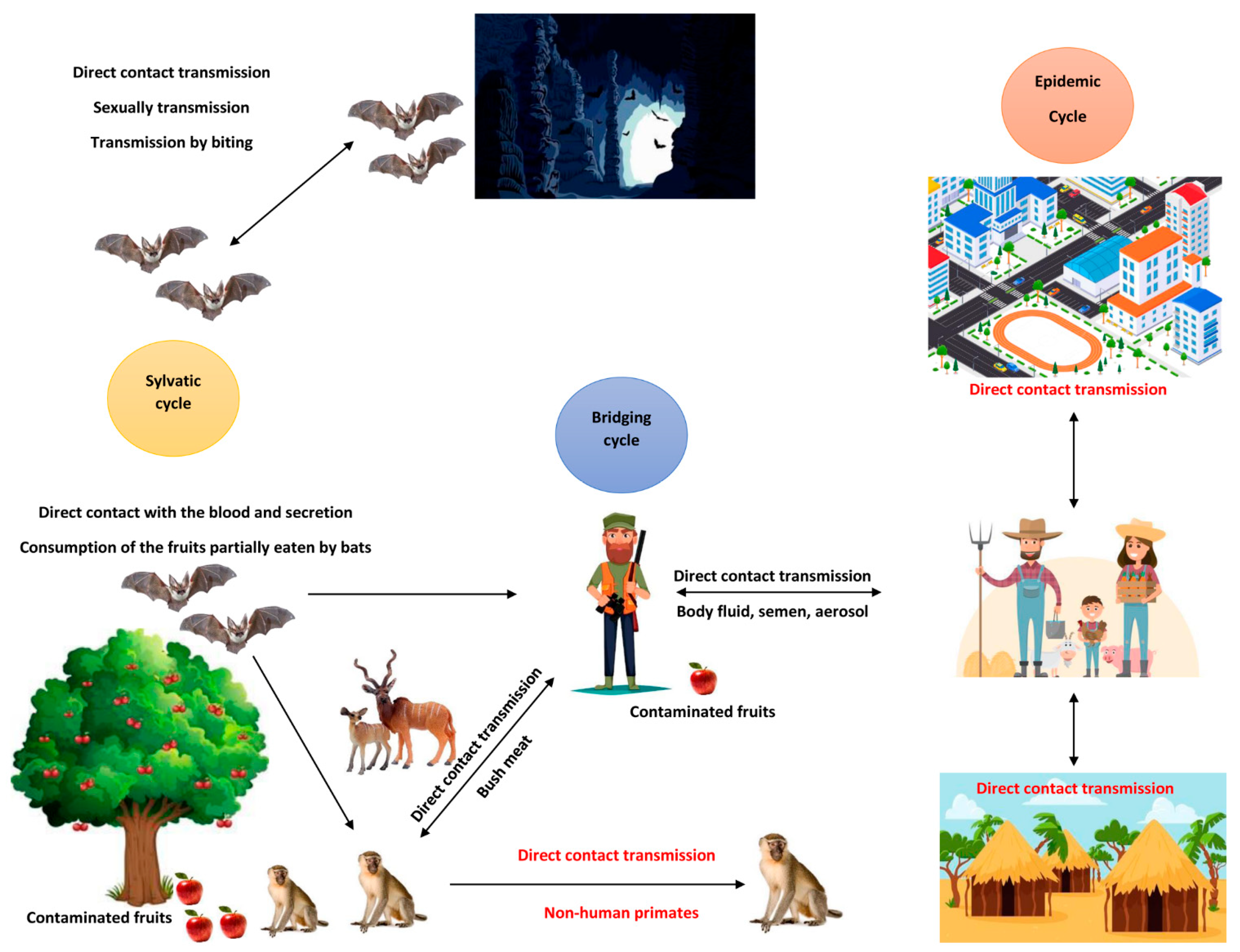
3. Natural Reservoirs and Routes of Transmission
4. Routes of Transmission
5. Clinical Presentations
6. Diagnosis
7. Case Management
8. Integrated Multisectoral One Health Strategy for Prevention and Control of MVD
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Di Paola, N.; Formenty, P.B.H.; Griffiths, A.; Hume, A.J.; Mühlberger, E.; Netesov, S.V.; Palacios, G.; et al. ICTV Virus Taxonomy Profile: Filoviridae 2024. J. Gen. Virol. 2024, 105, 001955. [Google Scholar] [CrossRef] [PubMed]
- Shifflett, K.; Marzi, A. Marburg virus pathogenesis – differences and similarities in humans and animal models. Virol. J. 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brauburger, K.; Hume, A.J.; Mühlberger, E.; Olejnik, J. Forty-Five Years of Marburg Virus Research. Viruses 2012, 4, 1878–1927. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Marburg Virus Disease. Available online: https://www.who.int/health-topics/marburg-virus-disease (accessed on 9 October 2024).
- Srivastava, S.; Sharma, D.; Kumar, S.; Sharma, A.; Rijal, R.; Asija, A.; Adhikari, S.; Rustagi, S.; Sah, S.; Al-Qaim, Z.H.; et al. Emergence of Marburg virus: A global perspective on fatal outbreaks and clinical challenges. Front. Microbiol. 2023, 14, 1239079. [Google Scholar] [CrossRef]
- The Global Alliance for Vaccines and Immunizations (GAVI). The Next Pandemic: Marburg? Available online: https://www.gavi.org/vaccineswork/next-pandemic/marburg (accessed on 10 October 2024).
- Prioritizing Diseases for Research and Development in Emergency Contexts. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 16 February 2021).
- The World Health Organization (WHO). Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness. 2024. Available online: https://www.who.int/publications/m/item/pathogens-prioritization-a-scientific-framework-for-epidemic-and-pandemic-research-preparedness (accessed on 13 November 2024).
- Rwagasore, E.; Muvunyi, C.M.; Butera, Y.; Nsanzimana, S.; Condo, J. Rwanda’s seven steps in seven days for managing Marburg virus. Nature 2024, 634, 545. [Google Scholar] [CrossRef] [PubMed]
- Ristanović, E.S.; Kokoškov, N.S.; Crozier, I.; Kuhn, J.H.; Gligić, A.S. A Forgotten Episode of Marburg Virus Disease: Belgrade, Yugoslavia, 1967. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef]
- Guito, J.C.; Prescott, J.B.; Arnold, C.E.; Amman, B.R.; Schuh, A.J.; Spengler, J.R.; Sealy, T.K.; Harmon, J.R.; Coleman-McCray, J.D.; Kulcsar, K.A.; et al. Asymptomatic Infection of Marburg Virus Reservoir Bats Is Explained by a Strategy of Immunoprotective Disease Tolerance. Curr. Biol. 2020, 31, 257–270.e5. [Google Scholar] [CrossRef]
- Amman, B.R.; Schuh, A.J.; Albariño, C.G.; Towner, J.S. Marburg Virus Persistence on Fruit as a Plausible Route of Bat to Primate Filovirus Transmission. Viruses 2021, 13, 2394. [Google Scholar] [CrossRef]
- Abir, M.H.; Rahman, T.; Das, A.; Etu, S.N.; Nafiz, I.H.; Rakib, A.; Mitra, S.; Bin Emran, T.; Dhama, K.; Islam, A.; et al. Pathogenicity and virulence of Marburg virus. Virulence 2022, 13, 609–633. [Google Scholar] [CrossRef]
- Butera, Y.; Mutesa, L.; Parker, E.; Muvunyi, R.; Umumararungu, E.; Ayitewala, A.; Musabyimana, J.P.; Olono, A.; Sesonga, P.; Ogunsanya, O.; et al. Genomic Characterization Uncovers Transmission Dynamics of Marburg Virus in Rwanda Following a Single Zoonotic Spillover Event. medRxiv 2024, 2024.11.01.24316374. [Google Scholar] [CrossRef]
- Schuh, A.J.; Amman, B.R.; Guito, J.C.; Graziano, J.C.; Sealy, T.K.; Kirejczyk, S.G.M.; Towner, J.S. Natural reservoir Rousettus aegyptiacus bat host model of orthonairovirus infection identifies potential zoonotic spillover mechanisms. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Timen, A. Response to Imported Case of Marburg Hemorrhagic Fever, the Netherlands. Emerg. Infect. Dis. 2009, 15, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Transmission of Colorado Tick Fever Virus by Blood Transfusion—Montana. Morbidity and Mortality Weekly Report 1975. Available online: https://stacks.cdc.gov/view/cdc/1031 (accessed on 10 October 2024).
- Amman, B.R.; Jones, M.E.B.; Sealy, T.K.; Uebelhoer, L.S.; Schuh, A.J.; Bird, B.H.; Coleman-McCray, J.D.; Martin, B.E.; Nichol, S.T.; Towner, J.S. Oral Shedding of Marburg Virus in Experimentally Infected Egyptian Fruit Bats (Rousettus aegyptiacus). J. Wildl. Dis. 2015, 51, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; van Vuren, P.J.; Masumu, J.; Leman, P.A.; Grobbelaar, A.A.; Birkhead, M.; Clift, S.; Swanepoel, R.; Kemp, A. Virological and Serological Findings in Rousettus aegyptiacus Experimentally Inoculated with Vero Cells-Adapted Hogan Strain of Marburg Virus. PLoS ONE 2012, 7, e45479. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T.; van Vuren, P.J.; Fenton, K.A.; Graves, K.; Grobbelaar, A.A.; Moolla, N.; Leman, P.; Weyer, J.; Storm, N.; McCulloch, S.D.; et al. Lack of Marburg Virus Transmission from Experimentally Infected to Susceptible In-Contact Egyptian Fruit Bats. J. Infect. Dis. 2015, 212, S109–S118. [Google Scholar] [CrossRef]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012, 8, e1002877. [Google Scholar] [CrossRef]
- Dhama, K.; Chandran, D.; Chakraborty, S.; Yatoo, M.I.; Islam, A.; Bhattacharya, M.; Chakraborty, C.; Harapan, H.; Chaicumpa, W. Zoonotic concerns of Marburg virus: Current knowledge and counteracting strategies including One Health approach to limit animal-human interface: An update. Int. J. Surg. 2022, 106, 106941. [Google Scholar] [CrossRef]
- The World Health Organization (WHO) Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 10 October 2024).
- European Centre for Disease Prevention and Control (ECDC) Factsheet for Health Professionals about Marburg Virus Disease. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/marburg-virus-disease/factsheet-health-professionals-about-marburg-virus (accessed on 20 October 2024).
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.G.; Hay, S.I. Mapping the zoonotic niche of Marburg virus disease in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 366–378. [Google Scholar] [CrossRef]
- Mehedi, M.; Groseth, A.; Feldmann, H.; Ebihara, H. Clinical Aspects of Marburg Hemorrhagic Fever. Futur. Virol. 2011, 6, 1091–1106. [Google Scholar] [CrossRef]
- Martini, G.A.; Schmidt, H.A. [Spermatogenic transmission of the “Marburg virus”. (Causes of “Marburg simian disease”)]. Klin Wochenschr. 1968, 46, 398–400. [Google Scholar] [CrossRef]
- Gear, J.S.; A Cassel, G.; Gear, A.J.; Trappler, B.; Clausen, L.; Meyers, A.M.; Kew, M.C.; Bothwell, T.H.; Sher, R.; Miller, G.B.; et al. Outbreake of Marburg virus disease in Johannesburg. BMJ 1975, 4, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Borchert, M.; Muyembe-Tamfum, J.J.; Colebunders, R.; Libande, M.; Sabue, M.; Van der Stuyft, P. Short communication: A cluster of Marburg virus disease involving an infant*. Trop. Med. Int. Health 2002, 7, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.-J.; Tugume, B.; et al. Risk Factors for Marburg Hemorrhagic Fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003, 9, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.A.; Glynn, A.R.; Steele, K.E.; Lackemeyer, M.G.; Garza, N.L.; Buck, J.G.; Mech, C.; Reed, D.S. Aerosol Exposure to the Angola Strain of Marburg Virus Causes Lethal Viral Hemorrhagic Fever in Cynomolgus Macaques. Veter. Pathol. 2010, 47, 831–851. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Daddario-DiCaprio, K.M.; Geisbert, J.B.; Reed, D.S.; Feldmann, F.; Grolla, A.; Ströher, U.; Fritz, E.A.; Hensley, L.E.; Jones, S.M.; et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 2008, 26, 6894–6900. [Google Scholar] [CrossRef]
- Lin, K.L.; Twenhafel, N.A.; Connor, J.H.; Cashman, K.A.; Shamblin, J.D.; Donnelly, G.C.; Esham, H.L.; Wlazlowski, C.B.; Johnson, J.C.; Honko, A.N.; et al. Temporal Characterization of Marburg Virus Angola Infection following Aerosol Challenge in Rhesus Macaques. J. Virol. 2015, 89, 9875–9885. [Google Scholar] [CrossRef]
- Mitu, R.A.; Islam, R. The Current Pathogenicity and Potential Risk Evaluation of Marburg Virus to Cause Mysterious “Disease X”—An Update on Recent Evidences. Environ. Health Insights 2024, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, L.; Matthews, E.; Piquet, A.L.; Henao-Martinez, A.; Franco-Paredes, C.; Tyler, K.L.; Beckham, D.; Pastula, D.M. Nervous System Manifestations of Arboviral Infections. Curr. Trop. Med. Rep. 2022, 9, 107–118. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Grolla, A.; Fukuma, A.; Feldmann, H.; Yasuda, J. Development and Evaluation of a Simple Assay for Marburg Virus Detection Using a Reverse Transcription-Loop-Mediated Isothermal Amplification Method. J. Clin. Microbiol. 2010, 48, 2330–2336. [Google Scholar] [CrossRef]
- Grolla, A.; Lucht, A.; Dick, D.; E Strong, J.; Feldmann, H. Laboratory diagnosis of Ebola and Marburg hemorrhagic fever. Bull. Soc. Pathol. Exot. 2005, 98, 205–209. [Google Scholar]
- Saijo, M.; Niikura, M.; Ikegami, T.; Kurane, I.; Kurata, T.; Morikawa, S. Laboratory Diagnostic Systems for Ebola and Marburg Hemorrhagic Fevers Developed with Recombinant Proteins. Clin. Vaccine Immunol. 2006, 13, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kortepeter, M.G.; Dierberg, K.; Shenoy, E.S.; Cieslak, T.J. Marburg Virus Disease: A Summary for Clinicians. Int. J. Infect. Dis. 2020, 99, 233–242. [Google Scholar] [CrossRef]
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; Van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Collins, D.; Dobo, S.; Walling, D.M.; Sheridan, W.P.; Taylor, R. Pharmacokinetics and Safety of the Nucleoside Analog Antiviral Drug Galidesivir Administered to Healthy Adult Subjects. Clin. Pharmacol. Drug Dev. 2022, 11, 467–474. [Google Scholar] [CrossRef]
- Bixler, S.L.; Bocan, T.M.; Wells, J.; Wetzel, K.S.; Van Tongeren, S.A.; Dong, L.; Garza, N.L.; Donnelly, G.; Cazares, L.H.; Nuss, J.; et al. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antivir. Res. 2018, 151, 97–104. [Google Scholar] [CrossRef]
- Porter, D.P.; Weidner, J.M.; Gomba, L.; Bannister, R.; Blair, C.; Jordan, R.; Wells, J.; Wetzel, K.; Garza, N.; Van Tongeren, S.; et al. Remdesivir (GS-5734) Is Efficacious in Cynomolgus Macaques Infected with Marburg Virus. J. Infect. Dis. 2020, 222, 1894–1901. [Google Scholar] [CrossRef]
- Cross, R.W.; Bornholdt, Z.A.; Prasad, A.N.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Abelson, D.M.; Kim, D.H.; Shestowsky, W.S.; Campbell, L.A.; et al. Combination therapy protects macaques against advanced Marburg virus disease. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Bornholdt, Z.A.; Prasad, A.N.; Woolsey, C.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Abelson, D.M.; Kim, D.H.; Shestowsky, W.S.; et al. Combination therapy with remdesivir and monoclonal antibodies protects nonhuman primates against advanced Sudan virus disease. J. Clin. Investig. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Darwish, H.; Darwish, I.A.; Al-Ghusn, A.I. Chapter Three—Remdesivir. Profiles Drug. Subst. Excip. Relat. Methodol. 2023, 48, 71–108. [Google Scholar] [CrossRef]
- Rwanda Ministry of Health Rwanda National Guidelines for Management of Marburg Virus Disease. 2024.
- Muvunyi, C.M.; Bigirimana, N.; Tuyishime, A.; Mukagatare, I.; Ngabonziza, J.C.; Ahmed, A. Initiatives and Strategies to Strengthen the National, Regional, and International Global Health Security: A Case Study of Rwanda Biomedical Centre. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4957490 (accessed on 10 October 2024).
- Gashema, P.; Musafiri, T.; Ndahimana, F.; Iradukunda, H.; Saramba, E.; Nyakatswau, S.T.; Gahamanyi, N.; Iradukunda, P.G.; Ahmed, A.; Dzinamarira, T.; et al. Mpox in East Africa: Learning from COVID-19 and Ebola to Strengthen Public Health Responses. Viruses 2024, 16, 1578. [Google Scholar] [CrossRef]
- Bockarie, M.J.; Hanson, J.; Ansumana, R.; Yeboah-Manu, D.; Zumla, A.; Lee, S.S. The re-emergence of Marburg virus Disease in West Africa: How prepared is the sub-region for preventing recurrent zoonotic outbreaks? Int. J. Infect. Dis. 2023, 130, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Gashegu, M.; Ahmed, A.; Clarisse, M.; Remera, E.; Tuyishime, A.; Rwagasore, E.; Muhizi, D.; Kanesa, N.; Ndayisenga, F.; Thadee, T.; et al. One Health Prioritization for Zoonotic Diseases of Public Health Importance in Rwanda. Lancet 2024, in press. [Google Scholar]
- Ali, Y.; Siddig, E.E.; Osman, M.; Mohamed, N.S.; Musa, A.; Ahmed, A. Preparedness, Prevention, Investigation, and Response to the Emergence of Mpox in Khartoum, Sudan in 2022. Preprints 2024, in press. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the first molecularly confirmed outbreak of Rift Valley fever among humans in Rwanda, calls for institutionalizing the One Health strategy. IJID One Health 2024, 4, 1–3. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Nsekuye, O.; Semakula, M.; Gashegu, M.; Rutayisire, R.; Ishema, L.; Musanabaganwa, C.; Butera, Y.; Nsanzimana, S.; et al. Rift Valley Fever Epizootic, Rwanda, 2022. Emerg. Infect. Dis. 2024, 30, 2191–2193. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, Y.; Ibrahim, N.A.; Mohamed, S.I.; Zinsstag, J.; Siddig, E.E.; Mohamed, N.S.; Muvunyi, C.M. One Health Response for Rift Valley Fever Outbreak in Sudan. Preprints 2024, in press. [Google Scholar]
- Nsengimana, I.; Juma, J.; Roesel, K.; Gasana, M.N.; Ndayisenga, F.; Muvunyi, C.M.; Hakizimana, E.; Hakizimana, J.N.; Eastwood, G.; Chengula, A.A.; et al. Genomic Epidemiology of Rift Valley Fever Virus Involved in the 2018 and 2022 Outbreaks in Livestock in Rwanda. Viruses 2024, 16, 1148. [Google Scholar] [CrossRef]
- Ali, Y.; Ahmed, A.; Siddig, E.E.; Mohamed, N.S. The role of integrated programs in the prevention of COVID-19 in a humanitarian setting. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 193–196. [Google Scholar] [CrossRef]
- Ssemanda, J.N.; Reij, M.W.; Bagabe, M.C.; Muvunyi, C.M.; Nyamusore, J.; Joosten, H.; Zwietering, M.H. Estimates of the burden of illnesses related to foodborne pathogens as from the syndromic surveillance data of 2013 in Rwanda. Microb. Risk Anal. 2018, 9, 55–63. [Google Scholar] [CrossRef]
- CDC About Marburg. Available online: https://www.cdc.gov/marburg/about/index.html (accessed on 10 October 2024).
- Wirsiy, F.S.; Nkfusai, C.N.; Bain, L.E. The SPIN Framework to Control and Prevent the Marburg Virus Disease Outbreak in Equatorial Guinea. Pan. Afr. Med. J. 2023, 44, 110. [Google Scholar] [CrossRef]
- Ahmed, A. Urgent call for a global enforcement of the public sharing of health emergencies data: Lesson learned from serious arboviral disease epidemics in Sudan. Int. Health 2020, 12, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mahmoud, I.; Eldigail, M.; Elhassan, R.M.; Weaver, S.C. The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens 2021, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO). International Health Regulations (2005); World Health Organization: Geneva, Switzerland, 2008; ISBN 92-4-158041-0. [Google Scholar]
- Brainard, J.; Hooper, L.; Pond, K.; Edmunds, K.; Hunter, P.R. Risk factors for transmission of Ebola or Marburg virus disease: A systematic review and meta-analysis. Leuk. Res. 2015, 45, 102–116. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Ali, Y.; Abdalrahman, S.; Ahmed, A.; Siddig, E.E. The use of cholera oral vaccine for containment of the 2019 disease outbreak in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 763–766. [Google Scholar] [CrossRef]
- Packham, A.; Taylor, A.E.; Karangwa, M.-P.; Sherry, E.; Muvunyi, C.; Green, C.A. Measles Vaccine Coverage and Disease Outbreaks: A Systematic Review of the Early Impact of COVID-19 in Low and Lower-Middle Income Countries. Int. J. Public Health 2024, 69, 1606997. [Google Scholar] [CrossRef] [PubMed]
- Manno, D. Developing a vaccine against Marburg virus disease. Lancet 2023, 401, 251–253. [Google Scholar] [CrossRef]
- O’Donnell, K.L.; Feldmann, F.; Kaza, B.; Clancy, C.S.; Hanley, P.W.; Fletcher, P.; Marzi, A. Rapid protection of nonhuman primates against Marburg virus disease using a single low-dose VSV-based vaccine. EBioMedicine 2023, 89, 104463. [Google Scholar] [CrossRef]
- Gear, J.H.S. Handbook of Viral and Rickettsial Hemorrhagic Fevers; Margaretha, I., Ed.; Prevention and Control of Viral Hemorrhagic Fevers; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-0-429-27673-6. [Google Scholar] [CrossRef]
- Heeney, J.L. Hidden reservoirs. Nature 2015, 527, 453–455. [Google Scholar] [CrossRef]
| Phase | Duration | Characteristics | Symptoms |
|---|---|---|---|
| Generalized Phase | Days 1 to 4 | Abrupt onset of nonspecific symptoms | High fever (39–40° C), severe headache, chills, myalgia, prostration, and malaise |
| Early Phase | Days 5 to 13 | 50–75% of patients experience gastrointestinal symptoms | Anorexia, abdominal discomfort, severe nausea, vomiting, diarrhea, maculopapular rash, and symptoms of hemorrhagic fever (petechiae, mucosal, and gastrointestinal bleeding, hemorrhage from venipuncture sites) |
| Convalescence Phase | After Day 13 | Survivors may skip the most severe symptoms and may not progress to this phase | Neurological symptoms may present (disorientation, agitation, seizures, and coma), recovery symptoms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muvunyi, C.M.; Ngabonziza, J.C.S.; Bigirimana, N.; Ndembi, N.; Siddig, E.E.; Kaseya, J.; Ahmed, A. Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks. Diseases 2024, 12, 309. https://doi.org/10.3390/diseases12120309
Muvunyi CM, Ngabonziza JCS, Bigirimana N, Ndembi N, Siddig EE, Kaseya J, Ahmed A. Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks. Diseases. 2024; 12(12):309. https://doi.org/10.3390/diseases12120309
Chicago/Turabian StyleMuvunyi, Claude Mambo, Jean Claude Semuto Ngabonziza, Noella Bigirimana, Nicaise Ndembi, Emmanuel Edwar Siddig, Jean Kaseya, and Ayman Ahmed. 2024. "Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks" Diseases 12, no. 12: 309. https://doi.org/10.3390/diseases12120309
APA StyleMuvunyi, C. M., Ngabonziza, J. C. S., Bigirimana, N., Ndembi, N., Siddig, E. E., Kaseya, J., & Ahmed, A. (2024). Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks. Diseases, 12(12), 309. https://doi.org/10.3390/diseases12120309







