The Unexpected Detection of Esophageal Varices Caused by Liver Cirrhosis in a 47-Year-Old Man Treated with a Growth Hormone in Childhood
Abstract
1. Introduction
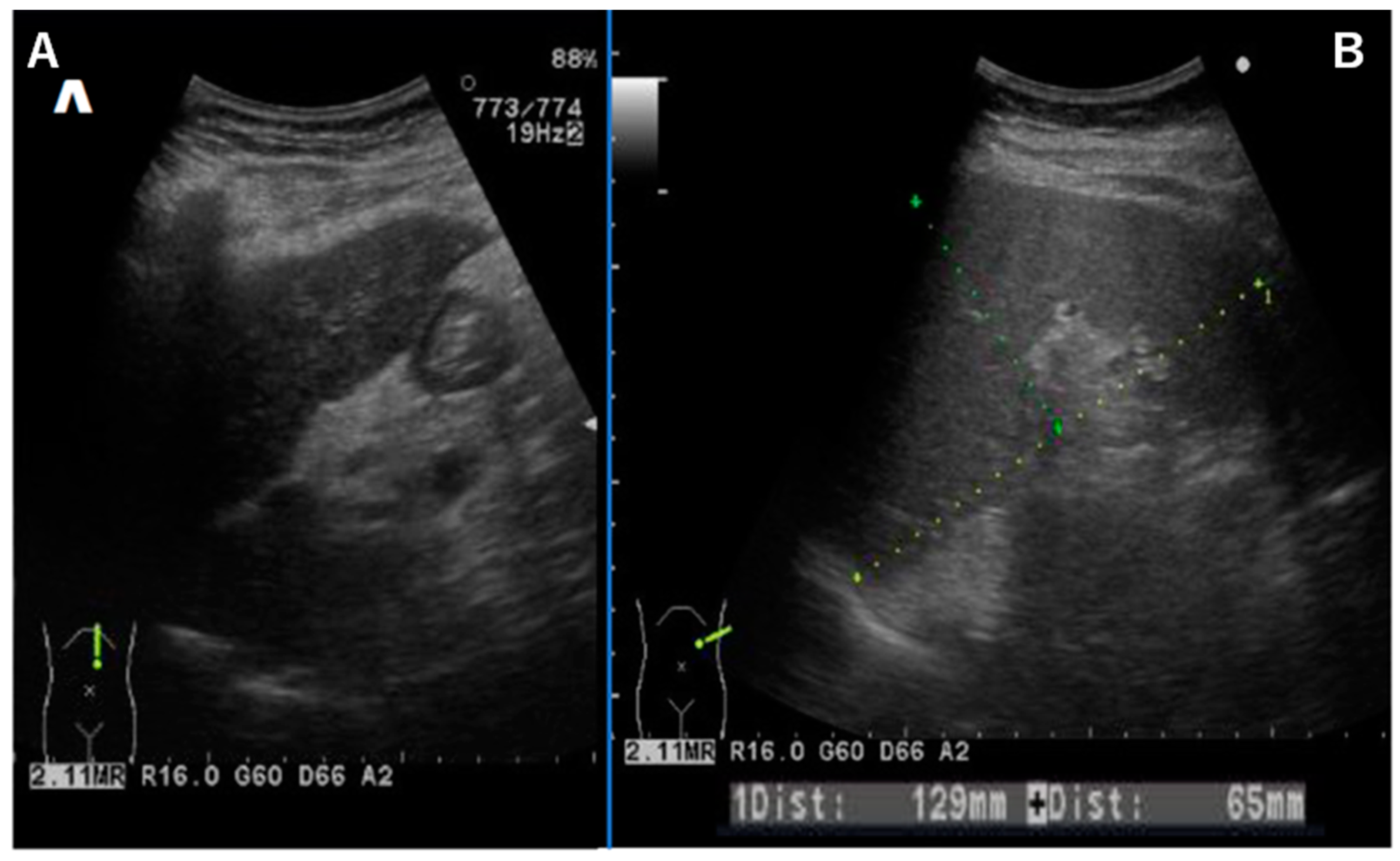
2. Case Report
3. Discussion
3.1. GH and IGF-1
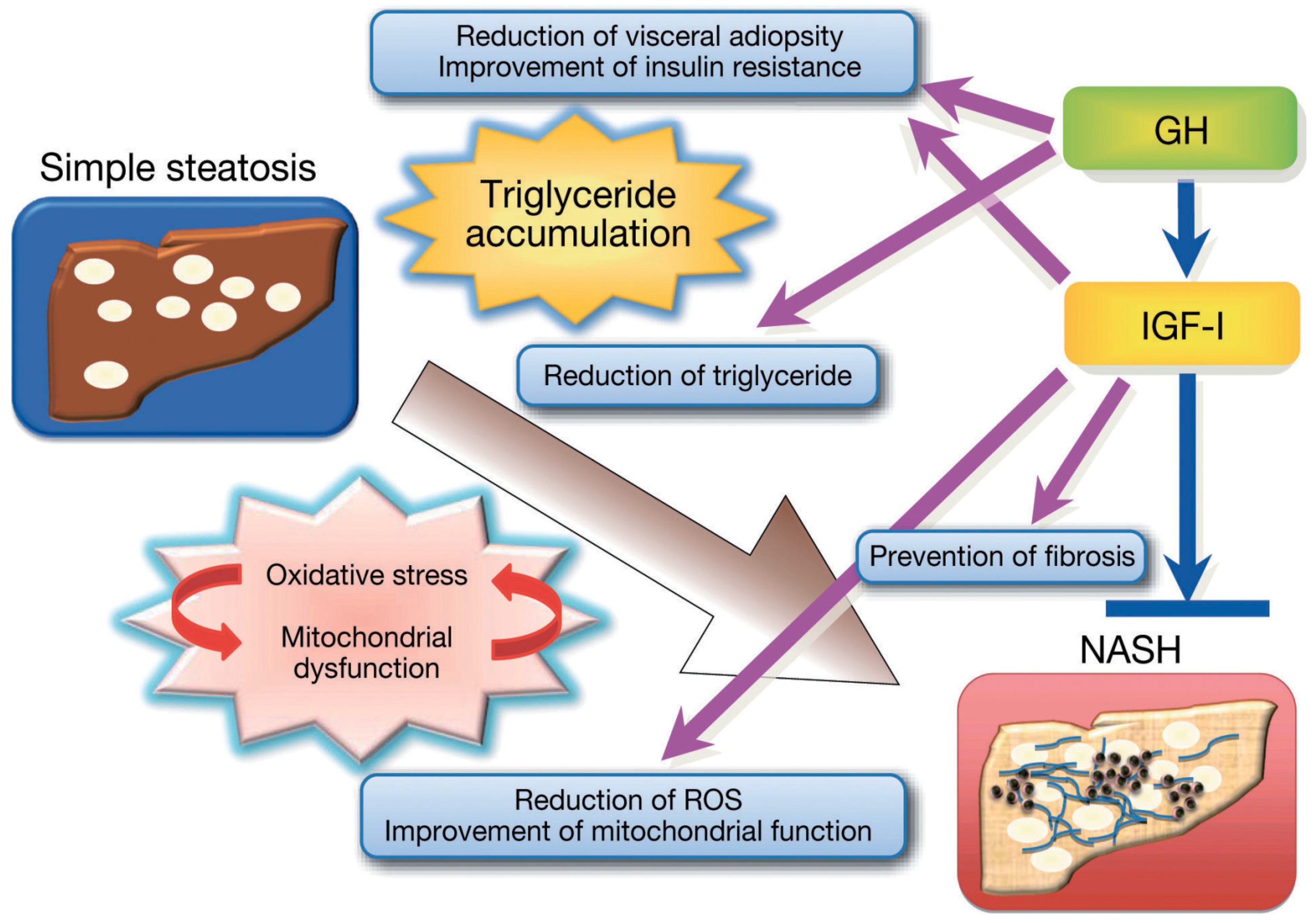
3.2. GH Action in the Liver
3.3. IGF-I Action in the Liver
3.4. SGA Impact on MASLD Development
3.5. Adult GH Deficiency (AGHD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic fatty liver disease in adults: Current concepts in etiology, outcomes, and management. Endocr. Rev. 2020, 41, bnz009. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, C.; Di Rosa, C.; Fintini, D.; Ferrara, P.; De Gara, L.; Khazrai, Y.M. Nutritional Approaches in Children with Overweight or Obesity and Hepatic Steatosis. Nutrients 2023, 15, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, W.; Karakas, S.; Sarkar, S. NASH in nondiabetic endocrine disorders. Metab. Syndr. Relat. Disord. 2018, 16, 315–320. [Google Scholar] [CrossRef]
- Doycheva, I.; Erickson, D.; Watt, K.D. Growth hormone deficiency and NAFLD: An overlooked and underrecognized link. Hepatol. Commun. 2022, 6, 2227–2237. [Google Scholar] [CrossRef]
- Johannsson, G.; Bengtsson, B.A. Growth hormone and the metabolic syndrome. J. Endocrinol. Investig. 1999, 22 (Suppl. S5), 41–46. [Google Scholar]
- Hanew, K.; Tachibana, K.; Yokoya, S.; Fujieda, K.; Tanaka, T.; Igarashi, Y.; Shimatsu, A.; Tanaka, H.; Tanizawa, T.; Teramoto, A.; et al. Clinical characteristics, etiologies and pathophysiology of patients with severe short stature with severe GH deficiency: Questionnaire study on the data registered with the foundation for growth science, Japan. Endocr. J. 2006, 53, 259–265. [Google Scholar] [CrossRef][Green Version]
- Fujisawa, I.; Kikuchi, K.; Nishimura, K.; Togashi, K.; Itoh, K.; Noma, S.; Minami, S.; Sagoh, T.; Hiraoka, T.; Momoi, T.; et al. Transection of the pituitary stalk: Development of an ectopic posterior lobe assessed with MR imaging. Radiology 1987, 165, 487–489. [Google Scholar] [CrossRef]
- Laron, Z. Growth hormone therapy: Emerging dilemmas. Pediatr. Endocrinol. Rev. 2011, 8, 364–373. [Google Scholar]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L. Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Boguszewski, C.L. Boguszewski MCDS: Growth hormone’s links to cancer. Endocr. Rev. 2019, 40, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. Essential roles of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the liver. Endocr. J. 2012, 59, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. Nonalcoholic fatty liver disease and adult growth hormone deficiency: An under-recognized association? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101816. [Google Scholar] [CrossRef]
- Adams, L.A.; Talwalkar, J.A. Diagnostic evaluation of nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2006, 40 (Suppl. S1), S34–S38. [Google Scholar]
- Yasui, K.; Hashimoto, E.; Tokushige, K.; Koike, K.; Shima, T.; Kanbara, Y.; Saibara, T.; Uto, H.; Takami, S.; Kawanaka, M.; et al. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol. Res. 2012, 42, 767–773. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Rudling, M.; Norstedt, G.; Olivecrona, H.; Reihner, E.; Gustafsson, J.A.; Angelin, B. Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc. Natl. Acad. Sci. USA 1992, 89, 6983–6987. [Google Scholar] [CrossRef]
- Moller, N.; Jorgensen, J.O. Effects of growth hor- mone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Fryburg, D.A.; Barrett, E.J. Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 1993, 42, 1223–1227. [Google Scholar] [CrossRef]
- Holt, R.I.; Simpson, H.L.; Sonksen, P.H. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet. Med. 2003, 20, 3–15. [Google Scholar] [CrossRef]
- Simpson, H.L.; Jackson, N.C.; Shojaee-Moradie, F.; Jones, R.H.; Russell-Jones, D.L.; Sonksen, P.H.; Dunger, D.B.; Umpleby, A.M. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Setser, J.; Zhao, H.; Stannard, B.; Haluzik, M.; Glatt, V.; Bouxsein, M.L.; Kopchick, J.J.; LeRoith, D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J. Clin. Investig. 2004, 113, 96–105. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol. Metab. 2009, 20, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Yakar, S.; LeRoith, D. Conditional knock- out of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc. Soc. Exp. Biol. Med. 2000, 223, 344–351. [Google Scholar]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Liu, J.L.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor, I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Ginsberg, S.; Webb, M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion—Preliminary report. Growth Horm. IGF Res. 2008, 18, 434–438. [Google Scholar] [CrossRef]
- Donaghy, A.; Ross, R.; Gimson, A.; Hughes, S.C.; Holly, J.; Williams, R. Growth hormone, insulin-like growth factor-1, and insulin like growth factor binding proteins 1 and 3 in chronic liver disease. Hepatology 1995, 21, 680–688. [Google Scholar]
- Caro, J.F.; Poulos, J.; Ittoop, O.; Pories, W.J.; Flickinger, E.G.; Sinha, M.K. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J. Clin. Investig. 1988, 81, 976–981. [Google Scholar] [CrossRef]
- Smith, B.W.; Adams, L.A. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011, 48, 97–113. [Google Scholar] [CrossRef]
- Nishizawa, H.; Takahashi, M.; Fukuoka, H.; Iguchi, G.; Kitazawa, R.; Takahashi, Y. GH-independent IGF-I action is essential to prevent the development of non- alcoholic steatohepatitis in a GH-deficient rat model. Biochem. Biophys. Res. Commun. 2012, 423, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Puche, J.E.; Garcia-Fernandez, M.; Muntane, J.; Rioja, J.; Gonzalez-Baron, S.; Castilla Cortazar, I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology 2008, 149, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Garcia-Fernandez, M.; Diaz-Sanchez, M.; Puche, J.E.; Delgado, G.; Conchillo, M.; Muntane, J.; Castilla-Cortazar, I. Mitochondrial protection by low doses of insulin-like growth factor- I in experimental cirrhosis. World J. Gastroenterol. 2008, 14, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Arturi, F.; Succurro, E.; Procopio, C.; Pedace, E.; Mannino, G.C.; Lugara, M.; Procopio, T.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J. Clin. Endocrinol. Metab. 2011, 96, E1640–E1644. [Google Scholar] [CrossRef] [PubMed]
- Volzke, H.; Nauck, M.; Rettig, R.; Dorr, M.; Higham, C.; Brabant, G.; Wallaschofski, H. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur. J. Endocrinol. 2009, 161, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Espejo, I.; Montero, J.L.; Costan, G.; Marchal, T.; Membrives, A.; Gallardo-Valverde, J.M.; Munoz-Castaneda, J.R.; Arevalo, E.; et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes. Surg. 2007, 17, 493–503. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakao, K.; Hamasaki, K.; Furukawa, R.; Tsuruta, S.; Ueda, Y.; Taura, N.; Shibata, H.; Fujimoto, M.; Toriyama, K.; et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth fac- tor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol. Int. 2007, 1, 287–294. [Google Scholar] [CrossRef]
- Fan, Y.; Menon, R.K.; Cohen, P.; Hwang, D.; Clemens, T.; DiGirolamo, D.J.; Kopchick, J.J.; Le Roith, D.; Trucco, M.; Sperling, M.A. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 2009, 284, 19937–19944. [Google Scholar] [CrossRef]
- Tateno, C.; Kataoka, M.; Utoh, R.; Tachibana, A.; Itamoto, T.; Asahara, T.; Miya, F.; Tsunoda, T.; Yoshizato, K. Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology 2011, 152, 1479–1491. [Google Scholar] [CrossRef]
- Ibáñez, L.; López-Bermejo, A.; Díaz, M.; Marcos, M.V.; Casano, P.; de Zegher, F. Abdominal fat partitioning and high-molecular- weight adiponectin in short children born small for gestational age. J. Clin. Endocrinol. Metab. 2009, 94, 1049–1052. [Google Scholar] [CrossRef]
- Soto, N.; Bazaes, R.A.; Peña, V.; Salazar, T.; Avila, A.; Iñiguez, G.; Ong, K.K.; Dunger, D.B.; Mericq, M.V. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: Results from a prospective cohort. J. Clin. Endocrinol. Metab. 2003, 88, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Childhood obesity: Rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin. Pediatr. Endocrinol. 2020, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Kanzaki, S.; Sato, M.; Kubo, T.; Seino, Y. Effect of growth hormone on fatty liver in panhypopituitarism. Arch. Dis. Child. 1997, 76, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Panera, N.; Agostoni, C.; Nobili, V. Intrauterine growth retardation and nonalcoholic Fatty liver disease in children. Int. J. Endocrinol. 2011, 2011, 269853. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Ventura, A.; D’Aniello, M.; Pepe, T.; Giordano, P.; Monteduro, M.; Cavallo, L. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: Influence of rapid weight catch-up growth. Horm. Res. Paediatr. 2013, 79, 103–109. [Google Scholar] [CrossRef]
- Bugianesi, E.; Bizzarri, C.; Rosso, C.; Mosca, A.; Panera, N.; Veraldi, S.; Nobilli, V. Low birthweight increases the likelihood of severe 190 steatosis in pediatric non-alcoholic fatty liver disease. Am. J. Gastroenterol. 2017, 112, 1277–1286. [Google Scholar] [CrossRef]
- Morrison, J.L.; Duffield, J.A.; Muhlhausler, B.S.; Gentili, S.; McMillen, I.C. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr. Nephrol. 2010, 25, 669–677. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: A ten year systematic review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar]
- Arisaka, O.; Ichikawa, G.; Nakayama, K.; Koyama, S.; Sairenchi, T. Low Birth Weight, Weight Gain Trajectory in Infancy, Adiposity Rebound, and Risk of Adult Coronary Heart Disease. J. Pediatr. 2023, 255, 261–262. [Google Scholar] [CrossRef]
- Chihara, K.; Koledova, E.; Shimatsu, A.; Kato, Y.; Kohno, H.; Tanaka, T.; Teramoto, A.; Bates, P.C.; Attanasio, A.F. An individualized GH dose regimen for long-term GH treatment in Japanese patients with adult GH deficiency. Eur. J. Endocrinol. 2005, 153, 57–65. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisaka, O.; Koyama, S.; Imataka, G.; Naganuma, J.; Arisaka, T.; Akatsuka, S. The Unexpected Detection of Esophageal Varices Caused by Liver Cirrhosis in a 47-Year-Old Man Treated with a Growth Hormone in Childhood. Diseases 2024, 12, 251. https://doi.org/10.3390/diseases12100251
Arisaka O, Koyama S, Imataka G, Naganuma J, Arisaka T, Akatsuka S. The Unexpected Detection of Esophageal Varices Caused by Liver Cirrhosis in a 47-Year-Old Man Treated with a Growth Hormone in Childhood. Diseases. 2024; 12(10):251. https://doi.org/10.3390/diseases12100251
Chicago/Turabian StyleArisaka, Osamu, Satomi Koyama, George Imataka, Junko Naganuma, Takahiro Arisaka, and Sei Akatsuka. 2024. "The Unexpected Detection of Esophageal Varices Caused by Liver Cirrhosis in a 47-Year-Old Man Treated with a Growth Hormone in Childhood" Diseases 12, no. 10: 251. https://doi.org/10.3390/diseases12100251
APA StyleArisaka, O., Koyama, S., Imataka, G., Naganuma, J., Arisaka, T., & Akatsuka, S. (2024). The Unexpected Detection of Esophageal Varices Caused by Liver Cirrhosis in a 47-Year-Old Man Treated with a Growth Hormone in Childhood. Diseases, 12(10), 251. https://doi.org/10.3390/diseases12100251






