Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective of the Study
- To analyze the differences in the results of bioelectrical impedance analysis (BIA) of body composition between male childhood brain tumor cancer survivors and healthy controls.
- 2.
- To evaluate the correlation between BIA results and the treatments performed (chemotherapy, radiotherapy, and steroid therapy).
2.2. Study Design and Inclusion and Exclusion Criteria
2.3. Disease- and Treatment-Related Data
- Anthropometric parameters at the time of diagnosis: age, weight, height, BMI, BMI percentile, cancer histology, site of disease, and presence of metastasis.
- Data related to the treatment: chemotherapy protocol, site and dose of radiotherapy, high dose of chemotherapy followed by autologous transplantation (ASCT), and duration of supportive steroid therapy during treatment.
- Presence of endocrinological disfunction occurred during or after the end of oncological treatment.
2.4. Anthropometric Characteristics
- Height, height-for-age percentile, and Z-score, calculated with a CDC growth charts-based percentile calculator available online (https://peditools.org/, last accessed on 19 February 2024).
- Weight (measured in the absence of clothing except undergarments with electronic scales), weight-for-age percentile, and Z-score, calculated with a CDC growth chart-based percentile calculator available online (https://peditools.org/, last accessed on 19 February 2024).
- BMI, calculated as (weight in kg)/(height in m)2, BMI-for-age percentile, and Z-score, calculated with a CDC growth chart-based percentile calculator available online (https://peditools.org/, last accessed on 19 February 2024).
- Neck, chest, arm, wrist, thigh, and calf circumference in cm; waist circumference (reported in cm; it was measured as the circumference in the smallest point between the last rib and the top of the iliac crest); and hip circumference (reported in cm; it was measured at the major circumference point at the posterior extension of the buttocks).
- Waist-to-hip ratio (WHR), expressed as the value obtained using the formula (waist circumference in cm)/(hip circumference in cm).
- Waist-to-height ratio (WHtR), expressed as the value obtained using the formula (waist circumference in cm)/(height in cm), with a value of WHtR > 0.5 considered indicative of central obesity.
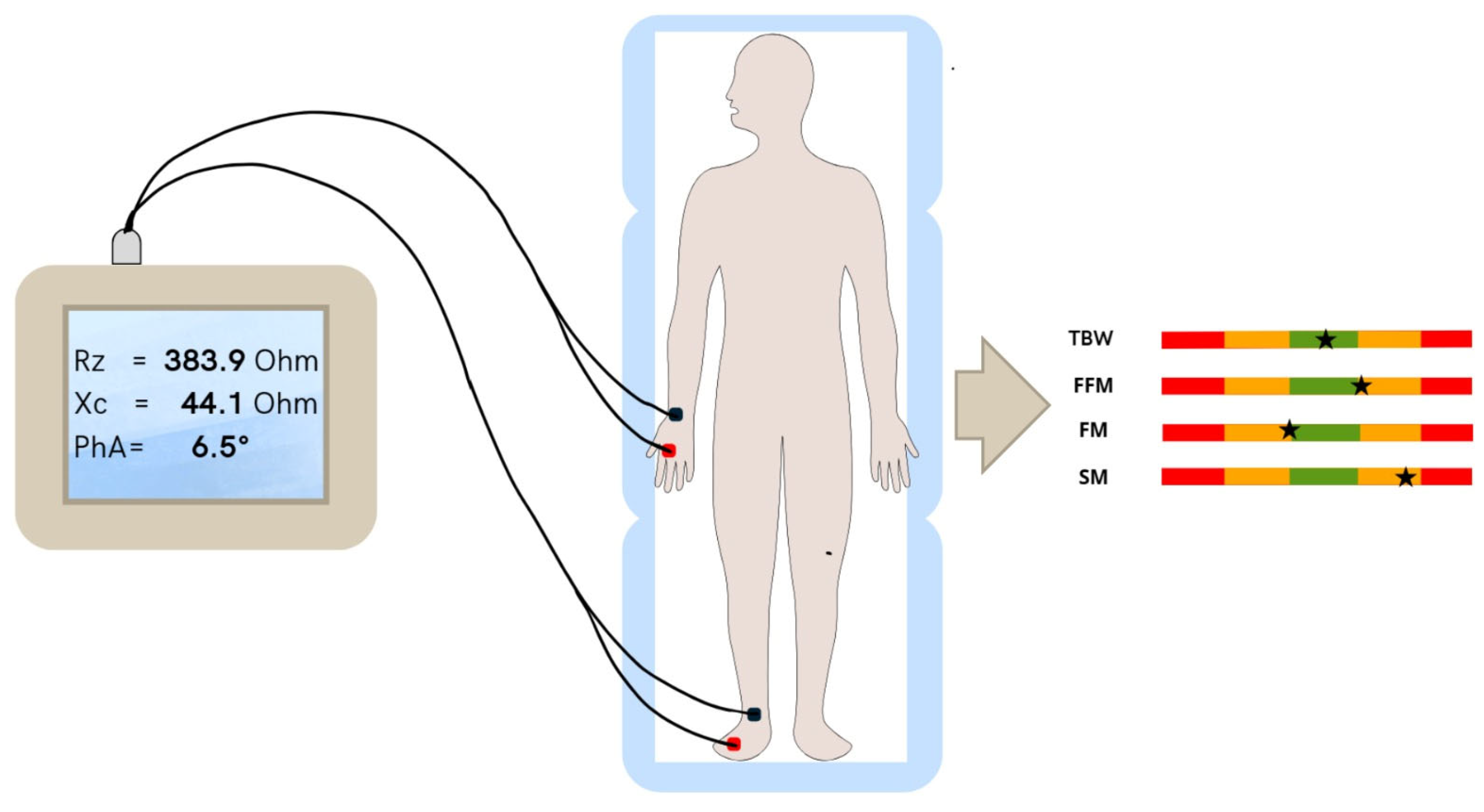
2.5. Body Composition Measurements
2.6. Sample Size and Statistical Analysis
3. Results
3.1. Disease- and Treatment-Related Data
3.2. Anthropometric Characteristics
3.3. BIA Results
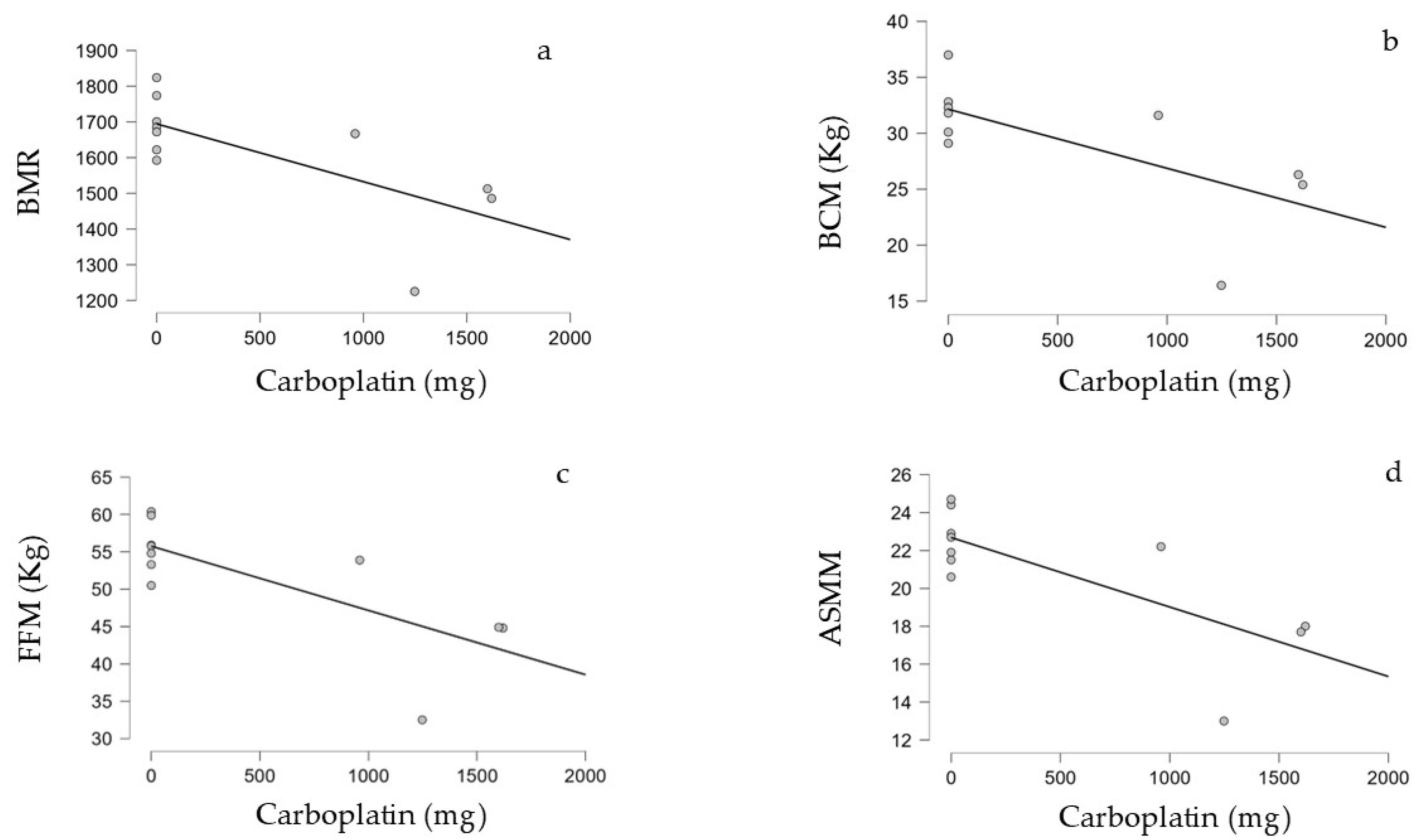
3.4. Correlation Between Treatment Performed and BIA Parameters in the Survivors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, M.; Phillips, P.C. Pediatric Brain Tumors. Contin. Lifelong Learn. Neurol. 2017, 23, 1727. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Tonorezos, E.S.; Landier, W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA 2023, 330, 1175. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Chow, E.J.; Goodman, P.J.; Leisenring, W.M.; Dietz, A.C.; Perkins, J.L.; Chow, L.; Sinaiko, A.; Moran, A.; Petryk, A.; et al. Impact of Treatment Exposures on Cardiovascular Risk and Insulin Resistance in Childhood Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari, K.K.; Lanning, M.; Tapanainen, P.; Knip, M. Long-Term Survivors of Childhood Cancer Have an Increased Risk of Manifesting the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3051–3055. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Del Vescovo, E.; Rivetti, S.; Triarico, S.; Attinà, G.; Mastrangelo, S.; Maurizi, P.; Ruggiero, A. Biomarkers Predictive of Metabolic Syndrome and Cardiovascular Disease in Childhood Cancer Survivors. J. Pers. Med. 2022, 12, 880. [Google Scholar] [CrossRef]
- Sofia, R.; Melita, V.; De Vita, A.; Ruggiero, A.; Romano, A.; Attinà, G.; Birritella, L.; Lamendola, P.; Lombardo, A.; Lanza, G.A.; et al. Cardiac Surveillance for Early Detection of Late Subclinical Cardiac Dysfunction in Childhood Cancer Survivors After Anthracycline Therapy. Front. Oncol. 2021, 11, 624057. [Google Scholar] [CrossRef]
- Follin, C.; Erfurth, E.M. Long-Term Effect of Cranial Radiotherapy on Pituitary-Hypothalamus Area in Childhood Acute Lymphoblastic Leukemia Survivors. Curr. Treat. Options Oncol. 2016, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Antal, Z.; Chemaitilly, W.; Cohen, L.E.; Follin, C.; Meacham, L.R.; Murad, M.H. Hypothalamic–Pituitary and Growth Disorders in Survivors of Childhood Cancer: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 2761–2784. [Google Scholar] [CrossRef]
- Sodero, G.; Agresti, P.; Triarico, S.; Romano, A.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Cipolla, C.; Ruggiero, A. Growth Hormone Replacement Therapy in Pediatric Brain Tumor Survivors. Minerva Pediatr. 2022, 74, 340–348. Available online: https://www.minervamedica.it/it/riviste/minerva-pediatrics/articolo.php?cod=R15Y2022N03A0340 (accessed on 27 January 2024). [CrossRef]
- Pluimakers, V.G.; van Waas, M.; Looman, C.W.N.; de Maat, M.P.; de Jonge, R.; Delhanty, P.; Huisman, M.; Mattace-Raso, F.U.S.; van den Heuvel-Eibrink, M.M.; Neggers, S.J.C.M.M. Metabolic Syndrome Detection with Biomarkers in Childhood Cancer Survivors. Endocr. Connect. 2020, 9, 676–686. [Google Scholar] [CrossRef]
- Pluimakers, V.G.; van Santen, S.S.; Fiocco, M.; Bakker, M.-C.E.; van der Lelij, A.J.; van den Heuvel-Eibrink, M.M.; Neggers, S.J.C.M.M. Can Biomarkers Be Used to Improve Diagnosis and Prediction of Metabolic Syndrome in Childhood Cancer Survivors? A Systematic Review. Obes. Rev. 2021, 22, e13312. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Jansen-Chaparro, S.; Saigi, I.; Bernal-Lopez, M.R.; Miñambres, I.; Gomez-Huelgas, R. Glucocorticoid-induced hyperglycemia. J. Diabetes 2014, 6, 9–20. [Google Scholar] [CrossRef]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial Dysfunction and Cardiovascular Risk Factors in Childhood Acute Lymphoblastic Leukemia Survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.H.J.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, Obesity, and Inflammation—Results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors Study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Ibarra, M.J.; Saucedo-Tamayo, M.D.S.; Alemán-Mateo, H.; Parra-Sánchez, H.; Othón-Ontiveros, P.; Hernández, J.; Caire-Juvera, G. Association Between Interleukin 6 and C-Reactive Protein Serum Levels and Body Composition Compartments and Components in Breast Cancer Survivors. Biol. Res. Nurs. 2024, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh Adipose Tissue Distribution Is Associated with Insulin Resistance in Obesity and in Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [CrossRef]
- Marcell, T.J. Sarcopenia: Causes, Consequences, and Preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M911–M916. [Google Scholar] [CrossRef]
- Chu, L.-W.; Tam, S.; Kung, A.W.C.; Lo, S.; Fan, S.; Wong, R.L.C.; Morley, J.E.; Lam, K.S.L. Serum Total and Bioavailable Testosterone Levels, Central Obesity, and Muscle Strength Changes with Aging in Healthy Chinese Men. J. Am. Geriatr. Soc. 2008, 56, 1286–1291. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Barnea, D.; Raghunathan, N.; Friedman, D.N.; Tonorezos, E.S. Obesity and Metabolic Disease After Childhood Cancer. Oncology 2015, 29, 849–855. [Google Scholar]
- Lee, J.; Hong, Y.; Shin, H.J.; Lee, W. Associations of Sarcopenia and Sarcopenic Obesity with Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-S. Low Skeletal Muscle Mass Is Associated with Insulin Resistance, Diabetes, and Metabolic Syndrome in the Korean Population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr. J. 2014, 61, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Di Paola, A.; Pota, E.; Argenziano, M.; Di Pinto, D.; Marrapodi, M.M.; Di Leva, C.; Di Martino, M.; Tortora, C. Biological Aspects of Inflamm-Aging in Childhood Cancer Survivors. Cancers 2021, 13, 4933. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-W.; Fleming, A.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Ladhani, S.; Socha, A.; Shinuda, J.; Jaber, S.; Burrow, S.; et al. Overweight, Obesity and Adiposity in Survivors of Childhood Brain Tumours: A Systematic Review and Meta-Analysis. Clin. Obes. 2018, 8, 55–67. [Google Scholar] [CrossRef]
- Wang, K.-W.; de Souza, R.J.; Fleming, A.; Singh, S.K.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Scheinemann, K.; Thabane, L.; et al. Adiposity in Childhood Brain Tumors: A Report from the Canadian Study of Determinants of Endometabolic Health in Children (CanDECIDE Study). Sci. Rep. 2017, 7, 45078. [Google Scholar] [CrossRef]
- Piqueras, P.; Ballester, A.; Durá-Gil, J.V.; Martinez-Hervas, S.; Redón, J.; Real, J.T. Anthropometric Indicators as a Tool for Diagnosis of Obesity and Other Health Risk Factors: A Literature Review. Front. Psychol. 2021, 12, 631179. [Google Scholar] [CrossRef]
- Sims, E.D.; Wang, K.-W.; Fleming, A.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Thabane, L.; Samaan, M.C. Tri-Ponderal Mass Index in Survivors of Childhood Brain Tumors: A Cross-Sectional Study. Sci. Rep. 2018, 8, 16336. [Google Scholar] [CrossRef]
- Karlage, R.E.; Wilson, C.L.; Zhang, N.; Kaste, S.; Green, D.M.; Armstrong, G.T.; Robison, L.L.; Chemaitilly, W.; Srivastava, D.K.; Hudson, M.M.; et al. Validity of Anthropometric Measurements to Characterize Obesity among Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer 2015, 121, 2036–2043. [Google Scholar] [CrossRef]
- Blijdorp, K.; van den Heuvel-Eibrink, M.M.; Pieters, R.; Boot, A.M.; Delhanty, P.J.D.; van der Lely, A.-J.; Neggers, S.J.C.M.M. Obesity Is Underestimated Using Body Mass Index and Waist-Hip Ratio in Long-Term Adult Survivors of Childhood Cancer. PLoS ONE 2012, 7, e43269. [Google Scholar] [CrossRef]
- Romano, A.; Sollazzo, F.; Rivetti, S.; Morra, L.; Servidei, T.; Lucchetti, D.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Zovatto, I.C.; et al. Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study. Cancers 2024, 16, 324. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of Tetrapolar Bioelectrical Impedance Method to Assess Human Body Composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.C.; Melahrinides, E.; Michielli, D.W.; Kalinski, M.I. Effects of Small Errors in Electrode Placement on Body Composition Assessment by Bioelectrical Impedance. Res. Q. Exerc. Sport 1994, 65, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Głąbska, D.; Cackowska, K.; Guzek, D. Comparison of the Body Composition of Caucasian Young Normal Body Mass Women, Measured in the Follicular Phase, Depending on the Carbohydrate Diet Level. Medicina 2018, 54, 104. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Slosman, D.O.; Pichard, C. Fat-Free and Fat Mass Percentiles in 5225 Healthy Subjects Aged 15 to 98 Years. Nutrition 2001, 17, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.D.; Boyden, C.M. Body Cell Mass and Limits of Hydration of the Fat-Free Body: Their Relation to Estimated Skeletal Weight. Ann. New York Acad. Sci. 1963, 110, 62–71. [Google Scholar] [CrossRef]
- Talluri, A.; Liedtke, R.; Mohamed, E.I.; Maiolo, C.; Martinoli, R.; De Lorenzo, A. The Application of Body Cell Massindex for Studying Muscle Mass Changes in Health and Diseaseconditions. Acta Diabetol. 2003, 40, s286–s289. [Google Scholar] [CrossRef]
- Rondanelli, M.; Talluri, J.; Peroni, G.; Donelli, C.; Guerriero, F.; Ferrini, K.; Riggi, E.; Sauta, E.; Perna, S.; Guido, D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a Useful Prognostic Factor to Describe Nutritional, Inflammation and Muscle Mass Status in Hospitalized Elderly?: Body Cell Mass Index Links in Elderly. Clin. Nutr. 2018, 37, 934–939. [Google Scholar] [CrossRef]
- Orgel, E.; Sea, J.L.; Mittelman, S.D. Mechanisms by Which Obesity Impacts Survival from Acute Lymphoblastic Leukemia. J. Natl. Cancer Inst. Monogr. 2019, 2019, 152–156. [Google Scholar] [CrossRef]
- Marriott, C.J.C.; Beaumont, L.F.; Farncombe, T.H.; Cranston, A.N.; Athale, U.H.; Yakemchuk, V.N.; Webber, C.E.; Barr, R.D. Body Composition in Long-Term Survivors of Acute Lymphoblastic Leukemia Diagnosed in Childhood and Adolescence: A Focus on Sarcopenic Obesity. Cancer 2018, 124, 1225–1231. [Google Scholar] [CrossRef]
- Suzuki, Y.; Aono, Y.; Kono, M.; Hasegawa, H.; Yokomura, K.; Naoi, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; et al. Cause of Mortality and Sarcopenia in Patients with Idiopathic Pulmonary Fibrosis Receiving Antifibrotic Therapy. Respirology 2021, 26, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Buğdaycı, O.; Eker, N. The Impact of Sarcopenia and Sarcopenic Obesity on Survival in Children with Ewing Sarcoma and Osteosarcoma. Pediatr. Radiol. 2023, 53, 854–861. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, W.E.; Murra, M.S.; Tufi, L.M.B.; Cavalcante, C.E.B.; de Oliveira, M.A.; da Costa, R.F.A.; Rosa, B.R.; da Silva, R.Z.M.; Ribeiro, R.C.; Ladas, E.J.; et al. Sarcopenia in Children with Wilms Tumor: A Marker of Undernutrition Which May Impact Adversely on Clinical Outcomes. J. Pediatr. Hematol. Oncol. 2023, 45, e817–e822. [Google Scholar] [CrossRef] [PubMed]
- Ritz, A.; Kolorz, J.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Koletzko, S.; Häberle, B.; Schmid, I.; Kappler, R.; Berger, M.; et al. Sarcopenia Is a Prognostic Outcome Marker in Children with High-Risk Hepatoblastoma. Pediatr. Blood Cancer 2021, 68, e28862. [Google Scholar] [CrossRef] [PubMed]
- Ritz, A.; Lurz, E.; Berger, M. Sarcopenia in Children with Solid Organ Tumors: An Instrumental Era. Cells 2022, 11, 1278. [Google Scholar] [CrossRef]
- Guo, M.; Zemel, B.S.; Hawkes, C.P.; Long, J.; Kelly, A.; Leonard, M.B.; Jaramillo, D.; Mostoufi-Moab, S. Sarcopenia and Preserved Bone Mineral Density in Paediatric Survivors of High-risk Neuroblastoma with Growth Failure. J. Cachexia Sarcopenia Muscle 2021, 12, 1024–1033. [Google Scholar] [CrossRef]
- Nakamura, N.; Kishimoto, K.; Ishida, T.; Nakamura, S.; Tamura, A.; Kozaki, A.; Saito, A.; Hasegawa, D.; Kosaka, Y. Muscle Mass Change during Chemotherapy in Children with High-Risk Neuroblastoma: A Retrospective Case Series of 24 Patients. Eur. J. Pediatr. 2021, 180, 3265–3271. [Google Scholar] [CrossRef]
- Kawakubo, N.; Kinoshita, Y.; Souzaki, R.; Koga, Y.; Oba, U.; Ohga, S.; Taguchi, T. The Influence of Sarcopenia on High-Risk Neuroblastoma. J. Surg. Res. 2019, 236, 101–105. [Google Scholar] [CrossRef]
- Romano, A.; Triarico, S.; Rinninella, E.; Natale, L.; Brizi, M.G.; Cintoni, M.; Raoul, P.; Maurizi, P.; Attinà, G.; Mastrangelo, S.; et al. Clinical Impact of Nutritional Status and Sarcopenia in Pediatric Patients with Bone and Soft Tissue Sarcomas: A Pilot Retrospective Study (SarcoPed). Nutrients 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Revuelta Iniesta, R.; Paciarotti, I.; Davidson, I.; McKenzie, J.M.; Brougham, M.F.H.; Wilson, D.C. Nutritional Status of Children and Adolescents with Cancer in Scotland: A Prospective Cohort Study. Clin. Nutr. ESPEN 2019, 32, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Brinksma, A.; Roodbol, P.F.; Sulkers, E.; Kamps, W.A.; de Bont, E.S.J.M.; Boot, A.M.; Burgerhof, J.G.M.; Tamminga, R.Y.J.; Tissing, W.J.E. Changes in Nutritional Status in Childhood Cancer Patients: A Prospective Cohort Study. Clin. Nutr. 2015, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Freeman, L.M.; Smith, D.E.; Abad, L.W.; Dinarello, C.A.; Kehayias, J.J. Adjuvant Arthritis as a Model of Inflammatory Cachexia. Arthritis Rheum. 1997, 40, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-α with Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Tumor Necrosis Factor Alpha Inhibits Signaling from the Insulin Receptor. Available online: https://www.pnas.org/doi/10.1073/pnas.91.11.4854 (accessed on 9 October 2024).
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Ruggiero, A.; Trombatore, G.; Triarico, S.; Arena, R.; Ferrara, P.; Scalzone, M.; Pierri, F.; Riccardi, R. Platinum com-pounds in children with cancer: Toxicity and clinical management. Anti-Cancer Drugs 2013, 24, 1007–1019. [Google Scholar] [CrossRef]
| Number (%) or Mean (SD) | |
|---|---|
| Age at diagnosis (years) | 9.6 (5) |
| Weight (kg) | 43.8 (22.3) |
| Weight percentile | 71.8 (33.9) |
| Weight Z-score | 0.9 (1.2) |
| Height (m) | 1.4 (0.3) |
| Height percentile | 52.8 (33.3) |
| Height Z-score | 0.1 (1.2) |
| BMI (kg/sqcm) | 21.1 (4.8) |
| BMI percentile | 87.9 (17.3) |
| Age at the enrollment (years) | 24.9 (3.9) |
| Time of follow up at the enrollment (months) | 171 (54) |
| Number of Patients (%) | |
|---|---|
| Histology | |
| Germ-cell tumor | 8 (57%) |
| Medulloblastoma | 4 (29%) |
| Ependymoma | 2 (14%) |
| Primary localization | |
| Anterior cranial fossa | 0 (0%) |
| Middle cranial fossa | 8 (57%) |
| Posterior cranial fossa | 6 (43%) |
| Number of Patients (%) or Mean (SD) | |
|---|---|
| Patients subjected to cranial radiotherapy | 14 (100%) |
| Patients subjected to spinal radiotherapy | 5 (36%) |
| Cranial radiotherapy dose (Gy) | 56.7 (16.6) |
| Spinal radiotherapy dose (Gy) | 30 (0) |
| Patients subjected to chemotherapy | 12 (86%) |
| Patients subjected to steroid therapy (more than 14 days) | 10 (71%) |
| Patients subjected to ASCT * | 2 (14%) |
| Relapsed disease | 2 (14%) |
| Cases | Controls | p Value | |
|---|---|---|---|
| [Mean (SD) or Number of Patients (%)] | [Mean (SD) or Number of Patients (%)] | ||
| Age (years) | 24.93 (3.89) | 24.64 (2.92) | 0.834 |
| Weight (kg) | 67.89 (10.89) | 76.81 (8.85) | 0.025 |
| Weight percentile | 48.23 (19.08) | 64.79 (23.91) | 0.015 * |
| Weight Z-score | −0.31 (1.12) | 0.41 (0.70) | 0.347 |
| Height (m) | 1.68 (0.10) | 1.77 (0.08) | 0.015 * |
| Height percentile | 28.67 (19.65) | 51.79 (33.23) | 0.007 * |
| Height Z-score | −1.19 (1.40) | 0.06 (1.13) | 0.434 |
| BMI (kg/m2) | 23.99 (3.22) | 24.59 (3.19) | 0.624 |
| BMI percentile | 57.34 (26.90) | 58.91 (26.29) | 0.877 |
| BMI Z-Score | 0.11 (1.12) | 0.31 (0.83) | 0.607 |
| Waist circumference (cm) | 83.04 (8.31) | 80.36 (9.83) | 0.079 |
| Hip circumference (cm) | 91.07 (7.44) | 87.86 (12.08) | 0.178 |
| WHR | 0.91 (0.05) | 0.92 (0.07) | <0.002 * |
| WHtR | 0.49 (0.05) | 0.45 (0.06) | 0.049 * |
| WHtR > 0.5 | 5 (36%) | 3 (21%) | 0.402 |
| Neck circumference (cm) | 36,214 (3.63) | 37,900 (1921) | 62 |
| Chest circumference (cm) | 94,500 (8644) | 91,846 (13,266) | 802 |
| Wrist circumference (cm) | 17,057 (1588) | 17,069 (781) | 554 |
| Arm circumference (cm) | 29,500 (3627) | 29,885 (2952) | 971 |
| Thigh circumference (cm) | 54,857 (14,347) | 53,808 (4381) | 388 |
| Calf circumference (cm) | 36,129 (3879) | 37,192 (2411) | 413 |
| Cases | Controls | p-Value | |
|---|---|---|---|
| [Mean (SD) or 50th Percentile (IQR)] | [Mean (SD) or 50th Percentile (IQR)] | ||
| BSA | 1.804 (0.214) | 1.901 (0.126) | 0.158 |
| BMI | 23.679 (3.715) | 24.900 (2.476) | 0.316 |
| XC | 68.273 (12.650) | 63.615 (6.764) | 0.262 |
| PA | 6.882 (0.946) | 7.438 (0.690) | 0.11 |
| BMR | 1614.636 (163.399) | 1828.177 (159.454) | 0.004 * |
| BCM (kg) | 29.280 (5.611) | 37.185 (5.498) | 0.003 * |
| BCMI (kg/m2) | 10.755 (1.545) | 11.900 (1.087) | 0.045 * |
| FFM (kg) | 51.518 (8.111) | 61.677 (7.179) | 0.004 * |
| FFM (%) | 80.064 (5.285) | 79.908 (9.186) | 0.961 |
| FFMI (kg/m2) | 18.609 (1.795) | 19.785 (1.442) | 0.089 |
| FM (kg) | 13.027 (3.966) | 14.554 (5.462) | 0.477 |
| FMI (kg/m2) | 4.745 (1.705) | 4.762 (2.087) | 0.984 |
| FM (%) | 19.936 (5.285) | 18.323 (6.139) | 0.502 |
| TBW (L) | 34.818 (6.938) | 44.869 (5.422) | <0.001 * |
| TBW (%) | 56.727 (5.603) | 59.069 (4.638) | 0.247 |
| SPA | 0.073 (1.210) | 0.798 (1.005) | 0.122 |
| SM (kg) | 28.100 (3.714) | 33.108 (4.162) | 0.007 * |
| SMI | 9.873 (0.852) | 10.608 (0.754) | 0.035 * |
| ASMM | 20.873 (3.437) | 25.592 (3.241) | 0.002 * |
| Rz | 527.00 (91.00) | 491.00 (56.00) | 0.018 * |
| Hydration | 72.700 (3.650) | 72.900 (0.475) | 0.084 |
| ECW (%) | 41.3 | 40.00 (1.800) | 0.118 |
| ECW (L) | 39.664 (2.842) | 39.985 (1.871) | 0.017 * |
| Carboplatin (Total Dose in mg) | ||
|---|---|---|
| BMR | Kendall’s Tau | −0.601 |
| p-value | 0.018 | |
| BCM (kg) | Kendall’s Tau | −0.599 |
| p-value | 0.025 | |
| FFM (kg) | Kendall’s Tau | −0.601 |
| p-value | 0.018 | |
| ASMM | Kendall’s Tau | −0.509 |
| p-value | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Sollazzo, F.; Corbo, F.; Attinà, G.; Mastrangelo, S.; Cordaro, S.; Modica, G.; Zovatto, I.C.; Monti, R.; Bianco, M.; et al. Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors. Diseases 2024, 12, 306. https://doi.org/10.3390/diseases12120306
Romano A, Sollazzo F, Corbo F, Attinà G, Mastrangelo S, Cordaro S, Modica G, Zovatto IC, Monti R, Bianco M, et al. Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors. Diseases. 2024; 12(12):306. https://doi.org/10.3390/diseases12120306
Chicago/Turabian StyleRomano, Alberto, Fabrizio Sollazzo, Fabio Corbo, Giorgio Attinà, Stefano Mastrangelo, Simona Cordaro, Gloria Modica, Isabella Carlotta Zovatto, Riccardo Monti, Massimiliano Bianco, and et al. 2024. "Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors" Diseases 12, no. 12: 306. https://doi.org/10.3390/diseases12120306
APA StyleRomano, A., Sollazzo, F., Corbo, F., Attinà, G., Mastrangelo, S., Cordaro, S., Modica, G., Zovatto, I. C., Monti, R., Bianco, M., Maurizi, P., Palmieri, V., & Ruggiero, A. (2024). Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors. Diseases, 12(12), 306. https://doi.org/10.3390/diseases12120306








