A Comprehensive Review of Moroccan Medicinal Plants for Diabetes Management
Abstract
1. Introduction
2. Methodology
- -
- Ethnobotanical surveys (38) of Moroccan medicinal plants used in diabetes management;
- -
- Publications related specifically to in vitro (30) and in vivo (97), or both (8), studies of the 10 most widely used Moroccan antidiabetic medicinal plants;
- -
- Studies published in peer-reviewed journals;
- -
- Research works that included clear experimental methods and statistical analyses.
3. Results
3.1. Traditional Uses and Plant Sources
| Family Name | Scientific Name | Local Name(s) | Region(s) | Used Part(s) | Mode(s) of Use | Citation Number | References |
|---|---|---|---|---|---|---|---|
| Aizoaceae | Opophytum theurkauffii Maire L. | âfzû | L | Leaves/Fruits | Dec/Pow | 1 | [19] |
| Alliaceae | Allium cepa L. | Bassla/Azalim | A-L, N-Q, T | Bulbs/Seeds/Roots | Pow/Raw | 22 | [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,51] |
| Allium sativum L. | Touma/Tiskert | A-L, O-Q | Bulbs/Roots | Raw/Mac/Dec | 19 | [18,19,21,24,25,26,27,29,30,31,32,33,34,35,36,37,38,39,51] | |
| Allium ampeloprasum var. porrum | Borro/Leborrou | D | Bulbs/Stems | Raw/Ing with water | 2 | [28,32] | |
| Aloeaceae | Aloe vera (L.) Burm. f. | Sebbar/Ssabra/Siber | D, F, H, K, L, T | Pulps/Leaves | Raw/Pow | 7 | [19,21,22,30,32,39,51] |
| Amaranthaceae | Anabasis aretioides Moq. & Coss. ex Bunge | Chajra ma yeharrekha rih/Salla | K, L | Aerial parts | Dec | 2 | [19,21] |
| Beta vulgaris L. | Lbarba | R | Seeds | Inf | 1 | [45] | |
| Spinacia oleracea L. | Sabanikh | D | Leaves | Nd | 1 | [51] | |
| Anacardiaceae | Pistacia atlantica Desf. | Btem/Igg/Drou | C, Q, H | Fruits | Inf/Dec | 3 | [25,41,44] |
| Pistacia lentiscus L. | Trou/Tidekt/Drou | D, E, F, K, N, O | Leaves/Gums/Barks | Raw/Inf/Dec | 6 | [21,23,24,34,39,51] | |
| Searsia albida (Schousb.) Moffett | Zewaya/anaffis | L | Fruits | Pow | 1 | [19] | |
| Apiaceae | Ammodaucus leucotrichus Coss. | Kamoun soufi | L, K, H, P | Seeds | Inf/Dec | 4 | [19,21,26,30] |
| Ammi majus L. | Atrilal/Trilal/Rjel l’aghrabe | V | Whole plant | Inf | 1 | [49] | |
| Ammi visnaga (L.) Lam. | Bachnikha/Barghanisse | A, C-E, G, I-K, N-P, T | Inflorescences/Fruits/Seeds | Dec/Mac/Ing/Inf | 15 | [17,18,20,21,22,23,24,26,29,32,33,34,35,37,51] | |
| Anethum foeniculum L. | Shamrah/Fennel | C | Nd | Nd | 1 | [33] | |
| Apium graveolens L. | Krafess | A, C, D, H, P, W | Seeds/Aerial parts | Inf/Dec/Mac | 6 | [26,29,30,32,33,52] | |
| Carum carvi L. | Lkarwya | A, C-E, G-L, Q | Seeds | Dec/Pow/Inf | 15 | [17,18,19,21,25,27,29,30,32,33,34,35,37,41,51] | |
| Coriandrum sativum L. | Kosbor | A-E, G-K, O, P, T, W | Seeds/Leaves | Inf/Dec/Ing | 16 | [17,20,21,22,26,28,29,30,31,32,33,34,35,37,51,52] | |
| Cuminum cyminum L. | Kamoun | C, D, F, K, L | Seeds | Pow/Ing | 6 | [19,21,32,33,39,51] | |
| Daucus carota L. | Khizou | K, L, O | Roots | Jui/Puree | 3 | [19,21,24] | |
| Eryngium ilicifolium Lam. | Tasnant/Iglifin | Q | Stems and leaves | Dec/Pow | 1 | [25] | |
| Ferula communis L. | L-kelḫ/Uffāl/Taggwelt | G, R | Fruits/Roots/Flowers/Leaves | Dec/Pow/Inf | 2 | [35,45] | |
| Foeniculum vulgare Mill. | Nafaa/Hebet hlawa | A, C-E, G-L, P, Q, W, X | Seeds/Fruits | Dec/Inf | 17 | [17,18,19,21,25,26,27,29,30,32,34,35,37,41,51,52,53] | |
| Pastinaca sativa L. | Left lmahfour | H, I, Q | Roots | Raw | 4 | [25,27,30,37] | |
| Petroselinum crispum (Mill.) Fuss | Maadnouss | A-D, K, I, H, L, P, W | Seeds/Leaves | Inf/Dec/Raw | 11 | [19,21,26,27,29,30,31,32,33,37,52] | |
| Petroselinum sativum Hoffm | Mԑadnūs/Imẓi | G | Aerial parts/Whole plants | Jui/Dec | 1 | [35] | |
| Pimpinella anisum L. | Habbat hlawa | C-E, G-I, K, L, P, Q, T | Seeds | Dec/Inf/Pow/Ing | 13 | [19,21,22,25,26,27,28,30,32,33,34,35,37] | |
| Ptychotis verticillata Duby | Nounkha | O | Aerial parts | Inf | 1 | [24] | |
| Ridolfia segetum (L.) Moris | Tebch | E, K, R | Seeds | Pow | 3 | [21,34,45] | |
| Apocynaceae | Apteranthes europaea (Guss.) Murb. | Oukan iddan | Q | Stems | Dec/Inf/Raw | 1 | [25] |
| Calotropis procera (Aiton) Dryand. | Turja | L | Leaves | Pow | 1 | [19] | |
| Caralluma europaea (Guss.) N.E.Br. | Daghmous | A, B, D, E, K, H, P, S, V | Aerial parts/Leaves/Rackets/Roots | Mac/Jui/Pow/Dec/Inf/Per | 10 | [21,26,29,30,31,32,34,44,46,49] | |
| Nerium oleander L. | Defla/Alili | A, C-L, N, P, Q, S, T, W, Y | Leaves | Dec/Inf/Mac/Fum | 23 | [17,18,19,21,22,23,25,26,27,32,33,34,35,36,37,39,41,44,46,48,50,51,52] | |
| Periploca laevigata subsp. Angustifolia (Labill.) Markgr. | Asllif | Q, S | Fruits/Leaves | Dec | 2 | [25,46] | |
| Arecaceae | Chamaerops humilis L. | Dum/Tiguezden | C-E, H, K, O, Y | Leaves/Fruits/Roots | Raw/Dec/Inf/Pow | 7 | [21,24,30,32,33,34,50] |
| Hyphaene thebaica (L.) Mart. | Dum/karur | L | Fruits | Pow | 1 | [19] | |
| Phoenix dactylifera L. | Tmar/Nkhil | E-H, K, L, P, J | Fruits/Seeds/Leaves/Pulps/Roots | Raw/Dec/Pow/Inf/Vin | 8 | [17,19,21,26,30,34,35,39] | |
| Aristolochiaceae | Aristolochia baetica L. | Tiswik nigrane/Berztem | S | Roots/Resins | Pow | 1 | [46] |
| Aristolochia longa subsp. Fontanesii Boiss. & Reut. | Berztem | A, G, H, K, L, T | Seeds | Pow/Dec | 6 | [18,19,21,22,30,35] | |
| Asparagaceae | Agave americana L. | Ssabra/Sayber | K | Leaves | Dec | 1 | [21] |
| Asparagus albus L. | Sekkum/Azzu | E, O | Young sprouts/Roots | Raw/Dec | 2 | [24,34] | |
| Asparagus officinalis L. | Saklaim | V | Stems | Coo in steamer, or water | 1 | [49] | |
| Asteraceae | Achillea odorata L. | Elqorte | E, K | Leaves and flowers | Inf | 2 | [21,34] |
| Achillea santolinoides Lag. | Chouihiya, El-qorte | E | Capitulum | Inf | 1 | [34] | |
| Anacyclus pyrethrum (L.) Lag. | Iguntas/Tagundecht/Takntist | O | Roots/Leaves | Inf/Pow | 1 | [24] | |
| Antennaria dioica (L.) Gaertn | Ouden elfar | K | Leaves | Dec | 1 | [21] | |
| Anvillea garcinii subsp. Radiata (Coss. & Durieu) | Negd | L, T | Leaves/Roots | Pow/Dec | 2 | [19,47] | |
| Artemisia abrotanum L. | Chih | K | Aerial parts | Dec | 1 | [21] | |
| Artemisia absinthium L. | Chiba | A-F, H, I, K, J, O, N, P, V | Aerial parts/Stems/Leaves | Inf | 17 | [17,18,20,21,23,26,27,29,30,31,32,33,34,37,39,49,51] | |
| Artemisia arborescens (Vaill.) L. | Šība/Šība šmaymiya | F | Aerial parts/Leaves | Inf | 1 | [35] | |
| Artemisia atlantica Coss. & Durieu | Chih ourika | K | Aerial parts | Inf | 1 | [21] | |
| Artemisia campestris L. | Chihi khorayss | E | Whole plant | Inf | 1 | [34] | |
| Artemisia herba-alba Asso | Izri/Chih dwidi | A, C-E, G-L, N-Q, S, T, W | Stems/leaves/Roots | Dec/Inf/Pow | 23 | [17,18,19,20,21,22,23,25,26,27,28,29,30,32,33,34,35,37,41,46,48,51,52] | |
| Artemisia herba alba Assac., | Chih | N | Aerial parts/Leaves | Dec/Inf/Pow | 1 | [23] | |
| Artemisia mesatlantica Maire | Chih elkhryassi | E, K | Whole plant/Aerial parts | Dec | 2 | [21,34,48] | |
| Artemisia reptans C. Sm. ex Link | Chihiya | L | Leaves | Dec | 1 | [19] | |
| Atractylis gummifera Salzm. ex L. | Addād/Ddād, | G | Roots | Inf | 1 | [35] | |
| Calendula arvensis Bieb., | Jemra Azwiwel | C, R | Flowers/Stems | Inf/Dec | 2 | [41,45] | |
| Centaurea maroccana Bal | Bejjaae nhal/Nogguir | D, K | Flowers | Inf | 2 | [21,51] | |
| Chamaemelum mixtum (L.) Alloni | Hellala | D | Flowers | Inf | 1 | [32] | |
| Chamaemelum nobile (L.) All. | Babounj | A, D, E, H, K, T | Leaves/Flowering tops | Dec/Inf | 6 | [21,22,29,30,32,34] | |
| Chrysanthemum coronarium L. | Hmessou | E | Flowers | Inf | 1 | [34] | |
| Cichorium intybus L. | Buaggad | L | Roots | Inf | 1 | [19] | |
| Cladanthus arabicus (L.) Cass. | Taafs | E, K | Flowers | Inf | 2 | [21,34] | |
| Cladanthus scariosus (Ball) Oberpr. & Vogt | Arzgi/irzgi | S | Flowers | Dec | 1 | [46] | |
| Cynara cardunculus L. | Kharchouf/Taggua | A, D, E, K, H, J, P, T, L | Aerial parts/Stems | Pow/Dec/Inf | 10 | [17,18,19,21,22,26,30,32,34,47] | |
| Cynara cardunculus subsp. scolymus (L.) | Lqoq | D, E, Q, T | Roots/Inflorescences | Dec/Inf | 4 | [25,32,34,47] | |
| Cynara humilis L. | Ṭimṭa/Ḥekk/Ḫeršūf | G | Roots | Dec/Pou | 1 | [35] | |
| Dittrichia viscosa (L.) Greuter | Terehla/Bagraman | B-D, E, K, O, S | Leaves/Stems/Fruits | Dec/Inf | 8 | [21,24,31,33,34,41,46,51] | |
| Echinops spinosissimus Turra | Taskra | Q, S, T | Flowers | Dec | 3 | [22,25,46] | |
| Helianthus annuus L. | Nouaratchamess | R, H | Roots/Seeds | Pow/Inf | 2 | [44,45] | |
| Inula conyza (Griess.) DC. | Terrehla | K | Roots | Dec | 1 | [21] | |
| Inula helenium L. | Terrehla damnatiya | K | Leaves/Flower | Dec | 1 | [21] | |
| Lactuca sativa L. | Khes/Lkhoss | E, K, H, P, R | Leaves | Raw/Inf | 5 | [21,26,30,34,45] | |
| Launaea arborescens (Batt.) Murb. | Iferskel/Moulbna | K, Q, L | Stems/Leaves/Roots Flowers | Pow/Dec/Inf | 3 | [19,21,25] | |
| Matricaria chamomilla L. | Mansania/Lbabounj | C, E, K, H, I, N | Leaves/Flowers | Dec/Inf | 7 | [21,23,27,33,34,37,41] | |
| Pallenis spinosa (L.) Cass. | Nugd/Nouged | E, K | Aerial parts/Whole plant | Dec/Inf | 2 | [21,34] | |
| Saussurea costus (Falc.) Lipschitz | Qist Hindi | W | Stems | Pow | 1 | [52] | |
| Scolymus hispanicus L. | Gurnina/Taghdiut | D, E, K, O, S | Stems/Leaves/Roots | Raw/Dec/Inf | 5 | [21,24,34,46,51] | |
| Scorzonera undulata Vahl | Tamtla | Q | Flowers | Raw | 1 | [25] | |
| Seriphidium herba-alba | Chih | X | Nd | Nd | 1 | [53] | |
| Sonchus arvensis L. | Kettan elhench/Tifaf | E, H, T | Leaves | Inf/Dec | 3 | [22,30,34] | |
| Sonchus asper (L.) Hill | Tifaf | R | Whole plants | Dec | 1 | [45] | |
| Sonchus tenerrimus L. | Tifaf | L, R | Leaves | Dec | 2 | [19,45] | |
| Stevia rebaudiana Willd. | Stevia | D, F | Leaves | Inf/Pow | 2 | [39,51] | |
| Silybum marianum L. | Chouka | D | Leaves/Fruits | Nd | 1 | [51] | |
| Tanacetum vulgare L. | Lbalssam | E, K, R | Stems/Leaves | Inf | 3 | [21,34,45] | |
| Taraxacum campylodes G.E. Haglund | Lhandba/Chlada | C, K | Flowers/Roots/Leaves | Dec/Pow | 2 | [21,41] | |
| Warionia saharae Benthem ex Benth. & Coss. | Afssas | Q, L, J | Leaves | Inf/Pow | 3 | [19,25,38] | |
| Berberidaceae | Berberis vulgaris subsp. Australis (Boiss.) Heywood | Arghis/Atizar | D, E, G, C, K | Leafy stem/Barks/Fruits | Dec | 5 | [21,33,34,35,51] |
| Brassicaceae | Anastatica hierochuntica L. | Chajarat Maryem/lkemcha | E, L, O, R, W | Stems/Leaves | Pow/Inf | 5 | [19,24,34,45,52] |
| Brassica napus L. | Left | L, H | Rhizomes | Jui | 2 | [19,30] | |
| Brassica nigra (L.) K. Koch | Elkhardel | K | Flowers | Pow/Inf | 1 | [21] | |
| Brassica oleracea L. | Krunb mkawar/Melfuf | C-E, H, K, L, O, P, R | Aerial parts/Fruits | Raw/Mac/Pou | 9 | [19,21,24,26,30,32,33,34,45] | |
| Brassica rapa L. | Left beldi | D, E, K, O | Roots/Leaves | Dec/Inf | 5 | [21,24,34,48,51] | |
| Diplotaxis pitardiana Maire | Kerkaz/Elharra | K, L | Flowers | Pow | 2 | [19,21] | |
| Eruca vesicaria (L.) Cav. | Ljerjir/Al girjir | D, E, H, L | Aerial parts | Jui/Pow | 3 | [19,30,34,51] | |
| Lepidium sativum L. | Hab errechad | A-L, P, W | Seeds | Mac/Pow/Dec/Inf | 18 | [17,18,19,21,26,27,28,29,30,31,32,33,34,35,37,39,41,51,52] | |
| Nasturtium officinale R.Br. | Gernunes | L | Leaves/stems | Mac | 1 | [19] | |
| Ptilotrichum spinosum (L.) Boiss. | Aguerbaz | O | Leaves/stems | Dec | 1 | [24] | |
| Raphanus raphanistrum subsp. sativus (L.) | Lfjel | A, D, E, K, H, I, Q, L, P | Roots/Bulbs | Raw/Inf/Mac | 10 | [19,21,25,26,27,29,32,34,37,51] | |
| Burseraceae | Boswellia sacra Flueck. | Louban Dakar/Salabane | D, E | Resins/Fruits | Inf/Ing/Dec | 2 | [32,34] |
| Commiphora myrrha (Nees) Engl. | Lmorra | A | Resins | Dec | 1 | [29] | |
| Buxaceae | Buxus balearica Lam. | Azazer/lbakous | K, O | Leaves | Dec | 2 | [21,24] |
| Buxus sempervirens L. | Lbeks | A | Leaves | Dec | 1 | [18] | |
| Cactaceae | Opuntia ficus indica (L.) Mill. | Lhndia/Aknari | A-D, F-H, J, K, L, O-Q, T | Stems/Roots/Flowers/Seeds/Fruits | Dec/Jui/Pow/Inf/Raw/Oil | 18 | [17,19,20,21,22,24,25,26,27,29,30,31,32,33,35,39,41,51] |
| Capparaceae | Capparis decidua (Forssk.) Edgew. | Ignin | L | Fruits | Pow | 1 | [19] |
| Capparis spinosa L. | Kabar/Taylulut | A, C-E, G, K, J, L, N, O, S, W | Aerial parts/Fruits/Roots | Pow/Dec/Inf | 12 | [17,18,19,21,23,24,34,35,41,46,51,52] | |
| Maerua crassifolia Forssk. | Atil/Sedra lkhadra | L | Leaves | Pow/Dec | 1 | [19] | |
| Caryophyllaceae | Herniaria glabra var. hirsuta (L.) Kuntze | Hrasset lehjer | G | Aerial parts | Dec/Pow | 1 | [35] |
| Paronychia argentea Lam. | Tahidourt n’imksaoum | S | Leafy stems | Inf | 1 | [46] | |
| Silene vivianii Steud. | Gern lebzal | L | Stems | Raw | 1 | [19] | |
| Corrigiola telephiifolia Pourr. | Sergina/Tasergint/Bakur al barbar | C, K, H, O, V | Roots | Pow | 5 | [21,24,30,33,49] | |
| Cannabaceae | Cannabis sativa L. | Al lkif | F | Seeds/Leaves/Flowers | Pow | 1 | [39] |
| Cistaceae | Cistus albidus L. | Boutour | O | Leaves | Dec | 1 | [24] |
| Cistus creticus L. | Irgel | K, Q, S | Leaves | Dec/Pow | 3 | [21,25,46] | |
| Cistus laurifolius L. | Agullid | E, K, S | Seeds/Flowers | Pow | 3 | [21,34,46] | |
| Cistus salviifolius L. | Irgel/Tirgelt | D, K, Q | Leaves/Seeds | Dec/Pow | 3 | [21,25,51] | |
| Cistus ladanifer L. | Touzalt | E | Leaves | Dec | 1 | [34] | |
| Chenopodiaceae | Atriplex halimus L. | Legtef | L | Leaves | Pow/Dec/Mac | 1 | [19] |
| Chenopodium ambrosioides L. | Mkhinza | A-C, E, G-J, W | Leaves/Aerial parts | Inf/Mac | 10 | [27,29,30,31,35,37,38,41,42,52] | |
| Hammada scoparia (Pomel) Iljin | Assay/Rremt | Q, M | Seeds/Leaves | Dec | 2 | [25,54] | |
| Salsola tetragona Delile | Laarad | L, J | Leaves and fruits | Pow | 2 | [19,43] | |
| Suaeda mollis Dest., | Adeghmous | J | Aerial parts | In meals | 1 | [43] | |
| Colchicaceae | Androcymbium gramineum (Cav.) J.F. Macbr. | Temrate leghrab | K | Bulbs | Inf | 1 | [21] |
| Convolvulaceae | Ipomoea batatas (L.) | Batata hlouwa | A | Roots | Raw | 1 | [29] |
| Cucurbitaceae | Bryonia dioica Jacq. | Terbouna | E | Stems/Fruits | Dec | 1 | [34] |
| Citrullus colocynthis (L.) Schrad. | Aferziz/lhdej | A, C-E, G, H, K, L, M, O-S | Seeds/Fruits | Dec/Cat/Pow/Ing | 15 | [18,19,21,24,25,26,28,30,32,33,34,35,45,46,54] | |
| Citrullus vulgaris Schard. | Dellah | E | Leaves | Inf/Mac | 1 | [34] | |
| Cucumis sativus L. | Lkhiar | A, B, D, E, G-I, K, L, O-Q | Fruits | Raw/Mac/Pow/Jui | 13 | [19,21,24,25,26,27,29,30,31,32,34,35,37] | |
| Cucumis melo var. flexuosus L. | Feqous | A | Fruits | Raw | 1 | [29] | |
| Cucurbita maxima Duchesne | Garaa lhamra | E, H, L | Leaves/Seeds | Dec/Pow | 3 | [19,30,34] | |
| Cucurbita pepo L. | Takhsait/curjt | D, F, K, H, L, O, N, Q, R | Fruits | Raw/Dec/Coo | 10 | [19,21,23,24,27,30,32,39,45,51] | |
| Cupressaceae | Juniperus phoenicea L. | Araar finiqui | A, D, E, K, L, O, R | Leaves/Aerial parts/Fruits/Barks | Pow/Dec Mac | 8 | [18,19,21,24,32,34,45,51] |
| Juniperus thurifera L | Tawayt | O | Leaves | Dec | 1 | [24] | |
| Juniperus oxycedrus L. | L arâar chrini | E | Leaves | Mac | 1 | [34] | |
| Tetraclinis articulata (Vahl) Mast. | Araar | C, F, K, G-I, K, N, P, T, V, W | Leaves/Aerial parts/Fruits | Inf/Mac/Pow/Dec | 13 | [21,22,23,26,27,30,33,35,37,39,41,49,52] | |
| Cynomoriaceae | Cynomorium coccineum L. | Tertut | L | Stems | Pow | 1 | [19] |
| Cyperaceae | Bolboschoenus maritimus (L.) Palla | Ssmar | K | Seeds | Dec | 1 | [21] |
| Cyperus longus L. | Arouk, esaad | E | Roots | Mac | 1 | [34] | |
| Cyperus rotundus L. | Tara | L | Leaves | Pow | 1 | [19] | |
| Dracaenaceae | Dracaena draco subsp. ajgal Benabid & Cuzin | Ajgal | Q | Stems/Leaves | Dec | 1 | [25] |
| Ephedraceae | Ephedra alata Decne. | Chdida | L | Leafy stem | Dec/Pow | 1 | [19] |
| Ephedra altissima Desf. | Tougel argan | H, Q | Stems/Leaves/whole plant | Dec | 2 | [25,27] | |
| Ephedra fragilis Desf. | Amater | S | Leafy stem | Dec | 1 | [46] | |
| Equisetaceae | Equisetum ramosissimum Desf | Dayl laawd | E | Stems | Dec | 1 | [34] |
| Ericaceae | Arbutus unedo L. | Sasnu/Barnnou | C-E, G, H, N, O | Leaves/Roots/Fruits | Dec/Inf | 6 | [23,24,27,34,35,41,51] |
| Vaccinium myrtillus L. | Oleik | D | Fruits | Nd | 1 | [51] | |
| Euphorbiaceae | Euphorbia officinarum subsp. echinus (Hook. f. & Coss.) Vindt | Tikiout/zakoum | E, K, L, O, Q | Fruits/Stems/Leaves | Mac/Dec/Pow/Jui | 5 | [19,20,21,25,34] |
| Euphorbia officinarum L. | Tikiout/Daghmouss | D, H, Q, W | Stems/Leaves | Pow | [25,30,51,52] | ||
| Euphorbia peplis L. | Hlliba | E, R | Whole plant | Inf | 2 | [34,45] | |
| Euphorbia resinifera O. Berg | Tikiwt | A, C, E, H, O, S | Leaves | A drop latex in a glass of water | 7 | [18,24,27,33,34,41,46] | |
| Mercurialis annua L. | Hurriga elmalssa | D, E, K, L | Leafy stem/Whole plant | Inf/Dec/Jui | 4 | [19,21,32,34] | |
| Ricinus communis L. | Awriwer/Lkharwaa | L | Seeds | Pou | 1 | [19] | |
| Fagaceae | Quercus coccifera L. | Elqermez | K | Leaves | Dec | 1 | [21] |
| Quercus suber L. | Belloute | A, B, D | Fruits | Dec/Raw | 3 | [29,31,32] | |
| Quercus ilex L. | Bellout, Kerrouch | C, E | Barks/Leaves | Dec | 2 | [33,34] | |
| Gentianaceae | Centaurium erythraea Rafn | Qusset elhayya/Ahchlaf ntawrra | C, D, G, K, N, O | Flowering/Aerial parts | Inf/Dec/Pow | 7 | [21,23,24,33,35,41,51] |
| Centaurium spicatum (L.) Fritsch | Gosset lhayya | E | Stems/Flowers | Inf | 1 | [34] | |
| Geraniaceae | Pelargonium odoratissimum L. | M’atarcha | X | Leaves | Dec | 1 | [53] |
| Pelargonium roseum Willd. | Laattercha | E | Leaves | Inf | 1 | [34] | |
| Iridaceae | Crocus sativus L. | Zaafran lhor | D, E, G, H, L | Stigmas/Flowers | Inf/Dec/Mac | 5 | [19,30,32,34,35] |
| Juglandaceae | Juglans regia L. | Swak/Gargaa | C, D, E, G, K, L, O, S | Leaves/Barks/Seeds/Flowers | Inf/Dec/Raw | 8 | [19,21,24,32,33,34,35,46] |
| Juncaceae | Juncus maritimus Lam. | Ssemar | K, L | Fruits/Stems | Dec | 2 | [19,21] |
| Lamiaceae | Ajuga iva (L.) Schreb. | Timerna nzenkhad/Chndkoura | A, C-E, G-I, K, L, N, P, Q, S, T | Stems/Leaves/Whole plant | Pow/Dec/Inf | 15 | [18,19,21,22,23,25,26,27,33,34,35,37,40,41,46] |
| Ballota hirsuta Benth | Merrou elhrami/Merrou | E, K | Leafy stem | Dec/Inf | 2 | [21,34] | |
| Calamintha officinalis Moench. | Manta | A, C, E, F, I | Aerial plants/Whole plant/Leaves/Stems/Flowers | Dec/Inf | 5 | [29,34,37,39,41] | |
| Calamintha nepeta subsp. Spruneri (Boiss.) Nyman | Nd | C | Nd | Nd | 1 | [33] | |
| Calamintha alpina L. | Fliyyo dial berr | D | Leaves | Dec | 1 | [28] | |
| Clinopodium alpinum (L.) Kuntze | Ziitra | D, L | Leaves | Dec | 2 | [19,28] | |
| Clinopodium nepeta subsp. glandulosum (Req.) Govaerts | Manta | N, T | Aerial parts | Inf/Dec | 2 | [22,23] | |
| Lavandula angustifolia Mill | Elkhzama zerqa/Elkhzama Fassiya | D, G, H, K, W | Aerial parts/Leafy stem | Inf/Dec/Pow | 6 | [21,30,32,35,51,52] | |
| Lavandula dentata L. | Timzeria/Lakhzama/Jaada | E, G, K, N, Q | Stems/Leaves/Whole plant | Dec/Pow/Inf/Raw/Pou | 5 | [21,23,25,34,35] | |
| Lavandula maroccana Murb. | Igazioen | E, Q, S | Stems/Leaves/Flowers | Dec/Inf | 3 | [25,34,46] | |
| Lavandula multifida L | Khilt lkheyl/Kohayla | E, G, L | Leaves/Inflorescence/Stems | Dec/Inf | 3 | [19,34,35] | |
| Lavandula stoechas L. | Imzeria/Tikenkert/Lhalhal | A, C, E, F, G, K, L, O, P, Q | Leaves/Flowers | Dec/Inf | 10 | [19,21,24,25,26,29,33,34,35,39] | |
| Marrubium vulgare L. | Mriwt/Ifzi | A, C, D, G-I, K, L, N-R, T, W | Leaves/Aerial parts | Dec/Inf/Pow | 21 | [18,19,20,21,22,23,24,25,26,27,28,29,30,32,33,35,37,41,45,51,52] | |
| Mentha pulegium L. | Fliou | A, C, D, F, G, K, L, O, Q, T | Leaves/Aerial parts | Dec/Inf | 12 | [18,19,21,22,24,25,28,29,32,33,35,39] | |
| Mentha piperita L. | Naanaa | D | Leaves/Aerial parts | Nd | 1 | [51] | |
| Melissa officinalis L. | Naanaa trunj | E | Leaves | Inf | 1 | [34] | |
| Mentha spicata L. | Nanaa/Liqama | D, E, K, L | Leaves/Leafy stem | Inf/Dec | 4 | [19,21,32,34] | |
| Mentha suaveolens Ehrh. | Mersita Timijja | D, E | Leaves/Whole plant | Inf | 3 | [28,32,34] | |
| Ocimum basilicum L. | Lahbaq | D, E, G, H, K, O | Stems/Whole plant/Leaves | Inf | 6 | [21,24,30,34,35,51] | |
| Origanum compactum Benth. | Azukenni/Zaater/Zaatar tadlawi | A-D, E, F, H, I, K, L, N, O, T | Stems/Leaves/Aerial parts | Dec/Inf/Pow/Mac | 13 | [19,21,22,23,24,29,30,31,33,34,37,39,51] | |
| Origanum elongatum (Bonnet) Emb. &Maire | Zaater | D, G | Leaves/Aerial plants | Inf | 3 | [28,32,35] | |
| Origanum majorana L. | Berdedouch | D, H, L | Leaves | Pow/Inf | 4 | [19,30,32,51] | |
| Origanum vulgare L. | Zaatar | C, P | Leaves | Inf | 2 | [26,33] | |
| Rosmarinus officinalis L. | Azir | A-I, K, L, N, O, Q, R, T, V, W | Leaves/Stems/Aerial plants | Pow/Dec/Inf/Mac | 22 | [18,19,21,22,23,24,25,28,29,30,31,32,33,34,35,37,39,41,45,49,51,52] | |
| Salvia officinalis L. | Salmia | A, C-E, G-I, K, L, O-T, V-X | Leaves/Aerial parts | Dec/Inf/Mac | 24 | [18,19,20,21,22,24,25,26,27,28,29,30,32,33,34,35,37,41,45,46,49,51,52,53] | |
| Salvia hispanica L. | Chia | D | Seeds | Nd | 1 | [51] | |
| Teucrium polium L. | Tawerart/Flyou lbour/jaaidia | A, E, H, Q, S | Leaves/Whole plant | Dec/Pow | 5 | [18,25,30,34,46] | |
| Thymus broussonetii Boiss. | Zietra | C, D, E | Stems/Leaves/Flowers | Inf/Mac/Dec | 3 | [28,34,41] | |
| Thymus algeriensis Boiss. & Reut. | Aduchen/Azukni/Zaitra | G, O | Stems/Leaves | Dec/Inf | 2 | [24,35] | |
| Thymus maroccanus Ball. | Tazoukennit | E, W | Leaves/Flowers | Inf/Mac | 2 | [34,52] | |
| Thymus munbyanus Boiss. & Reut | Aduchen/Azukni/Zaitra | O | Stems/Leaves | Dec/Inf | 1 | [24] | |
| Thymus satureioides Coss. | Asserkna/Ziitra | D, E, K, Q | Leaves | Inf/Dec/Pow/Mac | 4 | [21,25,32,34] | |
| Thymus vulgaris L. | Aduchen/Azukni/Zaitra | A, D-G, K, O, Q | Leaves/Aerial plants | Dec/Inf | 8 | [21,24,25,29,34,35,39,51] | |
| Thymus zygis L. | Aduchen/Azukni/Zaitra | G, O | Stems/Leaves | Dec/Inf | 2 | [24,35] | |
| Lauraceae | Cinnamomum cassia (L.) J. Presl | Qarfa | A, C-E, H, K, O, T | Barks | Dec/Inf | 8 | [18,21,22,24,30,33,34,51] |
| Cinnamomum verum J. Presl | Dar essini/Karfa | A, B, D, G, I, K, L, W | Barks | Mac/Inf/Dec/Pow | 9 | [19,21,28,29,31,32,35,37,52] | |
| Laurus nobilis L. | Ourak sidna moussa/Rand | B, D, E, F, I, H, K, P | Leaves | Inf/Dec | 8 | [21,26,30,31,34,37,39,51] | |
| Persea americana Mill. | Lavoca | A, D, H, L, O | Seeds/Fruits/Leaves | Pow/Ing/Raw | 7 | [18,19,20,28,30,32,51] | |
| Leguminosae | Acacia gummifera Willd. | Telh | E | Roots | Dec | 1 | [34] |
| Acacia nilotica (L.) Delile | Amur/Sllaha | L | Fruits | Pow | 1 | [19] | |
| Acacia senegal (L.) Willd. | Laalek | L | Gums | Pow | 1 | [19] | |
| Acacia tortilis (Forssk.) Hayne | Telh/Tadoute/Amrād | G, K, L, M | Roots/Fruits/Leaves | Dec/Pow | 4 | [19,21,35,54] | |
| Acacia albida Delile | Chok Telh | K, R | Roots | Dec | 2 | [21,45] | |
| Anagyris foetida L. | Ful gnawa | E, L | Seeds/Leaves | Pow/Inf | 2 | [19,34] | |
| Arachis hypogaea L. | Lgerta/Kawkaw | D, L | Seeds | Pow | 2 | [19,51] | |
| Cassia absus L. | El habba sawdae | E | Seeds | Pow | 1 | [34] | |
| Cassia fistula L. | ḫyār šambâr | G | Fruits | Dec | 1 | [35] | |
| Ceratonia siliqua L. | Tikida/Lkharoub | A, C-E, G-I, K, L, P, Q | Leaves/Seeds/Fruits | Dec/Inf/Pow/Raw | 14 | [19,21,25,26,27,28,29,32,33,34,35,37,41,51] | |
| Cicer arietinum L. | Lhemmes | A, D, E, H, L | Seeds | Dec/Pow/Inf | 4 | [19,27,29,34,51] | |
| Cytisus battandieri Maire | Akhamelel | C | Leaves | Dec | 1 | [41] | |
| Glycine max (L.) Merr. | Soja | A, C-H J, P, Q, S, W | Seeds | Mac/Raw/Inf/Dec/Pow | 14 | [17,25,26,27,29,30,32,34,35,39,41,46,51,52] | |
| Glycyrrhiza glabra L | Ark souss | D, E, F, I | Barks/Roots/Stems | Inf/Pow /Raw | 6 | [28,32,34,37,39,51] | |
| Lupinus albus L. | Tirms/Foul gnawa | A, C-E, G, H, K, L, O | Seeds | Pow/Inf/Dec | 12 | [18,19,20,21,27,29,32,33,34,35,41,51] | |
| Lupinus angustifolius L. | Ibawn dekouk | G, K, Q, S | Seeds | Pow /Dec | 4 | [21,25,35,46] | |
| Lupinus luteus L. | Kikel/Semqala | E, K | Seeds | Dec | 2 | [21,34] | |
| Lupinus pilosus L. | Rjel Djaja | R | Seeds | Inf | 1 | [45] | |
| Medicago sativa L. | Fassa | B, D, E, K, H, I, L, O, P | Aerial parts/Seeds/Leaves | Inf/Mac/Coo/Pow | 9 | [19,21,24,26,27,31,34,37,51] | |
| Ononis natrix L. | Hennet reg | L | Leaves | Dec | 1 | [19] | |
| Ononis tournefortii Coss. | Afezdad | L | Leaves | Dec | 1 | [19] | |
| Phaseolus aureus Roxb. | Soja | R | Seeds | Dec | 1 | [45] | |
| Phaseolus vulgaris L. | Lubya | D, E, K, L, O, R | Fruits/Seeds | Dec/Pow/Jui/Raw/Ing | 7 | [19,20,21,24,32,34,45] | |
| Retama monosperma (L.) Boiss. | Rtam | E | Roots/Leaves | Dec/Inf | 1 | [34] | |
| Retama raetam (Forssk.) Webb | Rtam/Allug | G, K | Roots/Leaves/Aerial plants | Dec/Pow | 2 | [21,35] | |
| Retama sphaerocarpa (L.) Boiss. | Rtem | J | Roots | Dec | 1 | [17] | |
| Senna alexandrina Mill. | Senameki | D | Leaves | Nd | 1 | [51] | |
| Trigonella foenum-graecum L. | Lhelba/Tifidas | A-L, N, O, P, Q, S, T, W | Seeds | Dec/Inf/Mac/Pow | 25 | [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,37,39,41,46,51,52] | |
| Vicia faba L. | Ful/Foul | A, D, L | Seeds | Pow | 3 | [19,29,32] | |
| Vicia sativa L. | Ayn larnab | L | Seeds | Pow | 1 | [19] | |
| Vigna radiata (L.) R.Wilczek | Soja | L | Seeds | Pow | 1 | [19] | |
| Vigna unguiculata (L.) Walp | Ful gnawa | G, K | Seeds | Dec/Pow/Mac | 2 | [21,35] | |
| Urginea maritima (L.) Baker | Bssallansal | C | Leaves | Dec | 1 | [41] | |
| Linaceae | Linum usitatissimum L. | Zariat elkattan | A-I, K, L, O, Q, R, T | Seeds | Dec/Pow/Inf | 17 | [19,21,22,24,25,28,29,30,31,32,33,34,35,37,39,45,51] |
| Lythraceae | Lawsonia inermis L. | Lhenna | F, K, G | Leaves | Dec/Cat/Pow/Inf | 3 | [21,35,39] |
| Punica granatum L. | Rman | A-G, I-L, O, Q, T | Pericarps/Barks/Fruits/Leaves | Dec/Inf/Pow | 16 | [17,18,19,21,22,24,25,29,31,32,33,34,35,37,39,51] | |
| Malvaceae | Abelmoschus esculentus (L.) Moench | Lmloukhia | B, D, E, O | Fruits/Flowers | Mac/Inf/Raw | 5 | [24,28,31,32,34] |
| Hibiscus sabdariffa L. | Karkadi/Bissam | C-E, K, L, S | Calyces/Leaves/Flowers | Inf | 6 | [19,21,33,34,46,51] | |
| Moraceae | Ficus abelii Miq | Karmous, Chriha | R | Leaves | Dec | 1 | [45] |
| Ficus carica L. | Tazart/Lkarmous/Karma/chriha/Elbakur | A-K, O, Q, R, T | Fruits/Leaves | Dec/Inf/Raw/Mac | 18 | [17,21,22,24,25,27,29,30,31,32,33,34,35,37,39,41,45,51] | |
| Ficus dottata Gasp. | Karmous, Chriha | R | Fruits | Other | 1 | [45] | |
| Morus alba L. | Tut lbari | A, D, G, K, O, R | Leaves | Inf | 6 | [18,21,24,35,45,51] | |
| Morus nigra L. | Šejrat t-tūt | G | Leaves | Inf | 1 | [35] | |
| Moringaceae | Moringa oleifera Lam. | Moringa | D | Leaves | Nd | 1 | [51] |
| Musaceae | Musa paradisiaca L. | Banan | L | Leaves | Dec | 1 | [19] |
| Myristicaceae | Myristica fragrans Houtt. | Lgouza | C, Q | Seeds | Pow | 2 | [25,41] |
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | Calitus | L | Leaves | Dec | 1 | [19] |
| Eucalyptus globulus Labill. | Calitus | A, C-E-I, K, N, O, T | Leaves/Fruits/Stems | Dec/Inf/Pow | 13 | [21,22,23,24,27,29,33,34,35,37,39,41,51] | |
| Eugenia caryophyllata Thunb | Qronfel | C-E | Cloves/Leaves/Flowers | Mac/Inf/Pow/Dec | 4 | [33,34,41,51] | |
| Jasminum fruticans L. | Yasmin | E | Leaves/Flowers | Mac/Inf | 1 | [34] | |
| Myrtus communis L. | Rihane | A, C-K, N, O | Leaves/Fruits/Flowers | Dec/Inf/Mas/Pow | 14 | [17,21,23,24,27,29,30,32,33,34,35,37,39,41] | |
| Syzygium aromaticum (L.) Merr. & L. M. Perry | Kranfal | A, D, K, H, I, L, N, Q | Fruits/Cloves/Seeds | Inf/Dec/Pow/Mac | 9 | [18,19,21,23,25,27,28,32,37] | |
| Nitrariaceae | Peganum harmala L. | Lharmel | C, E, G, I, H, J, K, O, T | Seeds | Inf/Pow/Mac | 9 | [17,21,22,24,30,34,35,37,41] |
| Oleaceae | Fraxinus angustifolia Vahl | Touzalt | O | Leaves | Inf | 1 | [24] |
| Fraxinus excelsior var.acuminata Schur | Lsān Eṭ-Ṭîr/Lsān L’uṣfūr/Ḥebb Derdār | G | Fruits/Stems/Barks | Dec/Inf/Pow | 1 | [35] | |
| Olea europaea L. | Jbouj/Azmour/Zitoun | A-H, J, K, L, O, P, Q, S, T, W, X | Leaves/Fruits/Flowers | Dec/Inf/Mac/Pow/Oil | 24 | [17,18,19,20,21,22,24,25,26,27,28,29,30,31,32,33,34,35,39,40,46,48,51,52,53] | |
| Olea europaea subsp. maroccana (Greuter & Burdet) | Zitūn/Zebbūj | G | Leaves/Fruits | Dec/Oil | 1 | [35] | |
| Olea europea subsp. europaea var. sylvestris (Mill) Lehr, | Jebbouj | I | Leaves | Dec | 1 | [37] | |
| Olea oleaster Hoffm.& Link. | Zabbouj | E | Leaves/Flowers | Inf | 1 | [34] | |
| Papaveraceae | Fumaria officinalis L. | Hachichat assebyane | E, K, R | Roots/Leaves | Dec/Inf | 3 | [21,34,45] |
| Papaver rhoeas L. | Belaaman | A, C, H, I, Q, S | Seeds | Pow | 6 | [25,27,29,37,41,46] | |
| Plantago ovata Forssk. | Katouna | C, D | Seeds | Inf | 2 | [41,51] | |
| Pedaliaceae | Sesamum indicum L. | Janjlan | A, D-J, L, N, Q, W | Seeds | Pow/Inf/Dec | 12 | [17,19,23,25,27,29,32,34,35,37,39,52] |
| Plantaginaceae | Globularia alypum L. | Ayen lerneb/Taselgha | A, C, E-H, K, L, O, S, T | Flowers/Leaves/Stems | Inf/Dec/Pou | 12 | [18,19,20,21,22,24,30,33,34,35,39,46] |
| Globularia repens Lam. | Ain lernab | P | Leaves | Dec | 1 | [26] | |
| Plumbaginaceae | Limonium sinuatum (L.) Mill. | Lgarsa | L | Leaves | Dec | 1 | [19] |
| Poaceae | Avena sativa L. | Khortal | D, E, K, O | Seeds | Pow/Inf/Dec | 5 | [21,24,32,34,51] |
| Avena sterilis L. | Waskone/Khortal | E, S | Seeds | Pow/Dec | 2 | [34,46] | |
| Castellia tuberculosa (Moris) Bor | Zwan lmkarkeb | E, K | Seeds | Dec | 2 | [21,34] | |
| Cynodon dactylon (L.) Pers. | Njem | L | Roots | Dec | 1 | [19] | |
| Hordeum vulgare L. | Chair/Zraa | D-F, K, L, Q | Aerial parts/Seeds/Whole plant | Inf/Pow /Mac/Dec | 7 | [19,21,25,32,34,39,51] | |
| Lolium perenne L. | Eziwane/Zouane | D, E, S, W | Seeds | Dec/Inf | 4 | [34,46,51,52] | |
| Lolium multiflorum Lam. | Zwane | A | Seeds | Pow | 1 | [29] | |
| Lolium rigidum Gaudin | Zwan | D | Seeds | Inf/Ing | 1 | [32] | |
| Panicum miliaceum L. | Tafssout | E, K | Seeds | Dec | 2 | [21,34] | |
| Panicum turgidum Forssk. | Umm rekba | L | Stems | Dec/Pow | 1 | [19] | |
| Pennisetum glaucum (L.) R.Br. | Illan | D, K, L, Q | Seeds | Inf/Pow | 4 | [19,21,25,51] | |
| Phalaris canariensis L. | Zouan | E, K, H, N, O, Q | Seeds/Fruits | Pow/Inf/Dec | 7 | [20,21,23,24,25,27,34] | |
| Phalaris paradoxa L. | Zwan/Senbūlt l-fār/Tigurramin | G | Seeds | Pow/Dec | 1 | [35] | |
| Polypogon monspeliensis (L.) Desf | Tugga | L | Fruits | Raw | 1 | [19] | |
| Sorghum bicolor (L.) Moench | Bachna | O, T | Seeds | Inf/Dec | 2 | [22,24] | |
| Triticum durum Desf. | Zraa/Lkamh | D, E, F, K | Seeds | Dec/Inf | 4 | [21,34,39,51] | |
| Triticum aestivum L. | Zraa | D, F | Seeds | Mac | 2 | [32,39] | |
| Triticum turgidum L. | Zraa | C | Nd | Nd | 1 | [33] | |
| Zea mays L. | Lahyat Adra | C, H, N, S | Stigmas | Pow | 4 | [23,27,33,46] | |
| Polygonaceae | Emex spinosa (L.) Campd. | Lhenzab | L | Leaves/Bulbs | Pow | 1 | [19] |
| Portulaca oleracea L. | Rejla | E, K, Q, R, S | Aerial parts/Whole plant | Dec/Coo | 5 | [21,25,34,45,46] | |
| Ranunculaceae | Nigella Sativa L. | Sanouj | A-L, N, O, Q, S, T, W | Seeds/Fruits | Inf/Dec/Pow/Ing | 40 | [17,18,19,20,21,22,23,24,25,27,28,29,30,31,32,33,34,35,37,39,41,46,51,52] |
| Resedaceae | Reseda lanceolata Lag. | Rġūwa/L-Ḫrūf/Islīḫ | G | Seeds/Leaves | Dec/Pow/Inf | 1 | [35] |
| Rhamnaceae | Ziziphus lotus (L.) Lam. | Nbeg/Azouggar/ssdra | A-D, E, G-L, Q, S, T | Leaves/Fruits/Roots | Dec/Pow/Inf | 17 | [17,18,19,21,22,25,27,29,30,31,33,34,35,37,41,46,51] |
| Ziziphus jujube Mill | Zafzouf | C | Leaves | Dec | 1 | [41] | |
| Rosaceae | Cydonia oblonga Mill. | Sferjel | J | Fruits | Raw | 1 | [17] |
| Chaenomeles sinensis (Dum.Cours.) Koehne | Sferjel | L | Roots | Dec | 1 | [19] | |
| Crataegus monogyna Jacq. | Za’zûr/Zu’rûr | C | Nd | Nd | 1 | [33] | |
| Eriobotrya japonica (Thunb.) Lindl. | Mzah | D, F, H, O, T | Leaves/Fruits | Inf/Dec/Raw/Jui | 5 | [22,24,30,32,39] | |
| Fragaria vesca L. | Fraiz berri | C | Fruits | Raw | 1 | [33] | |
| Malus communis (L.) Poir. | Etefah | D, E, G, S, R | Fruits | Jui/Raw/Vin | 4 | [32,35,45,46,48] | |
| Prunus armeniaca L. | Luz elhar | E, K | Seeds | Dec | 2 | [21,34] | |
| Prunus dulcis (Mill.) D.A. Webb | Louz imrzig/Louz morr | A-G, J, K, L, N, Q, S, T | Seeds/Leaves/Fruits | Raw/Dec/Pow | 16 | [17,19,21,22,23,25,28,29,31,32,33,34,35,39,41,46,51] | |
| Prunus cerasus L. | Red cherry | D, F | Seeds/Fruits | Jui/Raw | 2 | [39,51] | |
| Rubus fruticosus var. vulgaris (Weihe & Nees | Laalig/Toute | D, K | Leaves | Pow/Inf | 2 | [21,32] | |
| Rubus fruticosus var. ulmifolius, (Schott) | Laallik/Tabgha | E | Leaves/Fruits | Inf | 1 | [34] | |
| Rubiaceae | Rubia tinctorum L. | Fowwa | L | Roots | Pow | 1 | [19] |
| Coffea arabica L. | Qahwa | D, C | Seeds | Inf/Dec | 3 | [32,33,51] | |
| Rutaceae | Citrus medica var. limon L. | Lhamed beldî | D, E, G, K | Fruits/Flowers/Leaves | Jui/Inf/Mac/Raw/Dec | 5 | [21,32,34,35,51] |
| Citrus paradisi Macfad. | Pamblamus/Renj | D-F, H, K | Fruits | Jui/Raw | 5 | [21,30,32,34,39] | |
| Citrus sinensis (L.) Osbeck | Limun | F, L, P | Fruits | Raw /Jui | 3 | [19,26,39] | |
| Citrus aurantium L. | Larenj/Zenbue/trunj | A, C, E, J, H, K, L, N, O | Leaves/Fruits/Flowers | Jui/Inf/Dec | 9 | [17,18,19,20,21,23,30,34,41] | |
| Ruta graveolens L. | Lfijel | E, K, L | Roots | Dec/Inf | 3 | [19,21,34] | |
| Ruta chalepensis L. | Fjīla/L-Fījel/Āwermi | G | Aerial parts | Dec/Pow | 1 | [35] | |
| Ruta montana L. | Lfijel/Iwermi | A, E, J, K, N, O, T | Stems/Leaves | Dec/Inf/Pow | 7 | [17,18,21,22,23,24,34] | |
| Salicaceae | Salix alba L. | Salef lma | D, E, J | Leaves | Dec | 3 | [17,48,51] |
| Salvadoraceae | Salvadora persica L. | Siwak | D | Barks | Mac | 1 | [32] |
| Santalaceae | Viscum album L | Lenjbar | T | Seeds | Inf | 1 | [22] |
| Sapotaceae | Argania spinosa (L.) Skeels | Argan | B-D, F-H, K, L, O, Q, S, T | Seeds/Fruits/Leaves | Raw /Pow/Ing/Oil | 15 | [19,20,21,22,24,25,28,30,31,32,33,35,39,46,51] |
| Schisandraceae | Illicium verum Hook. f. | Badiana | K | Fruits | Dec | 1 | [21] |
| Solanaceae | Capsicum annuum L. | Felfel Hârr/soudania | C, E, L, N, O | Fruits | Raw | 5 | [19,23,24,33,34] |
| Datura stramonium L. | Sdag jmel/Metal | L | Seeds | Dec | 1 | [19] | |
| Lycopersicon esculentum Mill. | Maticha | E, K, L | Fruits | Raw | 3 | [19,21,34] | |
| Nicotiana tabacum L. | Nefha | N | Leaves | Dec | 1 | [23] | |
| Solanum melongena L. | Bdenjal | D | Fruits | Raw/Dec/Inf | 1 | [32] | |
| Withania frutescens (L.) Pauquy | Tirnet | E | Leaves | Inf | 1 | [34] | |
| Taxaceae | Taxus baccata L. | Guelguem/Aguelguimt | E, K | Roots | Dec | 2 | [21,34] |
| Theaceae | Camellia sinensis (L.) Kuntze | Attay | D, E, G-I, K, L, P, Q, T | Leaves/Seeds | Inf/Dec | 11 | [19,21,22,25,26,27,32,34,35,37,51] |
| Thymelaeaceae | Thymelaea hirsuta (L.) Endl. | Metnan | E, G, K | Leafy stem/Leaves | Pow/Inf | 3 | [21,34,35] |
| Thymelaea tartonraira (L.) All. | Talazazt | J | Leaves | Dec | 1 | [17] | |
| Thymelaea virgata (Desf.) Endl. | Metnan | E, K | Leafy stem | Dec | 2 | [21,34] | |
| Aquilaria malaccensis Lam | Taghriste | D, W | Barks | Inf/Dec/Mac | 2 | [32,52] | |
| Urticaceae | Urtica dioica L. | Taznagt/Tigzenin/Lhriga | C, D, G, H, J, K, N, Q, S, T | Stems/Leaves | Dec/Inf | 11 | [17,21,22,23,25,27,30,35,41,46,51] |
| Urtica pilulifera L. | Hurriga/Tisrakmaz | O | Leaves | Dec | 1 | [24] | |
| Urtica urens L. | Tikzint | E, I | Leaves/Stems | Pow/Dec | 2 | [34,37] | |
| Urtica membranacea Poir. ex Savigny | Ḥurrayga/Malssā | G | Leaves/Aerial parts | Pou/Dec | 1 | [35] | |
| Valerianaceae | Nardostachys jatamansi (D. Don) DC. | Underground part | W | Underground parts | Inf | 1 | [52] |
| Verbenaceae | Aloysia citriodora Palau | Alwiza/Louiza | E, D, L, N, O, T | Leaves | Dec/Inf | 6 | [19,20,22,23,32,34] |
| Verbena officinalis L. | Alwiza | B, D, I, H | Leaves | Dec/Inf | 4 | [28,30,31,37] | |
| Vitaceae | Vitis vinifera L. | Dalya/Zbib/Kerma/Adilite | E, J, K, L | Leaves | Dec | 4 | [17,19,21,34] |
| Xanthorrhoeaceae | Asphodelus microcarpus Salzm. & Viv. | Lberwag/blaluz/Tazia | E, K, L | Tubers | Raw/Dec | 3 | [19,21,34] |
| Asphodelus tenuifolius Cav. | Lehyat al aatrus/Tazya/Lberiwiga | K | Leaves | Dec | 1 | [21] | |
| Zingiberaceae | Zingiber officinale Roscoe. | Sekinjbir | A, C-E, H-J, L, N, T | Rhizomes | Dec/Inf/Pow /Mac | 12 | [17,19,22,23,28,29,30,32,33,34,37,51] |
| Curcuma longa L. | Kharqum | D, I | Stems/Rhizomes | Inf | 4 | [28,32,37,51] | |
| Zygophyllaceae | Tetraena gaetula (Emb. & Maire) Beier & Thulin | Aagaia | A, J, K, L, N, O, Q | Leaves/Roots/Seeds | Pow/Inf/Dec | 7 | [17,18,19,21,23,24,25] |
| Zygophyllum gaetulum Emb. &Maire | Aagaya | A, G | Aerial parts/Leaves | Dec/Inf | 2 | [29,35] |
3.1.1. Antidiabetic Plants Well-Known in Pharmacological Literature of Diabetes
3.1.2. Antidiabetic Plants Little Known in Pharmacological Literature on Diabetes
3.1.3. Antidiabetic Plants Unknown in Pharmacological Literature of Diabetes
3.2. Overview of Diabetes in Morocco
3.3. Phytochemical Composition of Antidiabetic Medicinal Plants
3.3.1. Trigonella foenum-graecum
3.3.2. Nerium oleander
3.3.3. Rosmarinus officinalis
3.3.4. Salvia officinalis
3.3.5. Olea europaea
3.3.6. Nigella sativa
3.3.7. Allium cepa
3.3.8. Artemisia herba-alba Asso
3.3.9. Allium sativum
3.3.10. Marrubium vulgare
- -
- Flavonoids. T. foenum-graecum, O. europeae, N. sativa, A. sativum, and A. cepa have been reported to be rich in flavonoids, including quercetin and kaempferol, which are known for their antioxidant and hypoglycemic effects;
- -
- Phenolic Acids. R. officinalis, S. officinalis, A. sativum, and M. vulgare contain significant amounts of phenolic acids such as rosmarinic acid, which is linked to glucose metabolism regulation and insulin sensitivity;
- -
- Terpenoids. Plants like T. foenum-graecum, N. oleander, O. europeae, N. sativa, A. cepa, A. herba-alba Asso, and M. vulgare have demonstrated a high content of terpenoids, which contribute to their antidiabetic and anti-inflammatory activities;
- -
- Alkaloids. Alkaloids have been identified in N. oleander, O. europeae, and N. sativa, which are known to influence insulin release and glucose absorption pathways.
3.4. In Vivo and In Vitro Antidiabetic Effects of Moroccan Medicinal Plants
| Plant Species | Used Parts | Extract/EO | Groups | Compounds | References |
|---|---|---|---|---|---|
| T. foenum-graecum | Leaves/seeds/stems/flowers | Aqueous extract | Flavonoids | Quercetin/kaempferol | [75] |
| Stems | Aqueous extract | Phenolic acids | Gallic acid/caffeic acid | [75,76] | |
| Seeds | EO | Terpenoids | Neryl acetate/camphor/β-pinene/α-selinene | [79,80] | |
| Seeds | 4-hydroxyisoleucine | Alkaloids | Trigonelline | [243] | |
| N. oleander | Seeds | Aqueous extract | Flavonoids | Rutin/kaempferol | [84,87] |
| Flower/Leaves | Ethanolic extract | Phenolic acids | Cinnamic acid/chlorogenic acid | [89] | |
| Flowers | EO | Terpenoids | Neriine/digitoxigenin | [86] | |
| Leaves/Seeds | Aqueous extract | Alkaloids | Oleandrin/odoroside | [83,84] | |
| R. officinalis | Aerial parts | Aqueous extract | Flavonoids | Luteolin/apigenin/diosmin | [96] |
| Aerial parts | Aqueous extract | Phenolic acids | Rosmarinic acid/caffeic acid | [96] | |
| Aerial parts | EO | Terpenoids | 1,8-cineole/α-pinene/camphor/carnosol/ursolic acid | [91,93] | |
| S. officinalis | Aerial parts | Aqueous extract | Flavonoids | Luteolin/apigenin | [111] |
| Powder | Aqueous extract | Phenolic acids | Rosmarinic acid/salvianolic acid | [113,114,115] | |
| Leaves | EO | Terpenoids | 1,8-cineole/α-β thujone/camphor | [108,109] | |
| O. europaea | Fruits | Oil | Flavonoids | Quercetin/luteolin/apigenin | [125] |
| Leaves | Oil/Aqueous extract | Phenolic acids | Hydroxytyrosol/oleuropein/verbascoside | [126,312] | |
| Leaves/stems/branches | Aqueous extract | Terpenoids | Maslinic acid/oleanolic acid | [127,133] | |
| Leaves | Aqueous extract | Alkaloids | Cinchonidine/cinchonine | [133] | |
| N. sativa | Seeds | Aqueous extract | Flavonoids | Quercetin/rutin/apigenin/catechin/nigelflavonoside B. | [144,145] |
| Seeds | Aqueous extract | Phenolic acids | Ferulic acid/gallic acid/vanillic acid/chlorogenic acid/p-coumaric acid | [144,145] | |
| Seeds | EO | Terpenoids | Thymoquinone/THQ/DHTQ/α-thujene/β-pinene/γ-terpinene. | [141] | |
| Seeds | Ethanolic extract | Alkaloids | Nigellicine/nigellimine/nigellidine | [147,148] | |
| A. cepa | Bulbs | Aqueous extract | Flavonoids | Quercetin 3-glucoside/quercetin 4′-glucoside/isorhamnetin | [163,164,165,166] |
| Onion skins | Ethanolic extract | Phenolic acids | Chlorogenic acid/vanillic acid/ferulic acid | [171] | |
| Roots | Methanol extract | Terpenoids | Allicin/disulfides/steroid saponins (alliospiroside A) | [169] | |
| A. herba-alba | Aerial parts | Aqueous extract | Flavonoids | Apigenin/catechin/luteolin. | [190] |
| Leaves/Aerial parts | Aqueous extract | Phenolic acids | Caffeic acid/tannins | [189,190] | |
| Leaves | EO | Terpenoids | α- β-thujone/camphor/terpinen-4-ol/ocimene | [184] | |
| A. sativum | Bulbs | Aqueous extract | Flavonoids | Quercetin (trace) | [203] |
| Bulbs | Aqueous extract | Phenolic acids | Chlorogenic acid/p-coumaric acid/4-hydroxybenzoic acid | [201] | |
| Bulbs | EO | Terpenoids | Allicin, diallyl disulfide, diallyl trisulfide, ajoene. | [197,198] | |
| Bulbs | Aqueous extract | Alkaloids | S-allyl cysteine | [198] | |
| M. vulgare | Aerial parts | Aqueous extract | Flavonoids | Apigenin/luteolin/chrysoeriol/diosmetin | [216] |
| Aerial parts | Aqueous extract | Phenolic acids | Gallic acid/gentisic acid/syringic acid/cinnamic acid/ferulic acid/p-coumaric acid | [215,216,217] | |
| Flowers/Aerial parts/Leaves | EO | Terpenoids | Marrubic acid/marrubiin/germacrene D/β-caryophyllene/bicyclogermacrene. | [220,221,222,223,224,225] |
| Family | Species | Extracts | Parts Used | Administrated Dose | Model/Experimental Methods | Key Results | References |
|---|---|---|---|---|---|---|---|
| Leguminosae | Trigonella foenum-graecum | Methanolic extract | Seeds | 2 g/kg | Oral glucose tolerance test Normal albino rats | Reduction in blood glucose | [235] |
| Hydroalcoholic extract | Seeds | 100 μL of extract for α-amylase/60 μL of extract for α-glucosidase | α-amylase and α-glucosidase inhibition assay | High inhibitory activity of α-amylase and α-glucosidase | [236] | ||
| Aqueous extract | Seeds | 300 mg/kg | STZ-induced diabetic rats | IM6E demonstrated strong α-glucosidase activity and moderate α-amylase and invertase inhibition activities under in vitro conditions | [237] | ||
| Ethanolic extract | Seeds | 1 g/kg | Normal and alloxan-induced diabetic rats | Decreased blood glucose to 12.40% level in alloxan-induced rats No acute toxicity | [238] | ||
| Aqueous extract | Seeds | 0.44/0.87/1.74 g/kg for 6 weeks | STZ-induced diabetic rats | Increases body weight and decreases fasting blood glucose | [239] | ||
| Aqueous extract | Seeds | 2.5 g/kg | Normal and alloxan induced diabetic rabbits | Reduction in plasma glucose levels in the fenugreek-treated rabbits | [240] | ||
| Ethanolic extract | Seeds | 25 g seed mucilage/rat/day | STZ-induced diabetic rats | Amelioration of the diabetic state | [241] | ||
| Aqueous extract | Seeds | 100 mg/kg | STZ-induced diabetic rats | Reduced blood glucose levels Urea levels decreased following daily intraperitoneal injection | [242] | ||
| Solution of 4-hydroxyisoleucine | Seeds | 50 mg/kg | Single and repeated injection STZ-induced type I diabetic rats | Levels of insulin are reduced by 65% | [243] | ||
| Hydroalcoholic extract | Seeds | 400 mg/kg | STZ-induced diabetic rats | Decreased blood glucose levels | [244] | ||
| Powder | Seeds | 5 g of dry FSP mixed with 95 g of powdered rat feed) for 21 days | Alloxan induced diabetic rats | FSP treatment increased insulin levels in diabetic rats to nearly 80% | [245] | ||
| Apocynaceae | Nerium oleander | Aqueous extract | Leaves | Nd | a-amylase inhibition assay | Breakdown of starch to maltose, maltotriose, various oligoglucans is mediated by α-amylase enzyme followed by subsequent α-glucosidase activity to finally yield glucose | [246] |
| Powder | Leaves | 16 g dry leaves/kg | Normal rats | Inhibitory activity of α-glucosidase Reduced the blood glucose level in maltose- and sucrose-loaded rats at very high dose of 16 g/kg | [247] | ||
| Methanolic extract | Leaves | 200 mg/kg | Alloxan induced diabetic rats | Reduced blood glucose level by 73.79% OGTT revealed increase in glucose tolerance by 65.72% No mortality was observed in the experiment | [248] | ||
| Methanolic extract | Flowers | Nd | Rats L6 myogenic cells | Decreasing the blood glucose level and inhibition of α-amylase | [249] | ||
| Plant extract | Nd | 250 mg/kg for 4 weeks | STZ-induced diabetic rats | Improvement in insulin and glucose levels | [250] | ||
| Ethanolic extract | Flowers | 225 mg/kg | STZ-induced diabetic rats | Decrease glucose level | [251] | ||
| Powder | Shoots | 375 μg/0.5 mL of distilled water for 12 weeks | High-fat-diet-fed STZ-induced diabetic rats | Reduced fasting blood glucose | [252] | ||
| Chloroform and ethanolic extract | Leaves | 50 mg to 5000 mg/kg | Alloxan-induced diabetic rats | Prevented body weight loss in diabetic rats No sub-acute glucose reduction | [253] | ||
| Lamiaceae | Rosmarinus officinalis | EO | Leaves | 250 µl | α-amylase inhibition assay | Inhibitory activity of α-amylase | [254] |
| Aqueous extract | Aerial parts | 100 µg/20 µL distilled water | α-glucosidase inhibition assay | High inhibitory activity of α-glucosidase | [255] | ||
| Ethanolic extract | Leaves | 100 mg of RAE | α-amylase inhibition assay | Inhibited amylase activity by 85% | [256] | ||
| Diethyl ether and n-butanol extract | Leaves | 800 mg/kg | α-glucosidase assay Oral glucose tolerance test Normal and STZ-induced diabetic rats | Inhibitory activity of α-glucosidase Decrease glucose level Inhibited glucose intestinal transport | [257] | ||
| Ethanolic extract | Leaves | 20 mg/0.6 water | Normal and STZ-induced diabetic rats | Strong α-glucosidase inhibitory | [258] | ||
| Powder | Leaves | 12% for 6 weeks | Normal and STZ-induced diabetic rats | Reduced fasting blood glucose | [259] | ||
| Ethylacetate extract | Nd | 300 mg/kg | Normal and alloxan-induced diabetic rats | Reduced fasting blood glucose | [260] | ||
| Aqueous extract | Leaves | 200 mg/kg for 21 days | Normal and STZ-induced diabetic rats | Reduced the glucose level | [261] | ||
| Aqueous extract | Leaves | 1.11 gm/mL/day | Normal and STZ-induced diabetic rats | Reduced blood glucose level Reduced fasting plasma glucose | [262] | ||
| Aqueous extract | Leaves | 200 mg/kg for 21 days | Normal and STZ-induced diabetic rats | Reduced fasting plasma glucose | [263] | ||
| Aqueous extract | Leaves | 200 mg/kg for 21 days | Normal and STZ-induced diabetic rats | Reduced fasting plasma glucose | [264] | ||
| Powder | Leaves | 5 g/100 g diet | Normal and STZ-induced diabetic rats | Reduced blood glucose level | [265] | ||
| Aqueous extract | Leaves | 200 mg/kg for 21 days | Normal and STZ-induced diabetic rats | Increased serum insulin, C-peptide while decreased ALT and aspartate aminotransferase | [266] | ||
| Aqueous extract | Leaves | 200 mg/kg/day | STZ-induced diabetic rats | Increased serum insulin level Reduced fasting plasma glucose | [267] | ||
| Aqueous extract | Leaves | 200 mg/kg for 21 days | STZ-induced diabetic rats | Reduced blood glucose level Reduced antioxidant status of diabetic rats | [268] | ||
| Rosmarinic acid | Leaves | 120–200 mg/kg | STZ-induced type 1 diabetes rats or high-fat-diet (HFD)-induced type 2 diabetes rats | Decreased plasma glucose levels and improved insulin sensitivity | [269] | ||
| Rosmarinic acid | Leaves | 577 µg/mL | STZ-induced diabetic rats High-fat-diet-induced diabetic rats | Reduced fasting plasma glucose Increased insulin levels without affecting liver glycogen levels | [270] | ||
| Ethanolic extract | Leaves | 200 mg/kg for 7 days | Alloxan-induced diabetic rats | Reduced fasting plasma glucose and increased serum insulin | [271] | ||
| Powder | Leaves | 20% of powder for 45 days | Alloxan-induced diabetic rats | Reduced fasting plasma glucose | [272] | ||
| Rosmarinic acid | Leaves | 100–200 mg/kg for 8 weeks | Alloxan-induced diabetic rats | Inhibited glomerular hypertrophy, glomerular number loss and glomerulosclerosis | [273] | ||
| Salvia officinalis | Aqueous extract | Aerial parts | Nd | α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [274] | |
| EO | Leaves | 5% to 75% | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [275] | ||
| Aqueous extract | Aerial parts | 50 µL | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [276] | ||
| Ethanolic extract | Leaves | 0–200 µg | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [112] | ||
| Water and ethanolic extract | Nd | 12% | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [277] | ||
| Ethylacetate extract | Aerial parts | 20–300 mg/mL | α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [278] | ||
| Methanolic extract | Leaves | 250 and 500 mg/kg for 21 days | α-glucosidase inhibition assay Oral glucose tolerance test Normal and alloxan-induced diabetic rats | Inhibitory activity of α-glucosidase Reduced postprandial blood glucose | [279] | ||
| Ethanolic extract | Leaves and flowers | 300 mg/kg | Alloxan induced diabetic rats | Reduced blood glucose and cholesterol | [280] | ||
| Ethanolic extract | Leaves | 0.2 and 0.4 g/kg for 14 days | Normal and STZ-induced diabetic rats | Reduction in serum glucose and increased plasma insulin in | [281] | ||
| Aqueous and ethanolic extracts | Leaves | 100 mg/kg for 14 days | Normal and alloxan-induced diabetes in white rats | Reduced blood glucose | [282] | ||
| Water ethanol extract | Leaves | 500 mg/kg | Normal and alloxan-induced diabetic mice | Reduced blood glucose | [283] | ||
| Aqueous extract | Leaves | 300 mg/kg for 5 weeks | Normal and alloxan-induced diabetes rats | Reduced blood glucose | [284] | ||
| Aqueous extract | Leaves | 400 and 600 mg/kg for 7 days | Alloxan-induced diabetic mice | Reduced fasting blood glucose | [285] | ||
| Methanolic extract | Leaves | 100–500 mg/kg | STZ-induced diabetic rats | Decreased serum glucose after 3 h of administration | [286] | ||
| Marrubium vulgare | Aqueous extract | Leaves | 400 mg/kg | α-amylase inhibition assay Normal rats | Inhibitory activity of pancreatic α-amylase Reduced blood glucose | [287] | |
| Hydro-alcoholic extract | Leaves | Nd | α-amylase inhibition assay | Inhibitory activity of pancreatic α-amylase | [288] | ||
| Methanolic extract | Aerial parts | 500 mg/kg for 28 days | STZ-induced diabetic rats | Increased plasma insulin Reduced blood glucose | [289] | ||
| Methanol, water and butanol extract | Whole plant | 1 and 2 mg/mL for 28 days | Cyclosporine A and STZ-induced diabetic rats | Induced autoimmune diabetes mellitus-type1 induced by cyclosporine A and STZ in mice | [290] | ||
| Aqueous extract | Aerial parts | 100, 200 and 300 mg/kg | Normal and alloxan-induced diabetes rats | Increased plasma insulin and tissue glycogen | [214] | ||
| Aqueous extract | Leaves | 300 mg/kg | Normal and alloxan-induced diabetes rats | Increased plasma insulin Reduced blood glucose | [291] | ||
| Ethanolic extract | Whole plant | 100 mg/kg | Normo-glycemic rats | Increased plasma insulin Reduced blood glucose | [292] | ||
| Oleaceae | Olea europaea | Alcoholic extract | Leaves | 0.1, 0.25 and 0.5 g/kg for 14 days | Normal and STZ-induced diabetic rats | Decreased the serum glucose Increased the serum insulin in diabetic rats | [293] |
| Nd | Leaves | 1 g/kg for 14 days | STZ-induced diabetic rats | Decreased blood glucose level | [294] | ||
| Alcoholic extract | Leaves | 1 g/kg | Single and repeated injection STZ-induced diabetic rats | Improved glucose homeostasis through the reduction of starch digestion and absorption | [295] | ||
| Aqueous extract | Leaves | 100 and 200 mg/kg | STZ-induced diabetic rats | Decreased serum glucose level | [296] | ||
| Powder | Leaves | 6.25% | STZ-induced diabetic rats | Decreased serum glucose level by 38% | [297] | ||
| Ethanolic extract | Leaves | 300 and 500 mg/kg/day | STZ-induced diabetic rats | Inhibited high-glucose-induced neural damage | [298] | ||
| Ethanolic extract | Leaves | 3 and 5 mg/kg | STZ-induced diabetic rats | Thymoquinone and oleuropein significantly decrease serum glucose levels | [299] | ||
| Aqueous extract | Leaves and fruits | 1 g/kg | Normal and STZ-induced diabetic rats | Decreased blood glucose level at 4th week compared to the diabetic control rats | [300] | ||
| Powder | Leaves | 17.8 mg/kg | STZ-induced diabetic rats | Reduced blood glucose tolerance curve | [301] | ||
| Aqueous extract | Leaves | 200 and 400 mg/kg | Normal and STZ-induced diabetic rats | Decreased serum insulin level | [302] | ||
| Ethanolic extract | Leaves | 200 and 400 mg/kg for 10 weeks | HFD STZ-induced diabetic rats | Increased serum insulin level | [303] | ||
| Aqueous extract | Leaves | 1% and 3% | STZ-induced diabetic rats | Exerted antihyperglycemic effects via AS160 inhibition | [304] | ||
| Aqueous extract | Leaves | 1 mg/mL 200 mg/kg | α-glucosidase inhibition assay Normal and STZ-induced diabetic rats | Strong α-glucosidase inhibitory activity Reduced blood glucose | [305] | ||
| Ethanolic extract | Leaves | 100 mg/kg | Normal and HFD rats | Reduced blood glucose and insulin levels | [306] | ||
| Alcoholic extract | Leaves | 8 and 16 mg/kg | Alloxan-induced diabetic rats | Decreased serum glucose level | [307] | ||
| Aqueous extract | Leaves | 3% and 6% | Alloxan-induced diabetes rats | Decreased blood glucose level | [308] | ||
| Aqueous extract | Leaves | 100–600 mg/kg | Normal and alloxan-induced diabetes rats | Decreased blood glucose level Increased plasma insulin level | [309] | ||
| Hydroethanolic extract | Leaves | 5–20 mg/kg for 40 days | Normal and alloxan-induced type 1 diabetic rats | Decreased blood glucose level | [310] | ||
| Ethanolic extract | Leaves | 600 mg/kg | Alloxan-induced diabetic rabbits | Reduced blood glucose level by 20% | [311] | ||
| Aqueous extract | Leaves | 20 mg/kg for 16 weeks | Normal and alloxan-induced diabetes rabbits | Decreased blood glucose level | [312] | ||
| Ethanolic extract | Leaves | 3.85 mg/ml | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [313] | ||
| Hydro-alcoholic extract | Oil | 500 to 31.25 mg/mL. | α-glucosidase and α-amylase inhibition assay | Inhibitory activity of α-glucosidase Less inhibitory activity of α-amylase | [314] | ||
| Ethyl acetate extract | Stems | 10 µL | α-amylase inhibition assay | Inhibitory activity of α-amylase | [315] | ||
| Hydro-alcoholic extract | Leaves | 100–600 µM | α-glucosidase and α-amylase inhibition assay | Inhibitory activity of α-glucosidase Less inhibitory activity of α-amylase | [134] | ||
| Ranunculaceae | Nigella Sativa | Aqueous extract | Seeds | 10–50 μL | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [316] |
| Ethanolic extract | Seeds | 2 g/kg for 4 weeks | Oral glucose tolerance test | Hypoglycemic and hypolipidemic activity | [299] | ||
| Aqueous extract | Seeds | 2 g/kg | Oral glucose tolerance test | Improved glucose tolerance in rats | [317] | ||
| Aqueous methanol Oil | Seeds | 810 mg/kg for 25 days 2.5 mL/kg for 25 days | Normal and alloxan-induced diabetes rats | Administration of the crude methanolic extract and the oil decreased significantly the blood glucose after 10 days of treatment | [318] | ||
| Methanolic extract/Oil | Seeds | 2.5 mL/kg for 24 days | Normal and alloxan-induced diabetes rabbits | Decreased blood glucose level | [319] | ||
| Ethanolic extract | Seeds | 20 and 40% of pulverized extract (for 24 days) | Normal and alloxan-induced diabetes rats | Decreased blood glucose level | [320] | ||
| Ethyl acetate fraction of Ethanolic extract | Seeds | 200–1000 mg/kg | Alloxan-induced type 2 diabetes rats | Reduced blood glucose level | [321] | ||
| Ethanolic extract | Seeds | 100, 200, and 400 mg/kg for 6 weeks | STZ-induced diabetic rats | Decreased serum glucose level | [322] | ||
| Methanolic extract | Seeds | 500 mg/kg | STZ-induced types 2 diabetic rats | Reduced postprandial glucose, and improved glucose tolerance in rats | [323] | ||
| Nd | Seeds | 0.5–1.5 mL | STZ-induced diabetic rats | Reduced serum glucose level | [324] | ||
| Ethanolic extract | Seeds | 300 and 600 mg/kg for 7 days | HFD STZ-induced diabetic rats | Reduced blood glucose level | [325] | ||
| Ethanolic extract | Seeds | 100 mg/kg for 28 days | STZ-induced diabetic rats | Decreased blood glucose level | [326] | ||
| Oil | Seeds | 400 mg/kg for 4 weeks | STZ-induced diabetic hamsters | Decreased blood glucose level | [327] | ||
| Oil | Seeds | 2 mg/kg for 30 days | STZ-induced diabetic rats | Reduced fasting blood glucose and increased insulin levels | [328] | ||
| Petroleum ether extract | Seeds | 2 g/kg for 4 weeks | STZ-induced diabetic rats | The petroleum ether extract exerted an insulin-sensitizing action | [329] | ||
| Ethanolic extract | Seeds Polys | 35–140 mg/kg for 4 weeks | HFD STZ-induced types 2 diabetic rats | Reduced fasting plasma glucose and increased serum insulin | [330] | ||
| Alliaceae | Allium cepa | Ethyl alcohol extract Quercetin | Skin | 1–3 mg/mL | α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [331] |
| Methanolic extract | Skin | Nd | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [332] | ||
| Ethanolic extract | Skin | 30 mg/mL 0.1–0.5 mg/mL | α-amylase inhibition assay α-glucosidase inhibition assay | Inhibitory activity of α-amylase α-glucosidase assay | [333] | ||
| Aqueous extracts | Skin | 0.01–10 mg/mL | α-amylase inhibition assay | Inhibitory activity of α-amylase | [334] | ||
| Hydroethanolic extract | Skin | 10 µg/mL | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [335] | ||
| Hydromethanolic extract | Skin | Nd | α-glucosidase inhibition assay | Inhibitory activity of α-glucosidase | [336] | ||
| EO | Bulbs | 100 mg/kg for 21 days | STZ-induced diabetic rats | Deceased blood glucose and increase in serum insulin | [337] | ||
| Ethanolic extract | Bulbs | 150 and 300 mg/kg | Normal and STZ-induced diabetic rats | Decreased fasting blood glucose Increased serum insulin levels | [338] | ||
| Ethanolic extract Quercetin | Bulbs | 0.5 or 1% for 8 weeks 0.1% for 8 weeks | Oral glucose tolerance test Normal and HFD STZ-induced diabetic rats | Improves insulin sensitivity by upregulating expressions of insulin receptor and glucose transporter | [339] | ||
| Powder | Bulbs | 0.5 and 2% for 4 weeks | Normal and HFD STZ-induced diabetic rats | Serum insulin concentrations and insulin resistance were dose-dependently increased in the onion-fed groups | [340] | ||
| Aqueous extract | Whole plant | 200–300 mg/kg for 6 weeks | Alloxan-induced diabetic rats | Reduced fasting blood glucose level by 75.4% at 300 mg/kg | [341] | ||
| Aqueous extract | Bulbs | 1 mL for 4 weeks | Normal and alloxan-induced diabetic rats | Reduced their plasma glucose levels by 70% | [342] | ||
| Powder | Bulbs | 12.5% for 15 days | Normal and HFD alloxan-induced diabetic rats | Reduced fasting blood glucose level | [343] | ||
| Allium sativum | Aqueous extract | Bulbs | 1250 µg/mL | α-amylase inhibition assay | Inhibitory activity of α-amylase | [344] | |
| Oil | Bulbs | 5–10% | α-amylase inhibition assay | Inhibitory activity of α-amylase | [346] | ||
| Polysaccharide | Bulbs | 0.5–4.0 mg/mL | α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [347] | ||
| Powder | Bulbs | Nd | Convective hot-air drying α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [348] | ||
| Allyl methyl sulfide | Bulbs | 50–200 mg/kg for 30 days | STZ-induced diabetic rats | Reduced blood glucose level Regulate insulin production and sensitivity in pancreatic β-cells | [349] | ||
| Ethanolic extract | Bulbs | 0.1–0.5 g/kg for 14 days | Normal and STZ-induced diabetic rats | Decreased serum glucose level | [350] | ||
| Aqueous extract | Bulbs | 500 mg/kg for 3 weeks | STZ-induced diabetic rats | Decreased serum glucose level | [351] | ||
| Polysaccharide | Bulbs | 1.25–5.0 g/kg for 5 weeks | STZ-induced diabetic rats | Reduced fasting blood glucose | [352] | ||
| Aqueous extract | Bulbs | 300 μL 200–400 mg/kg for 4 weeks | α-amylase inhibition assay Oral glucose tolerance Alloxan-induced diabetic rats | Inhibitory activity of α-amylase Decreased serum blood glucose level Increased plasma insulin level | [345] | ||
| Aqueous extract | Bulbs | 0.4 g/100 g for 4 weeks | Normal and alloxan-induced diabetic rats | Reduced their plasma glucose levels by 68% | [342] | ||
| Powder | Bulbs | 12.5% for 15 days | Normal and HFD alloxan-induced diabetic rats | Reduced fasting blood glucose level | [343] | ||
| Asteraceae | Artemisia herba-alba Asso | EO | Whole plants | 0.25–1 mg/mL | α-amylase and α-glucosidase inhibition assay | Inhibitory activity of α-amylase and α-glucosidase | [353] |
| Ethyl alcohol extract | Whole plants | 200 µL 500–4000 mg/kg | α-amylase inhibition assay Alloxan-induced diabetic rats | Inhibitory activity of α-amylase Decreased plasma glucose level | [354] | ||
| Aqueous extract | Aerial parts | 0.39 g/kg for 18 weeks | Alloxan-induced diabetic rats | Reduced blood glucose level | [355] | ||
| Aqueous extract | Aerial parts | 100–300 mg/kg for 15 days | Normal and alloxan-induced diabetic rats | Reduced blood glucose level | [356] | ||
| Aqueous extract | Aerial parts | 85 mg/kg | STZ-induced diabetic rabbits | Reduced blood glucose level | [357] | ||
| Ethyl alcohol extract | Aerial parts | 100–400 mg/kg for 14 weeks | STZ-induced diabetic rats | Reduced fasting blood glucose level Increased plasma insulin level | [358] | ||
| Aqueous extract | Aerial parts | 50 and 100 mg/kg | STZ-induced diabetic rabbits | Reduced blood glucose level | [359] | ||
| Aqueous extract | Whole plants | 50–100% for 10 days | Dexamethasone-induced diabetic rats | Decreased postprandial blood glucose | [360] | ||
| Hydroethanolic extract | Aerial parts | 2 g/kg 18 weeks | HFD-induced diabetic rats | Decreased the blood glucose level and serum insulin concentrations | [361] | ||
| Aqueous extract | Aerial parts | 0.39 g/kg for 14 weeks | Alloxan-induced diabetic rats | Reduced fasting serum glucose level | [362] | ||
| Aqueous extract | Aerial parts | 400 mg/kg for 3 weeks | Alloxan-induced diabetic rabbits | Reduced blood glucose level | [363] |
3.4.1. Trigonella foenum-graecum
3.4.2. Nerium oleander
3.4.3. Rosmarinus officinalis
3.4.4. Salvia officinalis
3.4.5. Marrubium vulgare
3.4.6. Olea europaea
3.4.7. Nigella sativa
3.4.8. Allium cepa
3.4.9. Allium sativum
3.4.10. Artemisia herba-alba Asso
- -
- T. foenum-graecum: Numerous studies have demonstrated its hypoglycemic potential, attributed to its saponins, alkaloids, and flavonoids. Clinical trials also show its promise in improving glucose tolerance.
- -
- O. europaea: The leaves contain high levels of oleuropein and hydroxytyrosol, known for their antidiabetic properties. These compounds have shown potent effects in animal models of diabetes.
- -
- N. sativa: Thymoquinone and other phenolics demonstrate strong insulinotropic and glucose-lowering effects in vivo.
- -
- A. herba-alba: The plant is rich in terpenoids, particularly thujone and camphor, which have shown antidiabetic effects in animal models. Its use in North Africa is well-established, and its traditional use is supported by modern pharmacological studies.
- -
- S. officinalis: This plant is widely recognized for its high levels of rosmarinic acid and flavonoids, which exhibit both hypoglycemic and antioxidant properties. In vivo studies confirm its potential as an adjunct in diabetes management.
3.5. Current Therapeutic Trajectory of Diabetes Management in Morocco
3.6. Comparison with Plant-Based Management of Diabetes in the Maghreb Region
4. Future Directions and Research Opportunities
5. Conclusions and Implications for Healthcare Practice
Funding
Conflicts of Interest
Correction Statement
References
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109279. [Google Scholar] [CrossRef]
- Lefèbvre, P. La pandémie de diabète: Un fléau cardiovasculaire et une menace pour les systèmes de santé et l’économie mondiale. Médecine Mal. Métab. 2008, 2, 169–179. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 August 2024).
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, D.S.; Mehta, A.; Sandesara, P.B.; Thobani, A.; Brandt, S.; Sperling, L.S. Strategies for appropriate selection of SGLT2-i vs. GLP1-RA in persons with diabetes and cardiovascular disease. Curr. Cardiol. Rep. 2019, 21, 100. [Google Scholar] [CrossRef]
- Marouf, A.; Joël, R. La Botanique de A à Z; Edition Dunod: Paris, France, 2007; pp. 66–82. [Google Scholar]
- Chang, C.L.; Lin, Y.; Bartolome, A.P.; Chen, Y.C.; Chiu, S.C.; Yang, W.C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Altern. Med. 2013, 2013, 378657. [Google Scholar] [CrossRef]
- Eddouks, M.; Ouahidi, M.L.; Farid, O.; Moufid, A.; Khalidi, A.; Lemhadri, A. L’utilisation des plantes médicinales dans le traitement du diabète au Maroc. Phytothérapie 2007, 5, 194–203. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef]
- Oridupa, O.; Saba, A. Diabetes mellitus in Nigeria and the on-going search for a cure from medicinal plants: A review. Afr. J. Diabetes Med. 2017, 25, 4–6. [Google Scholar]
- Jacob, B.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar]
- Shen, Q.; Zhang, L.; Liao, Z.; Wang, S.; Yan, T.; Shi, P.U.; Liu, M.; Fu, X.; Pan, Q.; Wang, Y.; et al. The genome of Artemisia annua provides insight into the evolution of Compositae family and artemisinin biosynthesis. Mol. Plant 2018, 27, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P. Compositae species with most prominent bioactivity and their potential applications: A review. Ind. Crop Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in South–Eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 270, 105–117. [Google Scholar] [CrossRef]
- Amrani, F.; Rhallab, A.; Alaoui, T.; Badaoui, K.; Chakir, S. Étude ethnopharmacologique de quelques plantes utilisées dans le traitement du diabète dans la région de Meknès-Tafilalet (Maroc). Phytothérapie 2010, 8, 161–165. [Google Scholar] [CrossRef]
- Ghourri, M.; Zidane, L.; Douira, A. Usage des plantes médicinales dans le traitement du Diabète Au Sahara marocain (Tan-Tan). J. Anim. Plant Sci. 2013, 17, 2388–2427. [Google Scholar]
- Bousta, D.; Boukhira, S.; Aafi, A.; Ghanmi, M.; El-Mansouri, L. Ethnopharmacological study of anti-diabetic medicinal plants used in the middle-atlas region of Morocco (Sefrou region). Int. J. Pharm. Res. Health Sci. 2014, 2, 75–79. [Google Scholar]
- Benkhnigue, O.; Akka, F.B.; Salhi, S.; Fadli, M.; Douira, A.; Zidane, L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J. Anim. Plant Sci. 2014, 23, 3539–3568. [Google Scholar]
- Alami, Z.; Aynaou, H.; Alami, B.; Hdidou, Y.; Latrech, H. Herbal medicines use among diabetic patients in oriental Morocco. J. Pharmacogn. Phytother. 2015, 7, 9–17. [Google Scholar]
- Orch, H.; Douira, A.; Zidane, L. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète, et des maladies cardiaques dans la région d’Izarène (Nord du Maroc). J. Appl. Biosci. 2015, 86, 7940–7956. [Google Scholar] [CrossRef]
- Hachi, M.; Ouafae, B.; Hachi, T.; Mohamed, E.B.; Imane, B.; Atmane, R.; Zidane, L. Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco). Lazaroa 2016, 37, 1–11. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western anti-atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Laadim, M.; Ouahidi, M.L.; Zidane, L.; El Hessni, A.; Ouichou, A.; Mesfioui, A. Ethnopharmacological survey of plants used for the treatment of diabetes in the town of Sidi Slimane (Morocco). J. Pharmacogn. Phytother. 2017, 9, 101–110. [Google Scholar]
- Mrabti, H.N.; Jaradat, N.; Kachmar, M.R.; Ed-Dra, A.; Ouahbi, A.; Cherrah, Y.; Faouzi, M.E.A. Integrative herbal treatments of diabetes in Beni Mellal region of Morocco. J. Integr. Med. 2019, 17, 93–99. [Google Scholar] [CrossRef]
- Skalli, S.; Hassikou, R.; Arahou, M. An ethnobotanical survey of medicinal plants used for diabetes treatment in Rabat, Morocco. Heliyon 2019, 5, e01421. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-safi, I.; Bari, A.; Grafov, A.; Bousta, D. Ethnobotanical survey about the management of diabetes with medicinal plants used by diabetic patients in region of Fez Meknes, Morocco. J. Ethnobot. Res. Appl. 2020, 19, 1–28. [Google Scholar] [CrossRef]
- Chetoui, A.; Kaoutar, K.; Boutahar, K.; El Kardoudi, A.; BenChaoucha-Chekir, R.; Chigr, F.; Najimi, M. Herbal medicine use among Moroccan type 2 diabetes patients in the Beni Mellal-Khenifra region. J. Herb. Med. 2021, 29, 100480. [Google Scholar] [CrossRef]
- Hinad, I.; S’hih, Y.; Elhessni, A.; Mesfioui, A.; Laarbi Ouahidi, M. Medicinal plants used in the traditional treatment of diabetes in Ksar Elkebir Region. Pan Afr. Med. J. 2022, 42, 1–12. [Google Scholar] [CrossRef]
- Sekkat, Z.L.; Hassikou, R.; Souad, S. Ethnobotanical study on the use of medicinal plants among diabetic patients in the Rabat-Salé-Kénitra region, Morocco. Ethnobot. Res. Appl. 2023, 26, 1–44. [Google Scholar] [CrossRef]
- Ghabbour, I.; Ghabbour, N.; Khabbach, A.; Louahlia, S.; Hammani, K. Ethnobotanical statistics of disease groups treated by medicinal plants used in the province of Taza (northern Morocco). Ethnobot. Res. Appl. 2023, 26, 1–23. [Google Scholar] [CrossRef]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Ennabili, A.; Maache, S.; Laamech, J.; Lyoussi, B. Ethnobotanical Study of Medicinal Plants used by Traditional Health Practitioners to Manage Diabetes Mellitus in Safi and Essaouira Provinces (Central-Western Morocco). Trop. J. Nat. Prod. Res. 2023, 7, 2178–2201. [Google Scholar]
- Insaf, M.; Ghizlane, H.; Ghada, B.; Fadoua, E.; Samiha, K.; Mohamed, F. Plant-based Bioproducts for the Control of Diabetes and Hypertension in Tangier-Tetouan Region (Morocco). Trop. J. Nat. Prod. Res. 2023, 7, 4016–4025. [Google Scholar]
- Arraji, M.; Al Wachami, N.; Boumendil, K.; Chebabe, M.; Mochhoury, L.; Laamiri, F.Z.; Barkaoui, M.; Chahboune, M. Ethnobotanical survey on herbal remedies for the management of type 2 diabetes in the Casablanca-Settat region, Morocco. BMC Complement. Med. Ther. 2024, 24, 160. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Fouad, Z.; Fatiha, E.A.; Larbi, E.G.; Lahcen, Z. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes and gout in the north of Morocco (Tangier, Tetouan and Chefchaouen cities). Plant Arch. 2019, 19, 2731–2737. [Google Scholar]
- Hseini, S.; Kahouadji, A. Étude ethnobotanique de la flore médicinale dans la région de Rabat (Maroc occidental). Lazaroa 2007, 28, 79–93. [Google Scholar]
- Naceiri Mrabti, H.; Bouyahya, A.; Naceiri Mrabti, N.; Jaradat, N.; Doudach, L.; Faouzi, M.E.A. Ethnobotanical survey of medicinal plants used by traditional healers to treat diabetes in the Taza region of Morocco. Evid. Based Complement. Altern. Med. 2021, 2021, 5515634. [Google Scholar] [CrossRef]
- Teixidor-Toneu, I.; Martin, G.J.; Ouhammou, A.; Puri, R.K.; Hawkins, J.A. An ethnomedicinal survey of a Tashelhit-speaking community in the High Atlas, Morocco. J. Ethnopharmacol. 2016, 188, 96–270. [Google Scholar] [CrossRef]
- El Rhaffari, L.; Zaid, A. Pratique de la phytothérapie dans le sud-est du Maroc (Tafilalet): Un savoir empirique pour une pharmacopée rénovée. Sources Savoir Médicaments Future 2002, 1, 293–318. [Google Scholar]
- Hachi, M.; Hachi, T.; Belahbib, N.; Dahmani, J.; Zidane, L. Contribution a L’etude Floristique et Ethnobotanique de la Flore medicinale utilisee Au Niveau de la ville de Khenifra (MAROC)/Contribution to the study and Floristic Ethnobotany flora medicinal use at the City OF Khenifra (Morocco). Int. J. Innov. Appl. Stud. 2015, 27, 754. [Google Scholar]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; Zidane, L. Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon 2019, 5, e02191. [Google Scholar] [CrossRef] [PubMed]
- Katiri, A.; Barkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in the Tizi n’Test region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Fakchich, J.; Elachouri, M. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar]
- Benlamdini, N.; Elhafian, M.; Rochdi, A.; Zidane, L. Etude floristique et éthnobotanique de la flore médicinale du Haut Atlas oriental (Haute Moulouya). J. Appl. Biosci. 2014, 78, 6771–6787. [Google Scholar] [CrossRef]
- Hilah, F.E.; Dahmani, J.; Zidane, L. Ethnobotanical study of medicinal plants used to control diabetes in population of the central plateau, Morocco. Plant Arch. 2021, 21, 560–564. [Google Scholar]
- Bouyahya, A.; Abrini, J.; Et-Touys, A.; Bakri, Y.; Dakka, N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur. J. Integr. Med. 2017, 13, 9–25. [Google Scholar] [CrossRef]
- El Kourchi, C.; Belhoussaine, O.; Harhar, H.; Bouyahya, A.; Kotra, V.; Rehman, I.U.; Ming, L.C.; Lua, P.L.; Tabyaoui, M. Medicinal and aromatic plants traditionally used to treat metabolic diseases in the Rabat region, Morocco. J. Res. Pharm. 2024, 28, 1293–1315. [Google Scholar] [CrossRef]
- El Boullani, R.; Barkaoui, M.; Lagram, K.; El Finti, A.; Kamel, N.; El Mousadik, A.; Serghini, M.A.A.; Msanda, F. The use of plants in the traditional treatment of diabetes patients: Survey in southern Morocco. Not. Sci. Biol. 2022, 14, 27322. [Google Scholar] [CrossRef]
- El-Ghazouani, F.; El-Ouahmani, N.; Teixidor-Toneu, I.; Yacoubi, B.; Zekhnini, A. A survey of medicinal plants used in traditional medicine by women and herbalists from the city of Agadir, southwest of Morocco. Eur. J. Integr. Med. 2021, 42, 101284. [Google Scholar] [CrossRef]
- Taha, D.; Bourais, I.; El Hajjaji, S.; Bouyahya, A.; Khamar, H.; Iba, N. Traditional medicine knowledge of medicinal plants used in Laayoune boujdour sakia el hamra region, Morocco. J. Herbs Spices Med. Plants 2022, 28, 351–369. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). Diabetes in Morocco. 2021. Available online: https://idf.org/our-network/regions-and-members/middle-east-and-north-africa/members/morocco/ (accessed on 5 August 2024).
- Lachguer, S.A.; Chakit, M.; Kossou, J.; Kachache, H.; Boujdi, R.; Bouziani, A.; Benkirane, H. Obesity and body composition determined by bioimpedance-metry in Moroccan adult population. Commun. Pract. 2024, 21, 393–400. [Google Scholar]
- International Diabetes Federation (IDF). IDF Diabetes Atlas 10th Edition. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 5 August 2024).
- International Diabetes Federation (IDF). IDF Diabetes Atlas 10th Edition. 2021. Available online: https://diabetesatlas.org/data/en/country/133/ma.html (accessed on 5 August 2024).
- Dinar, Y.; Belahsen, R. Diabetes mellitus in Morocco: Situation and challenges of diabetes care. J. Sci. Res. Rep. 2014, 3, 2477–2485. [Google Scholar] [CrossRef]
- Ministère Marocain de la Santé (MMS). Célébration de la Journée Mondiale de la Santé. 2016. Available online: https://www.sante.gov.ma/Documents/2016/04/Fiche%20Diab%C3%A8te%20Fran%C3%A7ais.pdf (accessed on 5 August 2024).
- Utz, B.; Assarag, B.; Lekhal, T.; Damme, W.V.; De Brouwere, V.D. Implementation of a new program of gestational diabetes screening and management in Morocco: A qualitative exploration of health workers’ perceptions. BMC Pregnancy Childbirth 2020, 20, 315. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. Techno. 2021, 115, 147–254. [Google Scholar]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytosci. 2020, 6, 18. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Mishra, N. Traditional Indian medicines used for the management of diabetes mellitus. J. Diabetes Res. 2013, 2013, 712092. [Google Scholar] [CrossRef]
- Al-Asadi, J.N. Therapeutic uses of fenugreek (Trigonella foenum-graecum L.). Am. J. Soc. Issues Hum. 2014, 2, 21–36. [Google Scholar]
- Ocvirk, S.; Kistler, M.; Khan, S.; Talukder, S.H.; Hauner, H. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh–an ethnobotanical survey. J. Ethnobiol. EthnoMed. 2013, 9, 43. [Google Scholar] [CrossRef]
- Savo, V.; Giulia, C.; Maria, G.P.; David, R. Folk phytotherapy of the amalfi coast (Campania, Southern Italy). J. Ethnopharmacol. 2011, 135, 376–392. [Google Scholar] [CrossRef]
- Bakhtiar, Z.; Hassandokht, M.; Naghavi, M.R.; Mirjalili, M.H. Variability in proximate composition, phytochemical traits and antioxidant properties of Iranian agro-ecotypic populations of fenugreek (Trigonella foenum-graecum L.). Sci. Rep. 2024, 14, 87. [Google Scholar] [CrossRef]
- US Department of Agriculture (USDA). Spices, Fenugreek Seeds, FDC ID 171324. Food Data Central. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171324/nutrients (accessed on 5 August 2024).
- Moradi, Z.; Zadeh, J.B. Fenugreek (Trigonella foenum-graecum L.) as a valuable medicinal plant. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 922–931. [Google Scholar]
- Srinivasan, K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev. Int. 2006, 22, 203–224. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Al-ali, S.; Tranchant, C.C.; Gammoh, S.; Alrosan, M.; Kubow, S.; Tan, T.C.; Ghatasheh, S. Current perspectives on fenugreek bioactive compounds and their potential impact on human health: A review of recent insights into functional foods and other high value applications. J. Food Sci. 2024, 89, 1835–1864. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Przybylski, R.; Rudzinska, M.; Rudzinska, M.; Acharya, S. Characterization of fenugreek (Trigonella foenum-graecum) seed lipids. J. Am. Oil Chem. Soc. 2011, 88, 1603–1610. [Google Scholar] [CrossRef]
- Han, Y.; Nishibe, S.; Noguchi, Y.; Jin, Z. Flavonol glycosides from the stems of Trigonella foenum-graecum. Phytochemistry 2001, 58, 577–580. [Google Scholar] [CrossRef]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- Sahu, P.K.; Cervera-Mata, A.; Chakradhari, S.; Patel, K.S.; Towett, E.K.; Quesada-Granados, J.J.; Martin-Ramos, P.; Rufian-Henares, J.A. Seeds as Potential Sources of Phenolic Compounds and Minerals for the Indian Population. Molecules 2022, 27, 3184. [Google Scholar] [CrossRef]
- Salam, S.G.A.; Rashed, M.M.; Ibrahim, N.A.; Rahim, E.A.A.; Aly, T.A.; Al-Farga, A. Phytochemical screening and in-vitro biological properties of unprocessed and household processed fenugreek (Trigonella foenum-graecum Linn.) seeds and leaves. Sci. Rep. 2023, 13, 7032. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Feki, A.; Allouche, N. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Dis. 2011, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Rajhi, I.; Baccouri, B.; Rajhi, F.; Hammami, J.; Souibgui, M.; Mhadhbi, H.; Flamini, G. HS-SPME-GC–MS characterization of volatile chemicals released from microwaving and conventional processing methods of fenugreek seeds and flours. Ind. Crop Prod. 2022, 182, 114824. [Google Scholar] [CrossRef]
- Chaudhary, K.; Prasad, D.; Sandhu, B. Preliminary pharmacognostic and phytochemical studies on Nerium oleander Linn. (White cultivar). J. Pharmacogn. Phytochem. 2015, 4, 185–188. [Google Scholar]
- Balkan, I.A.; Doğan, H.; Zengin, G.; Colak, N.; Ayaz, F.A.; Gören, A.; Kırmızıbekmez, H.; Yeşilada, E. Enzyme inhibitory and antioxidant activities of Nerium oleander L. fl ower extracts and activity guided isolation of the active components. Ind. Crop Prod. 2018, 112, 24–31. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential health benefits of natural products derived from truffles: A review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Farooqui, S.; Tyagi, T. Nerium oleander: It’s application in basic and applied science: A Review. Int. J. Pharm. Pharm. Sci. 2018, 10, 1–4. [Google Scholar] [CrossRef]
- Garima, Z.; Amla, B. A review on chemistry and pharmacological activity of Nerium oleander L. J. Chem. Pharm. Res. 2010, 2, 351–358. [Google Scholar]
- Al-Snai, A.E. Pharmacological and therapeutic effects of Lippia nodiflora (Phyla nodiflora). IOSR J. Pharm. 2019, 9, 15–25. [Google Scholar]
- Atay Balkan, İ.; Gören, A.C.; Kırmızıbekmez, H.; Yeşilada, E. Evaluation of the in vitro anti-inflammatory activity of Nerium oleander L. flower extracts and activity-guided isolation of the active constituents. Rec. Nat. Prod. 2018, 12, 128–141. [Google Scholar] [CrossRef]
- Sinha, S.N.; Biswas, K. A concise review on Nerium oleander L.—An important medicinal plant. Trop. Plant Res. 2016, 3, 408–412. [Google Scholar]
- Singhal, K.G.; Gupta, G.D. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4–induced liver injury in rats. Asian Pac. J. Trop. Med. 2012, 5, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ding, B.; Zhang, H.; Kong, B.; Xiong, Y.L. Textural and sensorial quality protection in frozen dumplings through the inhibition of lipid and protein oxidation with clove and rosemary extracts. J. Sci. Food Agric. 2019, 99, 4739–4747. [Google Scholar] [CrossRef] [PubMed]
- Peru Checklist. The Catalogue of the Flowering Plants and Gymnosperms of Peru; Missouri Botanical Garden: St. Louis, MO, USA, 2014. [Google Scholar]
- Carrubba, A.; Abbate, L.; Sarno, M.; Sunseri, F.; Mauceri, A.; Lupini, A.; Mercati, F. Characterization of Sicilian rosemary (Rosmarinus officinalis L.) germplasm through a multidisciplinary approach. Planta 2020, 251, 37. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Germplasm Resources Information Network (GRIN); National Germplasm Resources Laboratory: Beltsville, MD, USA, 2014. Available online: https://www.ars-grin.gov/ (accessed on 5 August 2024).
- Edinburgh, R.B.G. Flora Europea; Royal Botanic Garden Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Science OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibanez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultraperformance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A. 2010, 1217, 2512–2520. [Google Scholar] [CrossRef]
- Hanson, J. Rosemary, the beneficial chemistry of a garden herb. Sci. Progress 2016, 99, 83–91. [Google Scholar] [CrossRef]
- Del Bano, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Marín, M.P.; Del Río, J.A.; Ortuno, A.; Ibarra, I. Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis postulation of a biosynthetic pathway. J. Agric. Food Chem. 2004, 52, 4987–4992. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef] [PubMed]
- Kompelly, A.; Kompelly, S.; Vasudha, B.; Narender, B. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. J. Drug Deliv. Ther. 2019, 9, 323–330. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oil—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Máthé, I.; Hohmann, J.; Janicsák, G.; Nagy, G.; Rédei, D. Chemical diversity of the biological active ingredients of Salvia offi cinalis and some closely related species. Acta Pharm. Hung. 2007, 77, 37–43. [Google Scholar]
- Bernotiené, G.; Nivinskiené, O.; Butkiené, R.; Mockuté, D. Essential oil composition variability in sage (Salvia officinalis L.). Chemija 2007, 18, 38–43. [Google Scholar]
- Hadri, A.; Gomez del Rio, M.; Sanz, J.; Coloma, A.; Idaomar, M.; Ozanas, B. Cytotoxic activity of α-humulene and transcario-phyllene from Salvia offi cinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Jug-Dujaković, M.; Ristić, M.; Pljevljakušić, D.; Dajić-Stevanović, Z.; Liber, Z.; Hančević, K.; Radić, T.; Šatović, Z. High diversity in indigenous populations of Dalmatian sage (Salvia officinalis L.) in essential oil composition. Chem. Biodivers. 2012, 9, 2309–2323. [Google Scholar] [CrossRef]
- Stešević, D.; Ristić, M.; Nikolić, V.; Nedović, M.; Caković, D.; Satovic, Z. Chemotype Diversity of Indigenous Dalmatian Sage (Salvia offi cinalis L.) Populations in Montenegro. Chem. Biodivers. 2014, 11, 101–114. [Google Scholar] [CrossRef]
- Politi, M.; Ferrante, C.; Menghini, L.; Angelini, P.; Flores, G.A.; Muscatello, B.; Braca, A.; De Leo, M. Hydrosols from Rosmarinus officinalis, Salvia officinalis, and Cupressus sempervirens: Phytochemical Analysis and Bioactivity Evaluation. Plants 2022, 11, 349. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.N.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Añibarro-Ortega, M.; Finimundy, T.; Kostić, M.; Soković, M.; Teixeira, J.A.; et al. Phytochemical Composition and Bioactive Potential of Melissa officinalis L.; Salvia officinalis L. and Mentha spicata L. Extracts. Foods 2023, 12, 947. [Google Scholar] [CrossRef]
- Spréa, R.M.; Caleja, C.; Pinela, J.; Finimundy, T.C.; Calhelha, R.C.; Kostić, M.; Sokovic, M.; Prieto, M.A.; Pereira, E.; Amaral, J.S.; et al. Comparative study on the phenolic composition and in vitro bioactivity of medicinal and aromatic plants from the Lamiaceae family. Food Res. Int. 2022, 161, 111875. [Google Scholar] [CrossRef] [PubMed]
- Maliki, I.; Es-safi, I.; El Moussaoui, A.; Mechchate, H.; El Majdoub, Y.O.; Bouymajane, A.; Cacciola, F.; Mondello, L.; Elbadaouia, K. Salvia officinalis and Lippia triphylla: Chemical characterization and evaluation of antidepressant-like activity. J. Pharm. Biomed. Anal. 2021, 203, 114207. [Google Scholar] [CrossRef]
- Bracci, T.; Busconi, M.; Fogher, C.; Sebastiani, L. Molecular studies in olive (Olea europaea L.): Overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep. 2011, 30, 449–462. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Kaskoos, R.A. Pharmacognostic specifications of leaves of Olea europaea collected from Iraq. Am. J. Phytomed. ClinTher. 2013, 2, 153–160. [Google Scholar]
- Ayoub, L.; Hassan, F.; Hamid, S.; Abdelhamid, Z.; Souad, A. Phytochemical screening, antioxidant activity and inhibitorypotential of Ficus carica and Olea europaea leaves. Bioinformation 2019, 15, 226–232. [Google Scholar] [CrossRef]
- Kiritsakis, A.K. Olive Oil from the Tree to the Table, 2nd ed.; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 1998. [Google Scholar]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuno, A. Del Rio JA. Antioxidant activity of phenolics extracted from Olea europea L. leaves. Food. Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Haloui, E.; Marzouk, Z.; Marzouk, B.; Bouftira, I.; Bouraoui, A.; Fenina, N. Pharmacological Activities and Chemical Composition of the Olea europaea L. Leaf Essential Oils from Tunisia. J. Food Agric. Environ. 2010, 8, 204–208. Available online: https://www.wflpublisher.com/Abstract/1605 (accessed on 15 August 2024).
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, composition and antioxidant activity of Algerian wild olive (Olea europaea L. subsp. Oleaster) oil. Ind. Crops Prod. 2016, 83, 484–491. [Google Scholar] [CrossRef]
- Šarolić, M.; Gugić, M.; Tuberoso, C.I.G.; Jerković, I.; Šuste, M.; Marijanović, Z.; Kuś, P.M. Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules 2014, 19, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Luo, H. Extraction of Antioxidant Compounds from Olive (Olea europaea) Leaf. Thesis Massey Univ. 2011, pp. 1–17. Available online: https://mro.massey.ac.nz/handle/10179/3481 (accessed on 15 August 2024).
- Khlif, I.; Jellali, K.; Michel, T.; Halabalaki, M.; Skaltsounis, A.L.; Allouche, N. Characteristics, Phytochemical Analysis and Biological Activities of Extracts from Tunisian Chetoui Olea europaea Variety. J. Chem. 2015, 2015, e418731. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Nazlić, M.; Miletić, T.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Dunkić, V. Antioxidant Capacity of Free Volatile Compounds from Olea europaea L. cv. Oblica Leaves Depending on the Vegetation Stage. Antioxidants 2021, 10, 1832. [Google Scholar]
- Alagna, F.; Geu-Flores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and Characterization of the Iridoid Synthase Involved in Oleuropein Biosynthesis in Olive (Olea europaea) Fruits. J. Biol. Chem. 2016, 291, 5542–5554. [Google Scholar] [CrossRef]
- Maalej, A.; Bouallagui, Z.; Hadrich, F.; Isoda, H.; Sayadi, S. Assessment of Olea europaea L. fruit extracts: Phytochemical characterization and anticancer pathway investigation. Biomed. Pharmacother. 2017, 90, 179–186. [Google Scholar]
- Gariboldi, P.; Jommi, G.; Verotta, L. Secoiridoids from Olea europaea. Phytochemistry 1986, 25, 865–869. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef]
- Kamran, M. Olive (Olea europaea L.) Leaf Biophenols as Nutraceuticals. Doctor of Philosophy. Charles Sturt University. 2016, Volume 292. Available online: https://researchoutput.csu.edu.au/ws/portalfiles/portal/92488706/Kamran_Muhammad_thesis.pdf. (accessed on 15 August 2024).
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-Glucosidase and α-Amylase Enzyme Inhibitory of Hydroxytyrosol and Oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Ghosia, L.; Khan, A.; Tila, H.; Hussain, A.; Khan, A. Evaluation of Plants Extracts for Proximate Chemical Composition, Antimicrobial and Antifungal Activities. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 964–970. [Google Scholar]
- Celik, H.; Nadaroglu, H.; Senol, M. Evaluation of antioxidant, antiradicalic and antimicrobial activities of olive pits (Olea europaea L.). Bulg. J. Agric. Sci. 2014, 20, 1392–1400. [Google Scholar]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells through Modulation of Matrix Metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef]
- Shabana, A.; El-Menyar, A.; Asim, M.; Al-Azzeh, H.; Al Thani, H. Cardiovascular benefits of black cumin (Nigella Sativa). Cardiovasc. Toxicol. 2013, 13, 9–21. [Google Scholar] [CrossRef]
- Kehili, N.; Saka, S.; Aouacheri, O. L’effet phytoprotecteur de la nigelle (Nigella Sativa) contre la toxicité induite par le cadmium chez les rats. Phytothérapie 2018, 16, 194–203. [Google Scholar] [CrossRef]
- Sahak, M.K.A.; Kabir, N.; Abbas, G.; Draman, S.; Hashim, N.H.; Hasan Adli, D.S. The role of Nigella Sativa and its active constituents in learning and memory. Evid. Based Complement. Altern. Med. 2016, 1, 6075679. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.; Benouda, H.; Azghar, H.A.; Tahri, M.; Boufalja, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella Sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evid. Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef]
- Kabir, Y.; Akasaka-Hashimoto, Y.; Kubota, K.; Komai, M. Volatile compounds of black cumin (Nigella Sativa L.) seeds cultivated in Bangladesh and India. Heliyon 2020, 6, e05343. [Google Scholar] [CrossRef]
- Dalli, M.; Daoudi, N.E.; Azizi, S.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical composition analysis using HPLC-UV/GC-MS and inhibitory activity of different Nigella Sativa fractions on pancreatic α-amylase and intestinal glucose absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Farooq, M.A.; Kyunn, W.W. A new oleanane type saponin from the aerial parts of Nigella Sativa with anti-oxidant and anti-diabetic potential. Molecules 2020, 25, 2171. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman, S.M. Isolation and structure determination of nigellicine, a novel alkaloid from the seeds of Nigella Sativa. Tetrahedron Lett. 1985, 26, 2759–2762. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, S.M.; Zaman, K. Nigellimine: A new isoquinoline alkaloid from the seeds of Nigella Sativa. J. Nat. Prod. 1992, 55, 676–678. [Google Scholar] [CrossRef]
- Atta-ur-Rahman, S.M.; Hasan, S.S.; Choudhary, M.I.; Ni, C.Z.; Clardy, J. Nigellidine—A new indazole alkaloid from the seeds of Nigella Sativa. Tetrahedron Lett. 1995, 36, 1993–1996. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Al-Jassir, M. Chemical composition and microflora of black cumin (Nigella Sativa L.) seeds growing in Saudi Arabia. Food Chem. 1992, 45, 239–242. [Google Scholar] [CrossRef]
- Usmani, A.; Almoselhy, R.I. Current trends in Nigella Sativa L. (Black seed) from traditional to modern medicine with advances in extraction, formulation, quality control, regulatory status, and pharmacology. Int. J. Pharm. Chem. 2024, 11, 11–23. [Google Scholar] [CrossRef]
- Cheikh-Rouhoua, S.; Besbes, S.; Lognay, G.; Blecker, C.; Deroanne, C.; Attia, H. Sterol composition of black cumin (Nigella Sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J. Food Compos. Anal. 2008, 21, 162–168. [Google Scholar] [CrossRef]
- Mehta, B.K.; Verma, M.; Gupta, M. Novel lipid constituents identified in seeds of Nigella Sativa (Linn). J. Braz. Chem. Soc. 2008, 19, 458–462. [Google Scholar] [CrossRef]
- Bourgou, S.; Ksouri, R.; Bellila, A.; Skandrani, I.; Falleh, H.; Marzouk, B. Phenolic composition and biological activities of Tunisian Nigella Sativa L. shoots and roots. Comptes Rendus Biol. 2008, 331, 48–55. [Google Scholar] [CrossRef]
- Nickavar, B.; Mojab, F.; Javidnia, K.; Amoli, M.A. Chemical composition of the fixed and volatile oils of Nigella Sativa L. from Iran. Z. Natur. forsch. C. J. Biosci. 2003, 58, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Xu, F.; Ninomiya, K.; Matsuda, H.; Yoshikawa, M. Nigellamines A3, A4, A5, and C, new dolabellane-type diterpene alkaloids, with lipid metabolism-promoting activities from the Egyptian medicinal food black cumin. Chem. Pharm. Bull. 2004, 52, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella Sativa L. and Thymus vulgaris L.; and its anti-proliferative effect on HeLa cancer cell lines, Trop. J. Pharm. Res. 2019, 18, 37–42. [Google Scholar] [CrossRef]
- MatthauS, B.; ÖzCaN, M.M. Fatty acids, tocopherol, and sterol contents of some Nigella species seed oil. Czech J. Food Sci. 2011, 29, 145–150. [Google Scholar] [CrossRef]
- Hussain, S.; Rukhsar, A.; Iqbal, M.; ul Ain, Q.; Fiaz, J.; Akhtar, N.; Afzal, M.; Ahmad, N.; Ahmad, I.; Mnif, W.; et al. Phytochemical Profile, Nutritional and Medicinal Value of Nigella Sativa. Biocatal. Agric. Biotechnol. 2024, 60, 103324. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Prima, S.R.; Julianti, E.; Fidrianny, I. Update review: Etnopharmacological, bioactivity and phytochemical of Allium cepa L. Pharmacia 2023, 70, 717–724. [Google Scholar]
- Han, M.H.; Lee, W.S.; Jung, J.H.; Jeong, J.H.; Park, C.; Kim, H.J.; Kim, G.S.; Jung, J.M.; Kwon, T.K.; Kim, G.Y.; et al. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem. Toxicol. 2013, 62, 382–389. [Google Scholar] [CrossRef]
- Sato, A.; Zhang, T.; Yonekura, L.; Tamura, H. Antiallergic activities of eleven onions (Allium cepa) were attributed to quercetin 4′-glucoside using QuEChERS method and Pearson’s correlation coefficient. J. Funct. Foods 2015, 14, 581–589. [Google Scholar] [CrossRef]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion peel: Turning a food waste into a resource. Antioxidants 2021, 10, 304–321. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.K.; Kim, C.S.; Ha, M.T.; Ngo, Q.M.T.; Park, S.E.; Kwon, H.; Lee, D.; Choi, J.S.; Kim, J.A.; Min, B.S. Antioxidant and antidiabetic activities of flavonoid derivatives from the outer skins of Allium cepa L. J. Agric. Food Chem. 2020, 68, 8797–8811. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Prashanth, K.H.; Venkatesh, Y.P. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa). Carbohydr. Polym. 2015, 117, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gîtin, L.; Dinică, R.; Neagu, C.; Dumitrascu, L. Sulfur compounds identification and quantification from Allium spp. fresh leaves. J. Food Drug Anal. 2014, 22, 425–430. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Mahmoud, H.Y.; El-Sayed, M.; Tanaka, S.; Tran, L.S. Isolation and characterization of Cepa2, a natural alliospiroside A, from shallot (Allium cepa L. Aggregatum group) with anticancer activity. Plant Physiol. Biochem. 2017, 116, 167–173. [Google Scholar] [CrossRef]
- Ifesan, B. Chemical composition of onion peel (Allium cepa) and its ability to serve as a preservative in cooked beef. Int. J. Soc. Res. Methodol. 2017, 7, 25–34. [Google Scholar]
- Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturică, M.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-added crackers enriched with red onion skin anthocyanins entrapped in different combinations of wall materials. Antioxidants 2022, 11, 1048. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Kuete, V. Allium cepa. In Medicinal Spices and Vegetables from Africa; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 353–361. [Google Scholar]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N.; Levin, C.E.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020, 44, e14497. [Google Scholar] [CrossRef]
- Ramos, A.F.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Gabric, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Kovacevic, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Réggami, Y.; Benkhaled, A.; Boudjelal, A.; Berredjem, H.; Amamra, A.; Benyettou, H.; Larabi, N.; Senator, A.; Siracusa, L.; Ruberto, G. Artemisia herba-alba aqueous extract improves insulin sensitivity and hepatic steatosis in rodent model of fructose-induced metabolic syndrome. Arch. Physiol. Biochem. 2021, 127, 541–550. [Google Scholar] [CrossRef]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential oil and phenolic compounds of Artemisia herba alba (Asso.): Composition, Antioxidant, Antiacetylcholinesterase, and Antibacterial Activities. Int. J. Food Prop. 2016, 19, 1425–1438. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba-alba). Plants 2021, 10, 164. [Google Scholar] [CrossRef]
- Ouguirti, N.; Bahri, F.; Bouyahyaoui, A.; Wanner, J. Chemical characterization and bioactivities assessment of Artemisia herba-alba Asso essential oil from South-western Algeria. Nat. Volatiles Essent. Oils 2021, 8, 27–36. [Google Scholar] [CrossRef]
- Mrabti, H.N.; El Hachlafi, N.; Al-Mijalli, S.H.; Jeddi, M.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Phytochemical profile, assessment of antimicrobial and antioxidant properties of essential oils of Artemisia herba-alba Asso.; and Artemisia dracunculus L.: Experimental and computational approaches. J. Mol. Struct. 2023, 1294, 136479. [Google Scholar] [CrossRef]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical composition and antimicrobial activity of Artemisia herba-alba and Origanum majorana essential oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef] [PubMed]
- El Ouahdani, K.; Es-Safi, I.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bari, A.; Bousta, D. Thymus algeriensis and Artemisia herba-alba essential oils: Chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef] [PubMed]
- Benabdallah, A.; Betina, S.; Bouchentouf, S.; Boumendje, M.; Bechkr, S.; Bensouic, C.; Nicoli, F.; Vergine, M.; Negro, C. Chemical profiling, antioxidant, enzyme inhibitory and in silico modeling of Rosmarinus officinalis L. and Artemisia herba alba Asso. essential oils from Algeria. S. Afr. J. Bot. 2022, 147, 501–510. [Google Scholar]
- Almi, D.; Sebbane, H.; Lahcene, S.; Habera, F.; Laoudi, K.; Mati, A. Antibacterial and antioxidant activities of various extracts and essential oil from dried leaves of Artemisia herba-alba Asso of Tamanrasset (South Algeria). Int. J. Minor Fruits Med. Aromat. Plants 2022, 8, 47–55. [Google Scholar] [CrossRef]
- Bourgou, S.; Tammar, S.; Salem, N.; Mkadmini, K.; Msaada, K. Phenolic composition, essential oil, and antioxidant activity in the aerial part of Artemisia herba alba from several provenances: A comparative study. Int. J. F. Prop. 2016, 19, 549–563. [Google Scholar] [CrossRef]
- Benmeziane, B.; Haddadin, M.; AL-Domi, H. Extraction yield, phytochemicals analysis, and certain in vitro biological activities of Artemisia herba alba extracts. Jordan J. Agric. Sci. 2023, 19, 125–141. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in Barley (Hordeum vulgare L.). J. Agri. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, A.; Rashid, A.; Shahid, M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J. Food Drug Anal. 2015, 23, 109–115. [Google Scholar]
- Ashraf, A.; Sarfraz, A.R.; Mahmood, A. Phenolic compounds characterization Artemisia rutifolia spreng from Pakistani flora and their relationships with antioxidant and antimicrobial attributes. Int. J. F. Prop. 2017, 20, 2538–2549. [Google Scholar] [CrossRef]
- Morales-González, J.A.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Carmen Valadez-Vega, M.D.; ÁlvarezGonzález, I.; Morales-González, A.; Madrigal-Santillán, E. Garlic (Allium sativum L.): A brief review of its antigenotoxic effects. Foods 2019, 8, E343. [Google Scholar] [CrossRef]
- Varga-Visi, E.; Jócsák, I.; Ferenc, B.; Végvári, G. Effect of crushing and heating on the formation of volatile organosulfur compounds in garlic. CYTA J. Food 2019, 17, 796–803. [Google Scholar] [CrossRef]
- Al-Snafi, A. Pharmacological effects of Allium species grown in Iraq. An overview. Int. J. Pharm. Health Care Res. 2013, 1, 132–147. [Google Scholar]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic role of functional components in Alliums for preventive chronic disease in human being. Evid. Based Complement. Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.B.; Dam, S.M.; Le, N.T.T. Amelioration of single clove black garlic aqueous extract on dyslipidemia and hepatitis in chronic carbon tetrachloride intoxicated swiss albino mice. Int. J. Hepatol. 2018, 2018, 9383950. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Han, X.; Hu, W. Garlic-derived compound S-allylmercaptocysteine (SAMC) is active against anaplastic thyroid cancer cell line 8305C (HPACC). Technol. Health Care 2015, 23, S89–S93. [Google Scholar] [CrossRef] [PubMed]
- Țigu, A.B.; Moldovan, C.S.; Toma, V.-A.; Farcaș, A.D.; Moț, A.C.; Jurj, A.; Fischer-Fodor, E.; Mircea, C.; Pârvu, M. Phytochemical analysis and in vitro effects of Allium fistulosum L. and Allium sativum L. extracts on human normal and tumor cell lines: A comparative study. Molecules 2021, 26, 574. [Google Scholar] [CrossRef] [PubMed]
- Akbarpour, A.; Kavoosi, B.; Hosseinifarahi, M.; Tahmasebi, S.; Gholipour, S. Evaluation of yield and phytochemical content of different Iranian garlic (Allium sativum L.) ecotypes. Int. J. Hortic. Sci. Technol. 2021, 8, 385–400. [Google Scholar]
- Efiong, E.E.; Akumba, L.P.; Chukwu, E.C.; Olusesan, A.I.; Obochi, G. Comparative qualitative phytochemical analysis of oil, juice and dry forms of garlic (Allium sativum) and different varieties of onions (Allium cepa) consumed in Makurdi metropolis. Int. J. Plant Physiol. Biochem. 2020, 12, 9–16. [Google Scholar]
- Knoss, W. Marrubium vulgare (White Horehound): In vitro culture, and the production of diterpene marrubiin and other secondary metabolites. In Medicinal and Aromatic Plants XI. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 43, pp. 274–289. [Google Scholar]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A Phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.M.; Quirantes-Piné, R.; Madani, K.; Segura-Carretero, A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Prod. 2014, 61, 120–129. [Google Scholar] [CrossRef]
- Ahmed, B.; Masoodi, M.H.; Siddique, A.H.; Khan, S. A new monoterpene acid from Marrubium vulgare with potential antihepatotoxic activity. Nat. Prod. Res. 2010, 24, 1671–1680. [Google Scholar] [CrossRef]
- Verma, A.; Masoodi, M.; Ahmed, B. Lead findings from whole plant of Marrubium vulgare L. with hepatoprotective potentials through in silico methods. Asian Pac. J. Trop. Biomed. 2012, 2, S1308–S1311. [Google Scholar] [CrossRef]
- Neamah, S.I.; Sarhan, I.A.; Al-Shayea, O.N. Extraction and evaluation of the anti-inflammatory activity of six compounds of Marrubium vulgare L. Biosci. Res. 2018, 15, 2393–2400. [Google Scholar]
- Knoss, W.; Zapp, J. Accumulation of furanic labdane diterpenes in Marrubium vulgare and Leonorus cardiaca. Planta Med. 1998, 64, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, F.; Rasool, S.; Shah, Z.A.; Soomro, S.; Jabeen, A.; Mesaik, M.A.; Choudhary, M.I. Chemical constituents of Marrubium vulgare as potential inhibitors of nitric oxide and respiratory burst. Nat. Prod. Commun. 2014, 9, 903–906. [Google Scholar] [CrossRef]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. The diterpenoids of the genus Marrubium (Lamiaceae). Nat. Prod. Commun. 2006, 1, 585–592. [Google Scholar] [CrossRef]
- Ghedadba, N.; Hambaba, L.; Fercha, N.; Houas, B.; Abdessemed, S.; Mokhtar, S.M.O. Assessment of hemostatic activity of the aqueous extract of leaves of Marrubium vulgare L.; a Mediterranean Lamiaceae Algeria. Int. J. Health Sci. 2016, 2, 253–258. [Google Scholar]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef]
- Paunovic, V.; Kostic, M.; Djordjevic, S.; Zugic, A.; Djalinac, N.; Gasic, U.; Trajkovic, V.; Harhaji-Trajkovic, J. Marrubium vulgare ethanolic extract induces proliferation block, apoptosis and cytoprotective autophagy in cancer cells in vitro. Cell. Mol. Biol. 2016, 62, 108–114. [Google Scholar] [PubMed]
- Dewick, M.P. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-74168-9. [Google Scholar]
- Boulila, A.; Sanaa, A.; Salem, I.B.; Rokbeni, N.; M’rabet, Y.; Hosni, K.; Fernandez, X. Antioxidant properties and phenolic variation in wild populations of Marrubium vulgare L. (Lamiaceae). Ind. Crops Prod. 2015, 76, 616–622. [Google Scholar] [CrossRef]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.S.; Dhanlta, P.; Addison, J.B.; Tong, S.; Fiehn, O.; Zerbe, P. Functional characterization of the cytochrome P450 monooxygenase CYP71AU87 indicates a role in marrubiin byosynthesis in the medicinal plant Marrubium vulgare. BMC Plant Biol. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Demiroz Akbulut, T.; Aydin Kose, F.; Demirci, B.; Baykan, S. Chemical profile and cytotoxicity evaluation of aerial parts of Marrubium vulgare L. from different locations in Turkey. Chem. Biodivers. 2023, 20, e202201188. [Google Scholar] [CrossRef]
- Guedri Mkaddem, M.; Zrig, A.; Ben Abdallah, M.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Hegab, M.Y.; Madany, M.M.Y.; Hassan, A.H.A. Variation of the chemical composition of essential oils and total phenols content in natural populations of Marrubium vulgare L. Plants 2022, 11, 612. [Google Scholar] [CrossRef]
- Rezgui, M.; Majdoub, N.; Mabrouk, B.; Baldisserotto, A.; Bino, A.; Kaab, L.B.B. Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J. Mycol. Med. 2020, 30, 100927. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.; Hassani, A. Chemical composition of Marrubium vulgare L. essential oil from Algeria. Int. Lett. Chem. Phys. Astron. 2013, 8, 210–214. [Google Scholar] [CrossRef]
- Zawiślak, G. The chemical composition of the essential oil of Marrubium vulgare L. from Poland. Farmacia 2012, 60, 287–292. [Google Scholar]
- Zarai, Z.; Kadri, A.; Ben Chobba, I.; Ben Mansour, R.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 21, 161. [Google Scholar] [CrossRef]
- Michalak, M.; Stryjecka, M.; Zagórska-Dziok, M.; Żarnowiec, P. Biological Activity of Horehound (Marrubium vulgare L.) Herb Grown in Poland and Its Phytochemical Composition. Pharmaceuticals 2024, 17, 780. [Google Scholar] [CrossRef]
- Tlili, H.; Hanen, N.; Ben Arfa, A.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Doria, E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef]
- Benhaddou-Andaloussi, A.; Martineau, L.; Vuong, T.; Meddah, B.; Madiraju, P.; Settaf, A.; Haddad, P.S. The in vivo antidiabetic activity of Nigella Sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evid. Based Complement. Altern. Med. 2011, 1, 538671. [Google Scholar] [CrossRef]
- Liu, I.M.; Tzeng, T.F.; Liou, S.S.; Lan, T.W. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007, 81, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Malviya, N.; Jain, S.; Malviya, S.A.P.N.A. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010, 67, 113–118. [Google Scholar] [PubMed]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Nakhaee, A.; Sanjari, M. Evaluation of effect of acarbose consumption on weight losing in non-diabetic overweight or obese patients in Kerman. J. Res. Med. Sci. 2013, 18, 391. [Google Scholar] [PubMed]
- Kast, R. Acarbose related diarrhea: Increased butyrate upregulates prostaglandin E. Inflamm. Res. 2002, 51, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Inbaraj, S.D.; Muniappan, M. Correlation between the in-vitro and in-vivo antihyperglycemic effect of Ocimum Sanctum, Trigonella Foenum graecum and Curcuma longa. Pharmacogn. J. 2020, 12, 369–376. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Apreutesei, O.T.; Radu, G.L. In vitro Assessment of the Antidiabetic and Anti-Inflammatory Potential of Artemisia absinthium, Artemisia vulgaris and Trigonella foenum-graecum Extracts Processed Using Membrane Technologies. Molecules 2023, 28, 7156. [Google Scholar] [CrossRef]
- Laila, O.; Murtaza, I.; Muzamil, S.; Ali, S.I.; Ali, S.A.; Paray, B.A.; Gulnaz, A.; Vladulescu, C.; Mansoor, S. Enhancement of nutraceutical and anti-diabetic potential of fenugreek (Trigonella foenum-graecum). Sprouts with natural elicitors. Saudi Pharm. J. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Mowla, A.; Alauddin, M.; Rahman, M.A.; Ahmed, K. Antihyperglycemic effect of Trigonella foenum-graecum (fenugreek) seed extract in alloxan-induced diabetic rats and its use in diabetes mellitus: A brief qualitative phytochemical and acute toxicity test on the extract. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.L.; Li, X.S.; Zhang, J.; Liu, Y.H.; Wang, Z.L.; Zhang, R.J. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac. J. Clin. Nutr. 2007, 16, 422–426. [Google Scholar]
- Abdelatif, A.M.; Ibrahim, M.Y.; Mahmoud, A.S. Antidiabetic effects of fenugreek (Trigonella foenum-graecum) seeds in the domestic rabbit (Oryctolagus cuniculus). Res. J. Med. Plant 2012, 6, 449–455. [Google Scholar] [CrossRef]
- Kumar, G.S.; Shetty, A.K.; Sambaiah, K.; Salimath, P.V. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr. Res. 2005, 25, 1021–1028. [Google Scholar] [CrossRef]
- Baset, M.E.; Ali, T.I.; Elshamy, H.; El Sadek, A.M.; Sami, D.G.; Badawy, M.T.; Abou-Zekry, S.S.; Heiba, H.H.; Saadeldin, M.K.; Abdellatif, A. Anti-diabetic effects of fenugreek (Trigonella foenum-graecum): A comparison between oral and intraperitoneal administration-an animal study. Int. J funct. Nutr. 2020, 1, 2–9. [Google Scholar] [CrossRef]
- Haeri, M.R.; Limaki, H.K.; White, C.J.B.; White, K.N. Non-insulin dependent anti-diabetic activity of (2S, 3R, 4S) 4-hydroxyisoleucine of fenugreek (Trigonella foenum graecum) in streptozotocin-induced type I diabetic rats. Phytomedicine 2012, 19, 571–574. [Google Scholar] [CrossRef]
- Priya, V.; Jananie, R.K.; Vijayalakshmi, K. Antidiabetic effect of Trigonella foenum graecum in diabetic rats-an in vivo study. Pharm. Sci. Monitor. 2012, 3, 204. [Google Scholar]
- Kumar, P.; Kale, R.K.; McLean, P.; Baquer, N.Z. Antidiabetic and neuroprotective effects of Trigonella foenum-graecum seed powder in diabetic rat brain. Prague Med. Rep. 2012, 113, 33–43. [Google Scholar] [CrossRef]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complem. Altern. Med. 2011, 11, 5. [Google Scholar]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. 2007, 53, 166–173. [Google Scholar] [CrossRef]
- Dey, P.; Saha, M.R.; Chowdhuri, S.R.; Sen, A.; Sarkar, M.P.; Haldar, B.; Chaudhuri, T.K. Assessment of anti-diabetic activity of an ethnopharmacological plant Nerium oleander through alloxan induced diabetes in mice. J. Ethnopharmcol. 2015, 161, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Magdalene, M.; Kavitha, S.; Priya, V.V.; Gayathri, R. Evaluation of antidiabetic effect of Nerium oleander flowers-An in vitro study. Drug Invent. Today 2019, 12, 1313. [Google Scholar]
- Mwafy, S.N.; Yassin, M.M. Antidiabetic activity evaluation of glimepiride and Nerium oleander extract on insulin, glucose levels and some liver enzymes activities in experimental diabetic rat model. Pak. J. Biol. Sci. 2011, 14, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Battal, A.; Dogan, A.; Uyar, A.; Demir, A.; Keleş, Ö.F.; Celik, I.; Baloglu, M.C.; Aslan, A. Exploring of the ameliorative effects of Nerium (Nerium oleander L.) ethanolic flower extract in streptozotocin induced diabetic rats via biochemical, histological and molecular aspects. Mol. Biol. Rep. 2023, 50, 4193–4205. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.L.; Demirci, S.; Yazihan, N.; Uney, K.; Ermis Kaya, E. Nerium oleander distillate improves fat and glucose metabolism in high-fat diet-fed streptozotocin-induced diabetic rats. Int. J. Endocrinol. 2012, 2012, 947187. [Google Scholar] [CrossRef]
- Sikarwar, M.S.; Patil, M.B.; Kokate, C.K.; Sharma, S.; Bhat, V. Antidiabetic activity of Nerium indicum leaf extract in alloxan-induced diabetic rats. J. Young Pharma. 2009, 1, 330–335. [Google Scholar] [CrossRef]
- Sharma, A.D.; Kaur, I.; Kaur, J.; Chauhan, A. Chemical profiling and in-vitro anti-oxidant, anti-diabetic, anti-inflammatory, anti-bacterial and anti-fungal activities of essential oil from Rosmarinus officinalis L. Not. Sci. Biol. 2024, 16, 11756. [Google Scholar] [CrossRef]
- Gholam, H.A.; Falah, H.; Sharififar, F.; Mirtaj, A.S. The inhibitory effect of some Iranian plants extracts on the α glucosidase. Iran. J. Basic Med. Sci. 2008, 11, 1–9. [Google Scholar]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar]
- Belmouhoub, M.; Bribi, N.; Iguer-ouada, M. A-glucosidase inhibition and antihyperglycemic activity of flavonoids rich fractions of Rosmarinus officinalis in normal and streptozotocin diabetic mice. Orient. Pharm. Exp. Med. 2017, 17, 29–39. [Google Scholar] [CrossRef]
- Koga, K.; Shibata, H.; Yoshino, K.; Nomoto, K. Effects of 50% ethanol extract from rosemary (Rosmarinus officinalis) on α-glucosidase inhibitory activity and the elevation of plasma glucose level in rats, and its active compound. J. Food Sci. 2006, 71, S507–S512. [Google Scholar] [CrossRef]
- Kabubii, Z.N.; Mbaria, J.M.; Mathiu, P.M.; Wanjohi, J.M.; Nyaboga, E.N. Diet supplementation with rosemary (Rosmarinus officinalis L.) leaf powder exhibits an antidiabetic property in streptozotocin-induced diabetic male wistar rats. Diabetology 2024, 5, 12–25. [Google Scholar] [CrossRef]
- Benkhedir, A.; Boussekine, S.; Saker, H.; Gasmi, S.; Benali, Y. Beneficial effects of Rosmarinus officinalis and Thymus numidicus on key enzymes of carbohydrate metabolism in alloxan-induced diabetic rats. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e9507. [Google Scholar] [CrossRef]
- Khalil, O.A.; Ramadan, K.S.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. Afri. J. Pharm. Pharmacol. 2012, 6, 1031–1036. [Google Scholar]
- Al-Jamal, A.R.; Alqadi, T. Effects of rosemary (Rosmarinus officinalis) on lipid profile of diabetic rats. Jordan J. Biol. Sci. 2011, 4, 199–204. [Google Scholar]
- Alnahdi, H.S. Effect of Rosmarinus officinalis extract on some cardiac enzymes of streptozotocin-induced diabetic rats. J. Health Sci. 2012, 2, 33–37. [Google Scholar] [CrossRef]
- Emam, M.A. Comparative evaluation of antidiabetic activity of Rosmarinus officinalis L. and Chamomile recutita in streptozotocin induced diabetic rats. Agric. Biol. J. Am. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Soliman, G.Z. Effect of Rosmarinus officinalis on lipid profile of streptozotocin-induced diabetic rats. Egypt. J. Hosp. Med. 2013, 53, 809–815. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef]
- Nazem, F.; Farhangi, N.; Neshat-Gharamaleki, M. Beneficial effects of endurance exercise with Rosmarinus officinalis labiatae leaves extract on blood antioxidant enzyme activities and lipid peroxidation in streptozotocin-induced diabetic rats. Can. J. Diabetes 2015, 39, 229–234. [Google Scholar] [CrossRef]
- Header, E.; ElSawy, N.; El-Boshy, M.; Basalamah, M.; Mubarak, M.; Hadda, T.B. POM analyses of constituents of Rosmarinus officinalis and their synergistic effect in experimental diabetic rats. J. Bioanal. Biomed. 2015, 7, 18–23. [Google Scholar]
- Runtuwene, J.; Cheng, K.C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.R.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Devel. Ther. 2016, 10, 2193–2202. [Google Scholar] [PubMed]
- Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Almeida, M.J.; Wilson, J.M.; Pereira-Wilson, C. Rosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucose. Mol. Nutr. Food Res. 2011, 55, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Bakırel, T.; Bakırel, U.; Keleş, O.Ü.; Ülgen, S.G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kensara, O.; ElSawy, N.; Altaf, F.; Header, E. Hypoglycemic and hepato-protective effects of Rosmarinus officinalis in experimental diabetic Rats. UQU Med. J. 2010, 1, 98–113. [Google Scholar]
- Tavafi, M.; Ahmadvand, H.; Tamjidipoor, A. Rosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. Iran. J. Basic Med. Sci. 2011, 14, 275–283. [Google Scholar]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, antidiabetic, and antibacterial potentials and chemical composition of Salvia officinalis and Mentha suaveolens grown wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 2844880. [Google Scholar] [CrossRef]
- Chehade, S.; Kobeissy, M.; Kanaan, H.; Haddad, M. Comparison between the chemical compositions and the in-vitro antidiabetic and anti-inflammatory activities of Salvia Libanotica’ and Salvia officinalis’ leaves essential oils. Eur. J. Pharm. Med. Res. 2022, 93, 34–43. [Google Scholar]
- Hamza, A.A.; Ksiksi, T.S.; Shamsi, O.A.A.; Balfaqh, S.A. α-glucosidase inhibitory activity of common traditional medicinal plants used for diabetes mellitus. J. Dev. Drugs 2015, 4, 1000144. [Google Scholar] [CrossRef]
- Kwon, Y.I.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107. [Google Scholar]
- Mahdi, S.; Azzi, R.; Lahfa, F.B. Evaluation of in vitro α-amylase and α-glucosidase inhibitory potential and hemolytic effect of phenolic enriched fractions of the aerial part of Salvia officinalis L. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Moradabadi, L.; Kouhsari, S.M.; Sani, M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic Insulin and cardiac Glut-4 mRNAs expression. Iran. J. Pharm. Res. 2013, 12, 387. [Google Scholar] [PubMed]
- Bouteldja, R.; Doucene, R.; Aggad, H.; Abdi, F.Z.; Belkhodja, H.; Belal, A.; Abdali, M.; Zidane, K. Phytochemical screening, acute toxicity and antidiabetic activity of ethanolic extract of Salvia officinalis L. in Wistar Rat. Agric. Conspec. Sci. 2023, 88, 351–357. [Google Scholar]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Khashan, K.T.; Al-Khefaji, K.A. Effects of Salvia officinalis L. (sage) leaves extracts in normal and alloxan-induced diabetes in white rats. Int. J. Sci. Eng. Res. 2015, 6, 20–28. [Google Scholar]
- Alarcon-Aguilar, F.J.; Roman-Ramos, R.; Flores-Saenz, J.L.; Aguirre-Garcia, F. Investigation on the hypoglycaemic effects of extracts of four Mexican medicinal plants in normal and Alloxan-diabetic mice. Phytother. Res. 2002, 16, 383–386. [Google Scholar] [CrossRef]
- Mbiti, K.F.; Mwendia, M.C.; Mutai, K.J.; Matasyoh, C.J. Hypoglycaemic effects of Salvia officinalis extracts on alloxan-induced diabetic Swiss albino mice. J. Med. Plants Res. 2020, 14, 518–525. [Google Scholar]
- Salah, M.M.A.L.C.; Hussein, M.; Rana, I.; Khalid, L.B. Effect of Salvia officinalis L. (Sage) aqueous extract on liver and testicular function of diabetic albino male rats. J. Babylon Univ. Pure Appl. Sci. 2016, 24, 83–90. [Google Scholar]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar] [CrossRef]
- Gourich, A.A.; Touijer, H.; Drioiche, A.; Asbabou, A.; Remok, F.; Saidi, S.; Siddique, F.; Ailli, A.; Bourhia, M.; Salamatullah, A.M.; et al. Insight into biological activities of chemically characterized extract from Marrubium vulgare L. in vitro, in vivo and in silico approaches. Front. Chem. 2023, 11, 1238346. [Google Scholar] [CrossRef]
- Aazza, S.; El-Guendouz, S.; da Graça Miguel, M. Antioxidant and α-amylase Inhibition activities of six plants used in the management of diabetes in Morocco. Lett. Appl. Nanosci. 2023, 13, 17. [Google Scholar]
- Elberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel-Sattar, E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015, 3, 37–44. [Google Scholar] [CrossRef]
- Elmhdwi, M.F.; Muktar, M.A.; Attitalla, I.H. Hypoglycemic effects of Marrubium vulgare (Rubia) in experimentally induced autoimmune diabetes mellitus. Int. Res. J. Biochem. Bioinform. 2014, 44, 42–54. [Google Scholar]
- Ghlissi, Z.; Atheymen, R.; Sahnoun, Z.; Zeghal, K.; Mnif, H.; Hakim, A. The effect of Marrubium vulgare L on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of diabetic rats. Int. J. Pharma Chem. Res. 2015, 1, 97–106. [Google Scholar]
- Vergara-Galicia, J.; Aguirre-Crespo, F.; Tun-Suarez, A.; Aguirre-Crespo, A.; Estrada-Carrillo, M.; Jaimes-Huerta, I.; Flo-res-Flores, A.; Estrada-Soto, S.; Ortiz-Andrade, R. Acute hypoglycemic effect of ethanolic extracts from Marrubium vulgare. Phytopharmacology 2012, 3, 54–60. [Google Scholar]
- Eidi, A.; Eidi, M.; Darzi, R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother. Res. 2009, 23, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.E.; Mostofa, M.; Awal, M.A. Antidiabetic effects of Azadirachta indica, Trigonella foenum graecum, Olea europea and Glibenclamide in experimentally diabetic induced rat. J. Bangladesh Agril Univ. 2005, 3, 277–282. [Google Scholar]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef] [PubMed]
- El-Amin, M.; Virk, P.; Elobeid, M.A.; Almarhoon, Z.M.; Hassan, Z.K.; Omer, S.A.; Merghani, N.M.; Daghestani, M.H.; Al-Olayan, E.M. Anti-diabetic effect of Murraya koenigii (L) and Olea europaea (L) leaf extracts on streptozotocin induced diabetic rats. Pak. J. Pharm. Sci. 2013, 26, 359–365. [Google Scholar]
- Moghadam, M.G.; Masomi, Y.; Razavian, M.; Moradi, M. The effect of oral consumption of olive leaves on serum glucose level and lipid profile of diabetic rats. J. Basic Clin. Pathophysiol. 2013, 1, 37–42. [Google Scholar]
- Kaeidi, A.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M.; Rasoulian, B.; Hajializadeh, Z.; Afrazi, S. Olive (Olea europaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: In vitro and in vivo studies. J. Ethnopharmacol. 2011, 136, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sangi, S.M.A.; Sulaiman, M.I.; Abd El-wahab, M.F.; Ahmedani, E.I.; Ali, S.S. Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. Pharmacogn. Mag. 2015, 11, S251. [Google Scholar] [CrossRef] [PubMed]
- Laaboudi, W.; Ghanam, J.; Ghoumari, O.; Sounni, F.; Merzouki, M.; Benlemlih, M. Hypoglycemic and hypolipidemic effects of phenolic olive tree extract in streptozotocin diabetic rats. Int. J. Pharm. Pharma. Sci. 2016, 8, 287–291. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.R.; El-Beltagi, H.S.; Fayed, S.A.; El-Ansary, A.E. Hypoglycemic and iron status ameliorative effects of Olea europea CV.‘Picual’ leaves extract in streptozotocin induced diabetic rats. Fresen. Environ. Bull. 2017, 26, 6898–6908. [Google Scholar]
- Al-Attar, A.M.; Alsalmi, F.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019, 26, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Guex, C.G.; Reginato, F.Z.; de Jesus, P.R.; Brondani, J.C.; Lopes, G.H.H.; de Freitas Bauermann, L. Antidiabetic effects of Olea europaea L. leaves in diabetic rats induced by high-fat diet and low-dose streptozotocin. J. Ethnopharmacol. 2019, 235, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Al-Shudiefat, A.A.R.; Alturk, H.; Al-Ameer, H.J.; Zihlif, M.; Alenazy, M. Olive leaf extract of Olea europaea reduces blood glucose level through inhibition of as160 in diabetic rats. Appl. Sci. 2023, 13, 5939. [Google Scholar] [CrossRef]
- Mansour, H.M.; Zeitoun, A.A.; Abd-Rabou, H.S.; El Enshasy, H.A.; Dailin, D.J.; Zeitoun, M.A.; El-Sohaimy, S.A. Antioxidant and anti-diabetic properties of olive (Olea europaea) leaf extracts: In vitro and in vivo evaluation. Antioxidants 2023, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Mousa, H.M.; Farahna, M.; Ismail, M.S.; Al-Hassan, A.A.; Ammar, A.S.; Abdel-Salam, A.M. Anti-diabetic effect of olive leaves extract in alloxan-diabetic rats. J. Agric. Vet. Sci. 2014, 7, 183–192. [Google Scholar] [CrossRef]
- Benhabyles, N.; Arab, K.; Bouchenak, O.; Baz, A. Phytochemical screening, hypoglycemic and antihyperglycemic effect of flavonoids from the leaves of Algerian Olea europaea L. in normal and alloxan-induced diabetic rats. Int. J. Pharmacol. 2015, 11, 477–483. [Google Scholar] [CrossRef]
- Qadir, N.M.; Ali, K.A.; Qader, S.W. Antidiabetic effect of oleuropein from Olea europaea leaf against alloxan induced type 1 diabetic in rats. Braz. Arch. Biol. Technol. 2016, 59, e16150116. [Google Scholar] [CrossRef]
- Farah, H.S. Hypoglycemic, hypolipidemic and antioxidant activities of ethanolic extract of Olea europaea Linn. Int. J. Novel Res. Life Sci. 2015, 2, 33–37. [Google Scholar]
- Al-Azzawie, H.F.; Alhamdani, M.S.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- AlShaal, S.; Karabet, F.; Daghestani, M. Evaluation the antioxidant activity of Syrian ficus and olive leaf extracts and their inhibitory effects on α-glucosidase in vitro. Mor. J. Chem. 2020, 8, 235–243. [Google Scholar]
- Loizzo, M.R.; Lecce, G.D.; Boselli, E.; Menichini, F.; Frega, N.G. Inhibitory activity of phenolic compounds from extra virgin olive oils on the enzymes involved in diabetes, obesity and hypertension. J. Food Biochem. 2011, 35, 381–399. [Google Scholar] [CrossRef]
- Khlif, I.; Hamden, K.; Damak, M.; Allouche, N. A new triterpene from Olea europea stem with antidiabetic activity. Chem. Nat. Compd. 2012, 48, 799–802. [Google Scholar] [CrossRef]
- Alhodieb, F.S. In vitro hypoglycemic effects of black seed (Nigella Sativa). NeuroQuantology 2022, 20, 425. [Google Scholar]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella Sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef]
- Houcher, Z.; Boudiaf, K.; Benboubetra, M.; Houcher, B. Effects of methanolic extract and commercial oil of Nigella sativa L. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines 2007, 18, 8–18. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ilyas, H.F.; Shaukat, U.A.; Qadir, R.; Masood, S.; Batool, S.; Zahoor, S.; Saadia, M. Comparative study of hypoglycaemic and antioxidant potential of methanolic seed extract and oil of Nigella Sativa on alloxanized diabetic rabbits. Pak. J. Pharm. Sci. 2022, 35, 1755–1760. [Google Scholar]
- Chisom, S.A.; Chinyere, S.N.; Andrew, C.N. Evaluation of the effect of black seed (Nigella Sativa) on oxidative stress markers of Alloxan–induced diabetic wistar rat. IAA J. Biol. Sci. 2022, 8, 164–177. [Google Scholar]
- Sutrisna, E.; Azizah, T.; Wahyuni, S. Potency of Nigella Sativa linn. seed as antidiabetic (preclinical study). Res. J. Pharm. Technol. 2022, 15, 381–384. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Niazmand, S.; Mahmoudabady, M.; Rezaee, S.A.; Soukhtanloo, M.; Mosallanejad, R.; Hayatdavoudi, P. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 2019, 228, 142–147. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S. Nigella Sativa stimulates insulin secretion from isolated rat islets and inhibits the digestion and absorption of (CH2O) n in the gut. Biosci. Rep. 2019, 39, BSR20190723. [Google Scholar] [CrossRef]
- Sadiq, N.; Subhani, G.; Fatima, S.A.; Nadeem, M.; Zafer, S.; Mohsin, M. Antidiabetic effect of Nigella Sativa compared with metformin on blood glucose levels in streptozotocin induced diabetic albino wistar rats. Int. J. Basic Clin. Pharmacol. 2021, 10, 361–367. [Google Scholar] [CrossRef]
- Tariq, S.M.; Khan, K.; Sadiq, M.M.; Pooja, S.; Suyog, S.; Devendra, S.K. Nigella Sativa’s effect on biochemical as well as anthropometric parameters in diabetic rats on high fat diet. J. Med. Sci. Health 2023, 9, 16–22. [Google Scholar] [CrossRef]
- Khan, S.S.; Zaidi, K.U. Protective effect of Nigella Sativa seed extract and its bioactive compound thymoquinone on streptozotocin-induced diabetic rats. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 51–59. [Google Scholar] [CrossRef]
- Fararh, K.M.; Atoji, Y.; Shimizu, Y.; Shiina, T.; Nikami, H.; Takewaki, T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res. J. Vet. Sci. 2004, 77, 123–129. [Google Scholar] [CrossRef]
- Abdelrazek, H.M.; Kilany, O.E.; Muhammad, M.A.; Tag, H.M.; Abdelazim, A.M. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male wistar rats. Oxid. Med. Cell. Longev. 2018, 2018, 8104165. [Google Scholar] [CrossRef] [PubMed]
- Le, P.M.; Benhaddou-Andaloussi, A.; Elimadi, A.; Settaf, A.; Cherrah, Y.; Haddad, P.S. The petroleum ether extract of Nigella Sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J. Ethnopharmacol. 2004, 94, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, Q.; Niu, Y.; Jiang, S.; Zhou, L.I.; Wang, J.; Ma, C.; Kang, W. Effects of Nigella Sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int. J. Biol. Macromol. 2020, 159, 725–738. [Google Scholar] [CrossRef]
- Kim, S.H.; Jo, S.H.; Kwon, Y.I.; Hwang, J.K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in sd rats model. Int. J. Mol. Sci. 2011, 12, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Hwang, J.Y.; Kang, M.J.; Kim, Y.M.; Jung, S.H.; Lee, J.H.; Kim, J.I. Hypoglycemic effect of onion skin extract in animal models of diabetes mellitus. Food Sci. Biotech. 2008, 17, 130–134. [Google Scholar]
- Amba, E.; Ramya, R.; Lakshmi, A.; Sandhya, S.; Suganya, P.; Pratibha, R. Quantification of the anti-diabetic effect of Allium cepa. Cureus 2024, 16, e59174. [Google Scholar] [CrossRef]
- Gois Ruivo da Silva, M.; Skrt, M.; Komes, D.; Poklar Ulrih, N.; Pogačnik, L. Enhanced yield of bioactivities from onion (Allium cepa L.) skin and their antioxidant and anti-α-amylase activities. Int. J. Mol. Sci. 2020, 21, 2909. [Google Scholar] [CrossRef]
- Yang, S.J.; Paudel, P.; Shrestha, S.; Seong, S.H.; Jung, H.A.; Choi, J.S. In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: A comparative study. Food Sci. Nutr. 2019, 7, 205–215. [Google Scholar] [CrossRef]
- Nile, A.; Gansukh, E.; Park, G.S.; Kim, D.H.; Nile, S.H. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste–In vitro and in silico approach. Food Chem. 2021, 335, 127650. [Google Scholar] [CrossRef]
- El-Soud, N.A.; Khalil, M. Antioxidative effects of Allium cepa essential oil in streptozotocin induced diabetic rats. Maced. J. Med. Sci. 2010, 3, 344–351. [Google Scholar] [CrossRef]
- Abouzed, T.K.; del Mar Contreras, M.; Sadek, K.M.; Shukry, M.; Abdelhady, D.H.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A.; et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lim, Y.; Moon, M.S.; Kim, J.Y.; Kwon, O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr. Metab. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Choi, H.; Loots, D.T. Effects of dietary onion (Allium cepa L.) in a high-fat diet streptozotocin-induced diabetes rodent model. Ann. Nutr. Metab. 2008, 53, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C. Anti-diabetic effects of Allium cepa (onions) aqueous extracts on alloxan-induced diabetic Rattus novergicus. J. Med. Plants Res. 2011, 5, 1134–1139. [Google Scholar]
- El-Demerdash, F.M.; Yousef, M.I.; Abou El-Naga, N.I. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem. Toxicol. 2005, 43, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, A.J.; Maleki, M.; Motadayen, M.H.; Sirus, S. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J. Med. Sci. 2005, 59, 64–69. [Google Scholar]
- Panda, N.; Panigrahi, S.P.; Gupta, M.K.; Kumari, A.; Mehta, D.; Goswami, T.D.; Das, G.N.; Gangopadhyay, A. Phytochemical screening and pharmacological study of antidiabetic potential and bioactive compounds present in Allium sativum. J. Chem. Health Risks 2024, 14, 2646–2654. [Google Scholar]
- El Mahi, F.; Hasib, A.; Boulli, A.; Boussadda, L.; Abidi, O.; Aabdousse, J.; Khiraoui, A.; Ourouadi, S. In vitro and in vivo antidiabetic effect of the aqueous extract of garlic (Allium sativum L.) compared to glibenclamide on biochemical parameters in alloxan-induced diabetic mice. Int. J. Pharm. Sci. Rev. Res. 2023, 80, 106–113. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Ibrahim, A.; Dahiru, N.J.; Mohammed, H.U.S. A amylase inhibitory potential and mode of inhibition of oils from Allium sativum (garlic) and Allium cepa (onion). Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1179551420963106. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, C.; Yu, Y.B.; Wu, L.X.; Chen, T.T.; Wang, Z.W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef]
- Wongsa, P.; Bhuyar, P.; Tongkoom, K.; Spreer, W.; Müller, J. Influence of hot-air drying methods on the phenolic compounds/allicin content, antioxidant activity and α-amylase/α-glucosidase inhibition of garlic (Allium sativum L.). Eur. Food Res. Technol. 2023, 249, 523–535. [Google Scholar] [CrossRef]
- Sujithra, K.; Srinivasan, S.; Indumathi, D.; Vinothkumar, V. Allyl methyl sulfide, an organosulfur compound alleviates hyperglycemia mediated hepatic oxidative stress and inflammation in streptozotocin-induced experimental rats. Biomed. Pharmacother. 2018, 107, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Drobiova, H.; Thomson, M.; Al-Qattan, K.; Peltonen-Shalaby, R.; Al-Amin, Z.; Ali, M. Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase method. Evid. Based Complement. Altern. Med. 2011, 2011, 703049. [Google Scholar] [CrossRef]
- Xie, C.; Gao, W.; Li, X.; Luo, S.; Wu, D.; Chye, F.Y. Garlic (Allium sativum L.) polysaccharide ameliorates type 2 diabetes mellitus (T2DM) via the regulation of hepatic glycogen metabolism. NFS J. 2023, 31, 19–27. [Google Scholar] [CrossRef]
- Mohamed, J.; Ainane, T. In vitro antidiabetic activity of essential oil of two species of Artemisia: Artemisia herba-alba asso and Artemisia ifranensis. Pharm. Online 2021, 3, 812–820. [Google Scholar]
- Awad, N.E.; Seida, A.A.; El-Khayat, Z.; Shaffie, N.; Abd El-Aziz, A.M. Hypoglycemic activity of Artemisia herba-alba (Asso.) used in Egyptian traditional medicine as hypoglycemic remedy. J Appl. Pharm. Sci. 2012, 2, 30–39. [Google Scholar]
- Tastekin, D.; Atasever, M.; Adiguzel, G.; Keles, M.; Tastekin, A. Hypoglycaemic effect of Artemisia herba-alba in experimental hyperglycaemic rats. Bull Vet. Inst. Pulawy. 2006, 50, 235–238. [Google Scholar]
- Boudjelal, A.; Siracusa, L.; Henchiri, C.; Sarri, M.; Abderrahim, B.; Baali, F.; Ruberto, G. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Medica. 2015, 81, 696–704. [Google Scholar] [CrossRef]
- Iriadam, M.; Musa, D.; Gumushan, H.; Baba, F. Effects of two Turkish medicinal plants Artemisia herba-alba and Teucrium polium on blood glucose levels and other biochemical parameters in rabbits. J. Cell. Mol. Biol. 2006, 5, 19–24. [Google Scholar]
- Abdallah, H.M.; Abdel-Rahman, R.F.; Jaleel, G.A.A.; El-Kader, H.A.M.A.; El-Marasy, S.A. Pharmacological effects of ethanol extract of Artemisia herba alba in streptozotocin-induced type 1 diabetes mellitus in rats. Biochem. Pharmacol. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; Zaki, E.R.; Abdallah, H.M.; Arbid, M.S. Therapeutic effects of aqueous, methanol and ethanol extracts of Egyptian Artemisia herba-alba in STZ-induced diabetic neuropathy in rats. J. Appl. Pharm. Sci. 2017, 7, 180–187. [Google Scholar]
- Ahmad, Z.A.K.; Abdul-Hussian, B.A. Effect of Artemisia herb on induced hyperglycemia in wistar rats. Al-Qadisiyah J. Vet. Med. Sci. 2016, 15, 63–69. [Google Scholar]
- Hamza, N.; Berke, B.; Cheze, C.; Le Garrec, R.; Lassalle, R.; Agli, A.N.; Robinson, P.; Gin, H.; Moore, N. Treatment of high fat diet induced type 2 diabetes in C57BL/6J mice by two medicinal plants used in traditional treatment of diabetes in the east of Algeria. J. Ethnopharmacol. 2011, 133, 931–933. [Google Scholar] [CrossRef]
- Mansi, K.; Amneh, M.; Nasr, H. The hypolipidemic effects of Artemisia sieberi (A. herba-alba) in alloxan induced diabetic rats. Int. J. Pharmacol. 2007, 3, 487–491. [Google Scholar] [CrossRef]
- Declaration of Amnesty, Abdul Razak Hamoui, Asad al Abdulla. Effect of watery extract of Artemisia herba alba on blood glucose level and body weight in alloxan-diabetic rabbits. Assiut Vet. Med. J. 2010, 56, 1–11. [CrossRef]
- El Ansari, N.; Chadli, A.; El Aziz, S.; El Mghari, G.; El Achhab, Y.; Seqat, M.; Nejjari, C. Observational study of patients in morocco with uncontrolled type 2 diabetes treated with metformin and/or sulfonylurea with or without insulin. J. Endocrinol. Metab. 2015, 5, 321–327. [Google Scholar] [CrossRef]
- Triad, A.C. Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk. Clevel. Clin. J. Med. 2009, 76, S20–S27. [Google Scholar][Green Version]
- Kasilo, O.M.; Nikiema, J.B. World Health Organization perspective for traditional medicine. In Novel Plant Bioresources: Applications in Food, Medicine and Cosmetics; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 23–42. [Google Scholar]
- Aboufaras, M.; Selmaoui, K.; Ouzennou, N. Use of complementary traditional phytotherapy to manage cancer in Morocco: A decade-long review of ethnopharmacological studies. J. Herb. Med. 2021, 29, 100494. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
- Wannes, W.A.; Marzouk, B. Research progress of Tunisian medicinal plants used for acute diabetes. J. Acute Dis. 2016, 5, 357–363. [Google Scholar] [CrossRef]
- Abogmaza, F.A.; Keer, F.K.; Takrizzah, A.A.; Yahya, E.B. A Review on the Medicinal and Aromatic Plants Growing in Libya and Their Therapeutic Properties. Int. Res. J. Sci. Technol. 2020, 2, 327–334. [Google Scholar] [CrossRef]
- Al-Traboulsi, M.; Alaib, M.A. A Survey of Medicinal Plants of Wadi Al-Kouf in Al-Jabal Al-Akhdar, Libya. Nat. Croat. Period. Musei Hist. Nat. Croat. 2021, 30, 389–404. [Google Scholar] [CrossRef]
- Hamden, K.; Jaouadi, B.; Carreau, S.; Bejar, S.; Elfeki, A. Inhibitory effect of fenugreek galactomannan on digestive enzymes related to diabetes, hyperlipidemia, and liver-kidney dysfunctions. Biotechnol. Bioprocess Eng. 2010, 15, 407–413. [Google Scholar] [CrossRef]
- Hachouf, M.; Aouacheri, O.; Saka, S.; Marzocchi, A.; Tenore, G.C. Phytochemical, biochemical and physiological assessment of the protective effect of local Trigonella foenum graecum in rats administered a β-cell toxicant. Chem. Biodiversity 2024, e202401183. [Google Scholar] [CrossRef] [PubMed]
- Ghlissi, Z.; Hamden, K.; Saoudi, M.; Sahnoun, Z.; Zeghal, K.M.; El Feki, A.; Hakim, A. Effect of Nigella sativa seeds on reproductive system of male diabetic rats. Afr. J. Pharm. Pharmacol. 2012, 6, 1444–1450. [Google Scholar]
- Belmouhoub, M.; Tacherfıout, M.; Boukhalfa, F.; Khodja, Y.K.; Bachir-Bey, M. Traditional medicinal plants used in the treatment of diabetes: Ethnobotanical and ethnopharmacological studies and mechanisms of action. Int. J. Plant Based Pharm. 2022, 2, 145–154. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hammami, M.; Hadj, A.B.; Boudawara, T.; Dammak, M.; Zouari, S.; El Feki, A. Metabolic impairments and tissue disorders in alloxan-induced diabetic rats are alleviated by Salvia officinalis L. essential oil. Biomed. Pharmacother. 2018, 108, 985–995. [Google Scholar] [CrossRef]
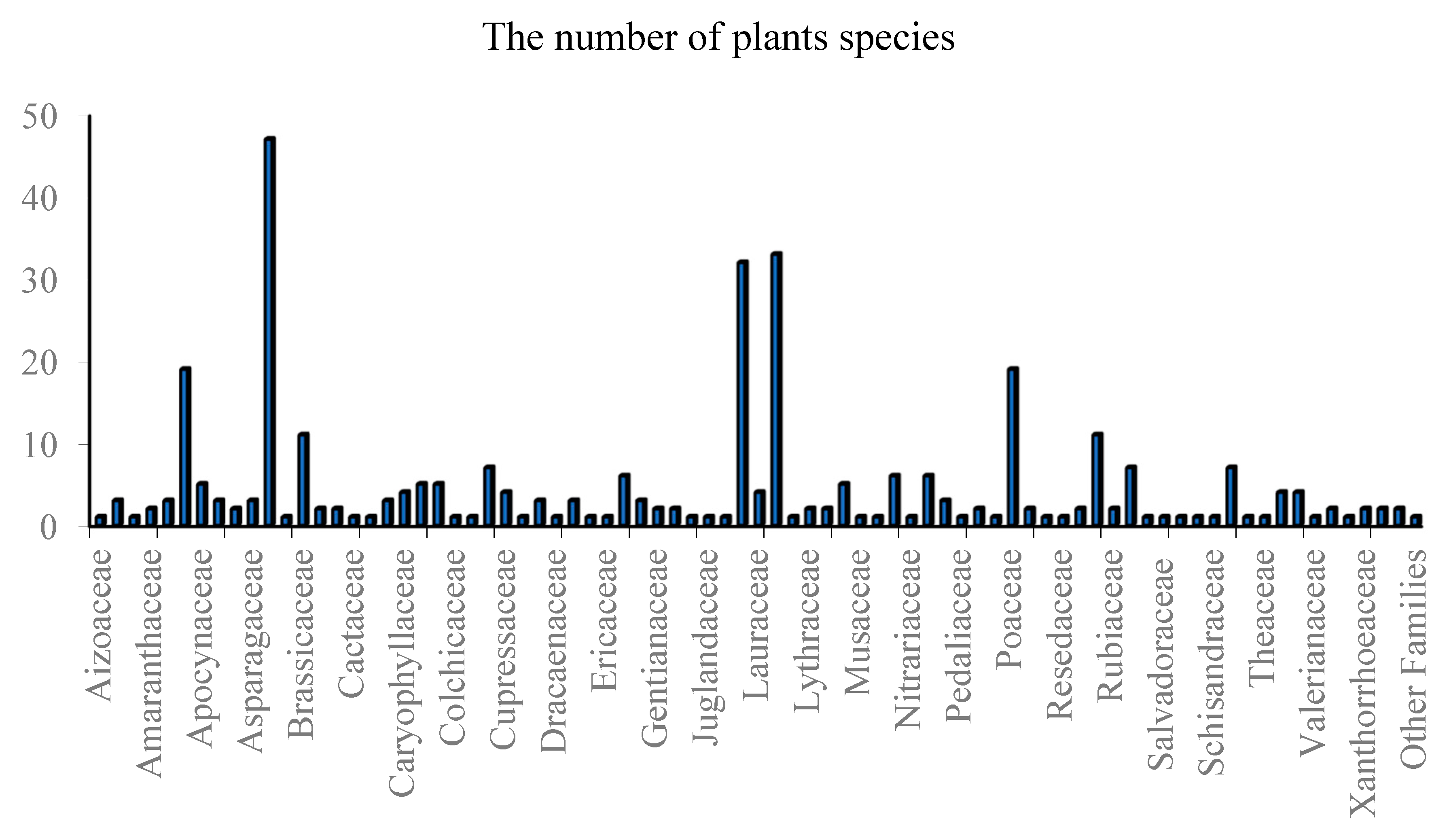
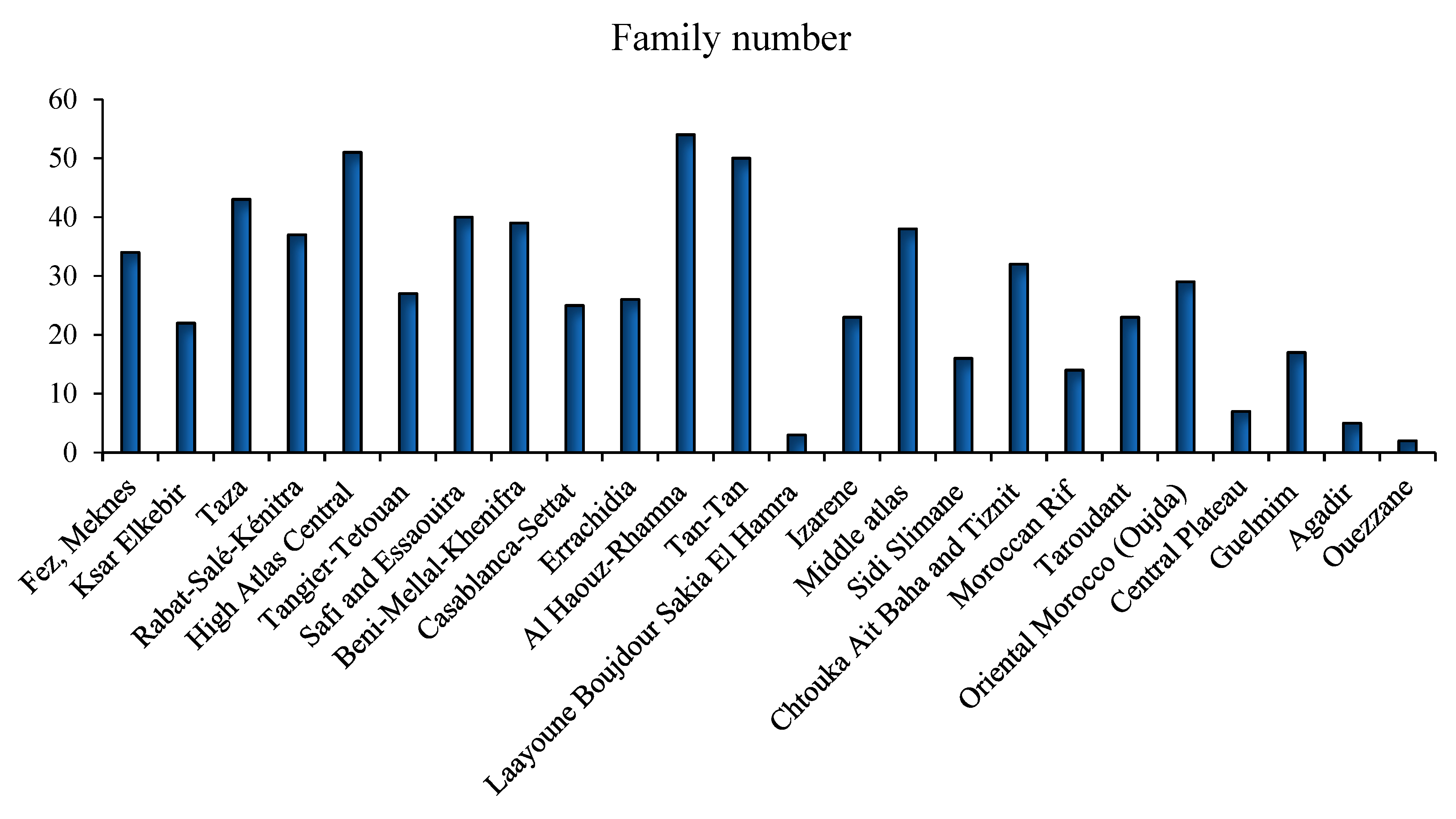
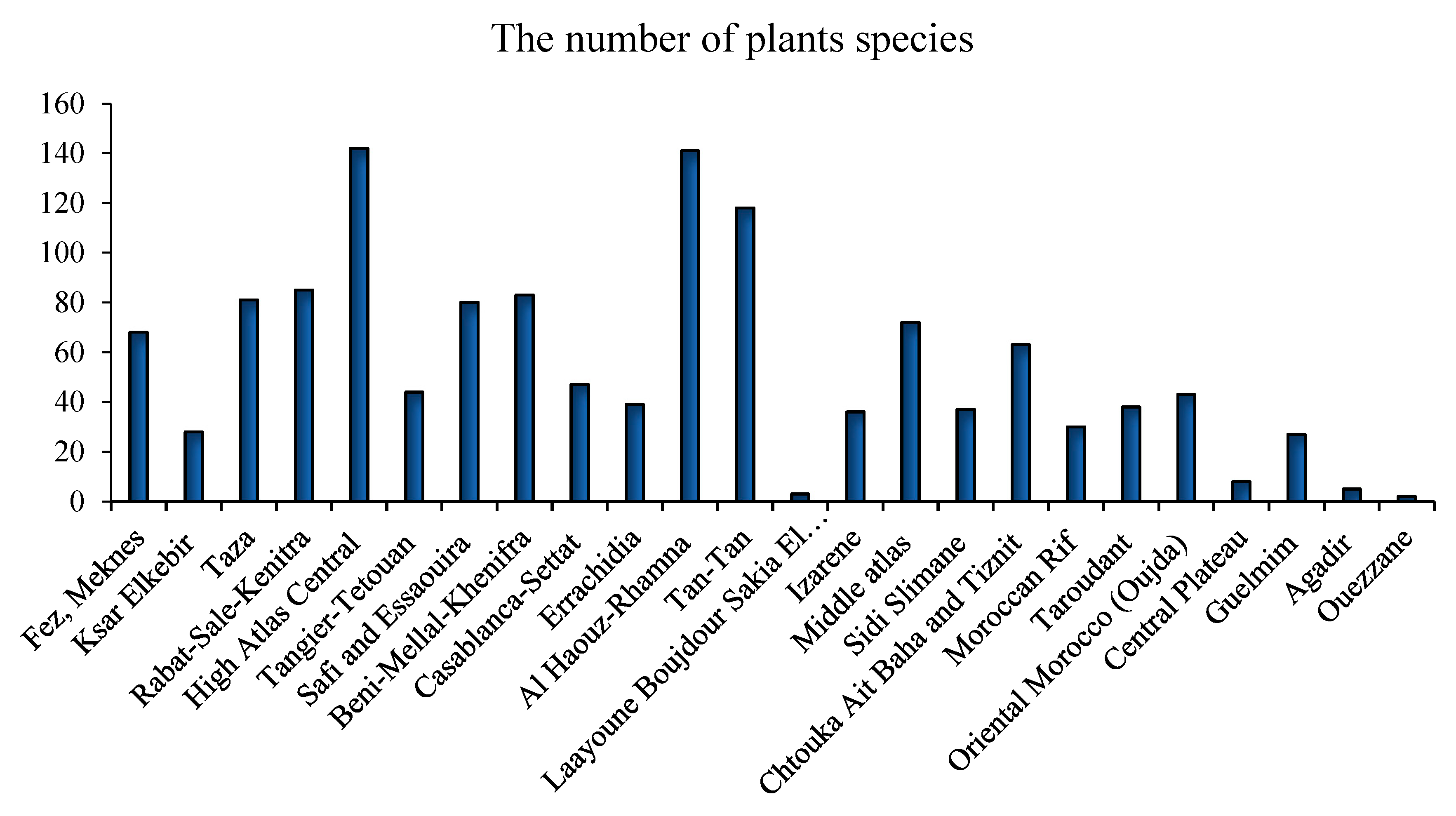
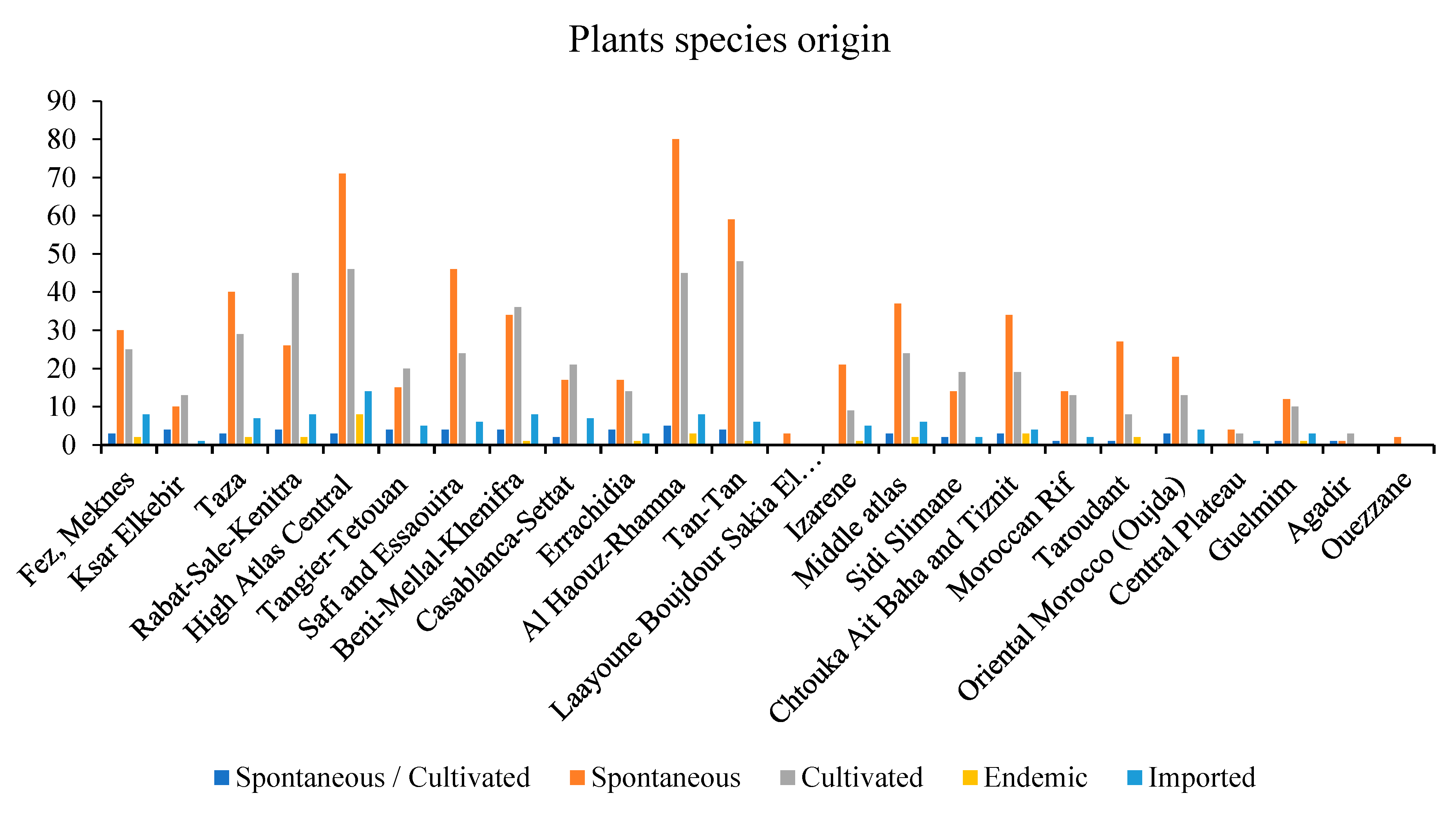



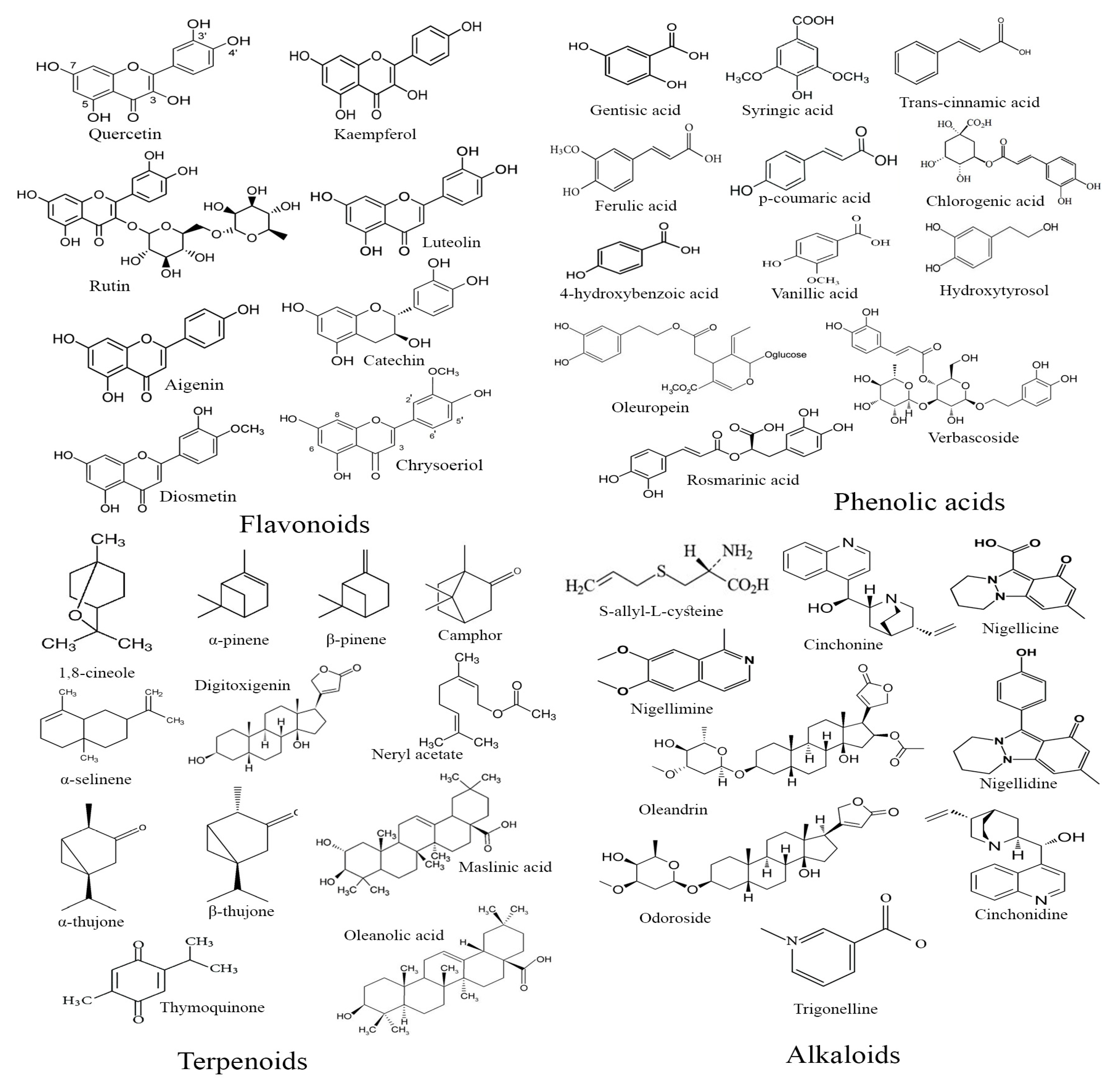
| Family Name | Scientific Name | Origin |
|---|---|---|
| Aizoaceae | Opophytum theurkauffii Maire L. | Spontaneous |
| Alliaceae | Allium cepa L. | Cultivated |
| Allium sativum L. | Cultivated | |
| Allium ampeloprasum var. porrum | Cultivated | |
| Aloeaceae | Aloe vera (L.) Burm.f. | Cultivated |
| Amaranthaceae | Anabasis aretioides Moq. & Coss. ex Bunge | Spontaneous |
| Beta vulgaris L. | Cultivated | |
| Spinacia oleracea L. | Cultivated | |
| Anacardiaceae | Pistacia atlantica Desf. | Spontaneous |
| Pistacia lentiscus L. | Spontaneous | |
| Searsia albida (Schousb.) Moffett | Spontaneous | |
| Apiaceae | Ammodaucus leucotrichus Coss. | Spontaneous |
| Ammi majus L. | Spontaneous | |
| Ammi visnaga (L.) Lam. | Spontaneous | |
| Anethum foeniculum L. | Cultivated | |
| Apium graveolens L. | Cultivated | |
| Carum carvi L. | Cultivated | |
| Coriandrum sativum L. | Cultivated | |
| Cuminum cyminum L. | Cultivated | |
| Daucus carota L. | Cultivated | |
| Eryngium ilicifolium Lam. | Spontaneous | |
| Ferula communis L. | Spontaneous | |
| Foeniculum vulgare Mill. | Cultivated | |
| Pastinaca sativa L. | Cultivated | |
| Petroselinum crispum (Mill.) Fuss | Cultivated | |
| Petroselinum sativum Hoffm | Cultivated | |
| Pimpinella anisum L. | Cultivated | |
| Ptychotis verticillata Duby | Cultivated | |
| Ridolfia segetum (L.) Moris | Spontaneous | |
| Apocynaceae | Apteranthes europaea (Guss.) Murb. | Spontaneous |
| Calotropis procera (Aiton) Dryand. | Spontaneous | |
| Caralluma europaea (Guss.) N.E.Br. | Spontaneous | |
| Nerium oleander L. | Spontaneous | |
| Periploca laevigata subsp. Angustifolia (Labill.) Markgr. | Spontaneous | |
| Arecaceae | Chamaerops humilis L. | Spontaneous |
| Hyphaene thebaica (L.) Mart. | Spontaneous | |
| Phoenix dactylifera L. | Cultivated | |
| Aristolochiaceae | Aristolochia baetica L. | Spontaneous |
| Aristolochia longa subsp. Fontanesii Boiss. & Reut. | Spontaneous | |
| Asparagaceae | Agave americana L. | Cultivated |
| Asparagus albus L. | Spontaneous | |
| Asparagus officinalis L. | Cultivated | |
| Asteraceae | Achillea odorata L. | Spontaneous |
| Achillea santolinoides Lag. | Spontaneous | |
| Anacyclus pyrethrum (L.) Lag. | Spontaneous | |
| Antennaria dioica (L.) Gaertn | Spontaneous | |
| Anvillea garcinii subsp. Radiata (Coss. & Durieu) Anderb. | Spontaneous | |
| Artemisia abrotanum L. | Cultivated | |
| Artemisia absinthium L. | Cultivated | |
| Artemisia arborescens (Vaill.) L. | Spontaneous | |
| Artemisia atlantica Coss. & Durieu | Spontaneous | |
| Artemisia campestris L. | Spontaneous | |
| Artemisia herba-alba Asso | Spontaneous | |
| Artemisia herba alba Assac. | Spontaneous | |
| Artemisia mesatlantica Maire | Endemic | |
| Artemisia reptans C. Sm. ex Link | Spontaneous | |
| Atractylis gummifera Salzm. ex L. | Spontaneous | |
| Calendula arvensis Bieb., | Spontaneous | |
| Centaurea maroccana Bal | Spontaneous | |
| Chamaemelum mixtum (L.) Alloni | Spontaneous | |
| Chamaemelum nobile (L.) All. | Spontaneous | |
| Chrysanthemum coronarium L. | Spontaneous | |
| Cichorium intybus L. | Cultivated | |
| Cladanthus arabicus (L.) Cass. | Spontaneous | |
| Cladanthus scariosus (Ball) Oberpr. & Vogt | Spontaneous | |
| Cynara cardunculus L. | Cultivated | |
| Cynara cardunculus subsp. scolymus (L.) | Cultivated | |
| Cynara humilis L. | Spontaneous | |
| Dittrichia viscosa (L.) Greuter | Spontaneous | |
| Echinops spinosissimus Turra | Spontaneous | |
| Helianthus annuus L. | Cultivated | |
| Inula conyza (Griess.) DC. | Spontaneous | |
| Inula helenium L. | Cultivated | |
| Lactuca sativa L. | Cultivated | |
| Launaea arborescens (Batt.) Murb. | Spontaneous | |
| Matricaria chamomilla L. | Spontaneous | |
| Pallenis spinosa (L.) Cass. | Spontaneous | |
| Saussurea costus (Falc.) Lipschitz | Spontaneous | |
| Scolymus hispanicus L. | Spontaneous | |
| Scorzonera undulata Vahl | Spontaneous | |
| Seriphidium herba-alba | Spontaneous | |
| Sonchus arvensis L. | Spontaneous | |
| Sonchus asper (L.) Hill | Spontaneous | |
| Sonchus tenerrimus L. | Spontaneous | |
| Stevia rebaudiana Willd. | Cultivated | |
| Silybum marianum L. | Spontaneous | |
| Tanacetum vulgare L. | Spontaneous | |
| Taraxacum campylodes G.E. Haglund | Spontaneous | |
| Warionia saharae Benthem ex Benth. & Coss. | Spontaneous | |
| Berberidaceae | Berberis vulgaris subsp. Australis (Boiss.) Heywood | Spontaneous |
| Brassicaceae | Anastatica hierochuntica L. | Spontaneous |
| Brassica napus L. | Cultivated | |
| Brassica nigra (L.) K. Koch | Cultivated | |
| Brassica oleracea L. | Cultivated | |
| Brassica rapa L. | Cultivated | |
| Diplotaxis pitardiana Maire | Spontaneous | |
| Eruca vesicaria (L.) Cav. | Spontaneous | |
| Lepidium sativum L. | Cultivated | |
| Nasturtium officinale R.Br. | Spontaneous | |
| Ptilotrichum spinosum (L.) Boiss. | Spontaneous | |
| Raphanus raphanistrum subsp. sativus (L.) | Cultivated | |
| Burseraceae | Boswellia sacra Flueck. | Imported |
| Commiphora myrrha (Nees) Engl. | Cultivated | |
| Buxaceae | Buxus balearica Lam. | Cultivated |
| Buxus sempervirens L. | Cultivated | |
| Cactaceae | Opuntia ficus indica (L.) Mill. | Spontaneous/Cultivated |
| Capparaceae | Capparis decidua (Forssk.) Edgew. | Cultivated |
| Capparis spinosa L. | Spontaneous | |
| Maerua crassifolia Forssk. | Cultivated | |
| Caryophyllaceae | Herniaria glabra var. hirsuta (L.) Kuntze | Spontaneous |
| Paronychia argentea Lam. | Spontaneous | |
| Silene vivianii Steud. | Spontaneous | |
| Corrigiola telephiifolia Pourr. | Spontaneous | |
| Cannabaceae | Cannabis sativa L. | Spontaneous/Cultivated |
| Cistaceae | Cistus albidus L. | Spontaneous |
| Cistus creticus L. | Spontaneous | |
| Cistus laurifolius L. | Spontaneous | |
| Cistus salviifolius L. | Spontaneous | |
| Cistus ladanifer L. | Spontaneous | |
| Chenopodiaceae | Atriplex halimus L. | Spontaneous |
| Chenopodium ambrosioides L. | Spontaneous | |
| Hammada scoparia (Pomel) Iljin | Spontaneous | |
| Salsola tetragona Delile | Spontaneous | |
| Suaeda mollis Dest., | Spontaneous | |
| Colchicaceae | Androcymbium gramineum (Cav.) J.F. Macbr. | Spontaneous |
| Convolvulaceae | Ipomoea batatas (L.) | Cultivated |
| Cucurbitaceae | Bryonia dioica Jacq. | Spontaneous |
| Citrullus colocynthis (L.) Schrad. | Spontaneous | |
| Citrullus vulgaris Schard. | Cultivated | |
| Cucumis sativus L. | Cultivated | |
| Cucumis melo var. flexuosus L. | Cultivated | |
| Cucurbita maxima Duchesne | Cultivated | |
| Cucurbita pepo L. | Cultivated | |
| Cupressaceae | Juniperus phoenicea L. | Imported |
| Juniperus thurifera L | Spontaneous | |
| Juniperus oxycedrus L. | Imported | |
| Tetraclinis articulata (Vahl) Mast. | Spontaneous | |
| Cynomoriaceae | Cynomorium coccineum L. | Spontaneous |
| Cyperaceae | Bolboschoenus maritimus (L.) Palla | Spontaneous |
| Cyperus longus L. | Imported | |
| Cyperus rotundus L. | Spontaneous | |
| Dracaenaceae | Dracaena draco subsp. ajgal Benabid & Cuzin | Cultivated |
| Ephedraceae | Ephedra alata Decne. | Spontaneous |
| Ephedra altissima Desf. | Spontaneous | |
| Ephedra fragilis Desf. | Spontaneous | |
| Equisetaceae | Equisetum ramosissimum Desf | Spontaneous |
| Ericaceae | Arbutus unedo L. | Spontaneous |
| Vaccinium myrtillus L. | Cultivated | |
| Euphorbiaceae | Euphorbia officinarum subsp. echinus (Hook. f. & Coss.) Vindt | Spontaneous |
| Euphorbia officinarum L. | Spontaneous | |
| Euphorbia peplis L. | Spontaneous | |
| Euphorbia resinifera O. Berg | Endemic | |
| Mercurialis annua L. | Spontaneous | |
| Ricinus communis L. | Spontaneous | |
| Fagaceae | Quercus coccifera L. | Spontaneous |
| Quercus suber L. | Spontaneous | |
| Quercus ilex L. | Imported | |
| Gentianaceae | Centaurium erythraea Rafn | Spontaneous |
| Centaurium spicatum (L.) Fritsch | Cultivated | |
| Geraniaceae | Pelargonium odoratissimum | Cultivated |
| Pelargonium roseum Willd. | Cultivated | |
| Iridaceae | Crocus sativus L. | Cultivated |
| Juglandaceae | Juglans regia L. | Cultivated |
| Juncaceae | Juncus maritimus Lam. | Cultivated |
| Lamiaceae | Ajuga iva (L.) Schreb. | Spontaneous |
| Ballota hirsuta Benth | Spontaneous | |
| Calamintha officinalis Moench. | Spontaneous | |
| Calamintha nepeta subsp. Spruneri (Boiss.) Nyman | Spontaneous | |
| Calamintha alpina L. | Spontaneous | |
| Clinopodium alpinum (L.) Kuntze | Spontaneous | |
| Clinopodium nepeta subsp. glandulosum (Req.) Govaerts | Spontaneous | |
| Lavandula angustifolia Mill | Spontaneous | |
| Lavandula dentata L. | Spontaneous | |
| Lavandula maroccana Murb. | Endemic | |
| Lavandula multifida L. | Spontaneous | |
| Lavandula stoechas L. | Spontaneous | |
| Marrubium vulgare L. | Spontaneous | |
| Mentha pulegium L. | Spontaneous | |
| Melissa officinalis L. | Spontaneous | |
| Mentha spicata L. | Spontaneous | |
| Mentha piperita L. | Cultivated | |
| Mentha suaveolens Ehrh. | Spontaneous | |
| Ocimum basilicum L. | Cultivated | |
| Origanum compactum Benth. | Spontaneous | |
| Origanum elongatum (Bonnet) Emb. & Maire | Spontaneous | |
| Origanum majorana L. | Spontaneous | |
| Origanum vulgare L. | Spontaneous | |
| Rosmarinus officinalis L. | Imported | |
| Salvia officinalis L. | Cultivated | |
| Salvia hispanica L. | Cultivated | |
| Teucrium polium L. | Spontaneous | |
| Thymus broussonetii Boiss. | Endemic | |
| Thymus algeriensis Boiss. & Reut. | Spontaneous | |
| Thymus maroccanus Ball. | Endemic | |
| Thymus munbyanus Boiss. & Reut | Spontaneous | |
| Thymus satureioides Coss. | Endemic | |
| Thymus vulgaris L. | Spontaneous | |
| Thymus zygis L. | Spontaneous | |
| Lauraceae | Cinnamomum cassia (L.) J. Presl | Imported |
| Cinnamomum verum J. Presl | Cultivated | |
| Laurus nobilis L. | Spontaneous | |
| Persea americana Mill. | Cultivated | |
| Leguminosae | Acacia gummifera Willd. | Endemic |
| Acacia nilotica (L.) Delile | Cultivated | |
| Acacia senegal (L.) Willd. | Cultivated | |
| Acacia tortilis (Forssk.) Hayne | Spontaneous | |
| Acacia albida Delile | Cultivated | |
| Anagyris foetida L. | Cultivated | |
| Arachis hypogaea L. | Cultivated | |
| Cassia absus L. | Imported | |
| Cassia fistula L. | Cultivated | |
| Ceratonia siliqua L. | Imported | |
| Cicer arietinum L. | Cultivated | |
| Cytisus battandieri Maire | Cultivated | |
| Glycine max (L.) Merr. | Cultivated | |
| Glycyrrhiza glabra L. | Imported | |
| Lupinus albus L. | Spontaneous | |
| Lupinus angustifolius L. | Spontaneous | |
| Lupinus luteus L. | Spontaneous | |
| Lupinus pilosus L. | Spontaneous | |
| Medicago sativa L. | Cultivated | |
| Ononis natrix L. | Spontaneous | |
| Ononis tournefortii Coss. | Spontaneous | |
| Phaseolus aureus Roxb. | Cultivated | |
| Phaseolus vulgaris L. | Cultivated | |
| Retama monosperma (L.) Boiss. | Spontaneous | |
| Retama raetam (Forssk.) Webb | Spontaneous | |
| Retama sphaerocarpa (L.) Boiss. | Spontaneous | |
| Senna alexandrina Mill. | Cultivated | |
| Trigonella foenum-graecum L. | Spontaneous | |
| Vicia faba L. | Spontaneous | |
| Vicia sativa L. | Spontaneous | |
| Vigna radiata (L.) R. Wilczek | Cultivated | |
| Vigna unguiculata (L.) Walp | Cultivated | |
| Urginea maritima (L.) Baker | Cultivated | |
| Linaceae | Linum usitatissimum L. | Cultivated |
| Lythraceae | Lawsonia inermis L. | Spontaneous |
| Punica granatum L. | Cultivated | |
| Malvaceae | Abelmoschus esculentus (L.) Moench | Cultivated |
| Hibiscus sabdariffa L. | Spontaneous | |
| Moraceae | Ficus abelii Miq | Cultivated |
| Ficus carica L. | Spontaneous/Cultivated | |
| Ficus dottata Gasp. | Cultivated | |
| Morus alba L. | Spontaneous | |
| Morus nigra L. | Spontaneous | |
| Moringaceae | Moringa oleifera Lam. | Cultivated |
| Musaceae | Musa paradisiaca L. | Cultivated |
| Myristicaceae | Myristica fragrans Houtt. | Cultivated |
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | Cultivated |
| Eucalyptus globulus Labill. | Imported | |
| Eugenia caryophyllata Thunb | Cultivated | |
| Jasminum fruticans L. | Spontaneous | |
| Myrtus communis L. | Imported | |
| Syzygium aromaticum (L.) Merr. & L. M. Perry | Cultivated | |
| Nitrariaceae | Peganum harmala L. | Spontaneous |
| Oleaceae | Fraxinus angustifolia Vahl | Spontaneous |
| Fraxinus excelsior var. acuminata Schur | Cultivated | |
| Olea europaea L. | Spontaneous/Cultivated | |
| Olea europaea subsp. maroccana (Greuter & Burdet) | Spontaneous/Cultivated | |
| Olea europea L. subsp. europaea var. sylvestris (Mill) Lehr, | Cultivated | |
| Olea oleaster Hoffm. & Link. | Spontaneous | |
| Papaveraceae | Fumaria officinalis L. | Spontaneous |
| Papaver rhoeas L. | Spontaneous | |
| Plantago ovata Forssk. | Spontaneous | |
| Pedaliaceae | Sesamum indicum L. | Imported |
| Plantaginaceae | Globularia alypum L. | Spontaneous |
| Globularia repens Lam. | Spontaneous | |
| Plumbaginaceae | Limonium sinuatum (L.) Mill. | Spontaneous |
| Poaceae | Avena sativa L. | Cultivated |
| Avena sterilis L. | Cultivated | |
| Castellia tuberculosa (Moris) Bor | Spontaneous | |
| Cynodon dactylon (L.) Pers. | Spontaneous | |
| Hordeum vulgare L. | Cultivated | |
| Lolium perenne L. | Cultivated | |
| Lolium multiflorum Lam. | Spontaneous | |
| Lolium rigidum Gaudin | Spontaneous | |
| Panicum miliaceum L. | Spontaneous | |
| Panicum turgidum Forssk. | Spontaneous | |
| Pennisetum glaucum (L.) R.Br. | Spontaneous | |
| Phalaris canariensis L. | Spontaneous | |
| Phalaris paradoxa L. | Spontaneous | |
| Polypogon monspeliensis (L.) Desf | Spontaneous | |
| Sorghum bicolor (L.) Moench | Spontaneous | |
| Triticum durum Desf. | Cultivated | |
| Triticum aestivum L. | Cultivated | |
| Triticum turgidum L. | Spontaneous | |
| Zea mays L. | Cultivated | |
| Polygonaceae | Emex spinosa (L.) Campd. | Spontaneous |
| Portulaca oleracea L. | Spontaneous | |
| Ranunculaceae | Nigella Sativa L. | Spontaneous |
| Resedaceae | Reseda lanceolata Lag. | Spontaneous |
| Rhamnaceae | Ziziphus lotus (L.) Lam. | Spontaneous |
| Ziziphus jujube Mill | Spontaneous | |
| Rosaceae | Cydonia oblonga Mill. | Cultivated |
| Chaenomeles sinensis (Dum.Cours.) Koehne | Cultivated | |
| Crataegus monogyna Jacq. | Cultivated | |
| Eriobotrya japonica (Thunb.) Lindl. | Cultivated | |
| Fragaria vesca L. | Cultivated | |
| Malus communis (L.) Poir. | Cultivated | |
| Prunus armeniaca L. | Cultivated | |
| Prunus dulcis (Mill.) D.A. Webb | Spontaneous | |
| Prunus cerasus L. | Cultivated | |
| Rubus fruticosus var. vulgaris (Weihe & Nees | Spontaneous | |
| Rubus fruticosus var. ulmifolius, (Schott) | Spontaneous | |
| Rubiaceae | Rubia tinctorum L. | Spontaneous |
| Coffea arabica L. | Cultivated | |
| Rutaceae | Citrus medica var. limon L. | Cultivated |
| Citrus paradisi Macfad. | Cultivated | |
| Citrus sinensis (L.) Osbeck | Cultivated | |
| Citrus aurantium L. | Imported | |
| Ruta graveolens L. | Spontaneous | |
| Ruta chalepensis L. | Spontaneous | |
| Ruta montana L. | Spontaneous | |
| Salicaceae | Salix alba L. | Cultivated |
| Salvadoraceae | Salvadora persica L. | Cultivated |
| Santalaceae | Viscum album L | Spontaneous |
| Sapotaceae | Argania spinosa (L.) Skeels | Cultivated |
| Schisandraceae | Illicium verum Hook.f. | Cultivated |
| Solanaceae | Capsicum annuum L. | Cultivated |
| Datura stramonium L. | Spontaneous/Cultivated | |
| Lycopersicon esculentum Mill. | Cultivated | |
| Nicotiana tabacum L. | Cultivated | |
| Solanum americanum Mill. | Spontaneous/Cultivated | |
| Solanum melongena L. | Cultivated | |
| Withania frutescens (L.) Pauquy | Cultivated | |
| Taxaceae | Taxus baccata L. | Spontaneous |
| Theaceae | Camellia sinensis (L.) Kuntze | Imported |
| Thymelaeaceae | Thymelaea hirsuta (L.) Endl. | Spontaneous |
| Thymelaea tartonraira (L.) All. | Spontaneous | |
| Thymelaea virgata (Desf.) Endl. | Endemic | |
| Aquilaria malaccensis Lam | Cultivated | |
| Urticaceae | Urtica dioica L. | Spontaneous |
| Urtica pilulifera L. | Spontaneous | |
| Urtica urens L. | Spontaneous | |
| Urtica membranacea Poir. ex Savigny | Spontaneous | |
| Valerianaceae | Nardostachys jatamansi (D. Don) DC. | Imported |
| Verbenaceae | Aloysia citriodora Palau | Cultivated |
| Verbena officinalis L. | Spontaneous/Cultivated | |
| Vitaceae | Vitis vinifera L. | Spontaneous/Cultivated |
| Xanthorrhoeaceae | Asphodelus microcarpus Salzm. & Viv. | Spontaneous |
| Asphodelus tenuifolius Cav. | Spontaneous | |
| Zingiberaceae | Zingiber officinale Roscoe. | Cultivated |
| Curcuma longa L. | Cultivated | |
| Zygophyllaceae | Tetraena gaetula (Emb. & Maire) Beier & Thulin | Endemic |
| Zygophyllum gaetulum Emb. & Maire | Spontaneous |
| Scientific Name | Type 1 Diabetes | Type 2 Diabetes | Gestational Diabetes Mellitus |
|---|---|---|---|
| Allium cepa L. | + | + | - |
| Allium sativum L. | + | + | - |
| Allium ampeloprasum var. porrum | - | + | - |
| Aloe vera (L.) Burm.f. | - | + | - |
| Beta vulgaris L. | - | + | - |
| Pistacia atlantica Desf. | - | + | - |
| Pistacia lentiscus L. | + | - | - |
| Ammi visnaga (L.) Lam. | + | + | - |
| Anethum foeniculum L. | - | + | - |
| Apium graveolens L. | + | + | - |
| Carum carvi L. | - | + | + |
| Coriandrum sativum L. | + | + | - |
| Cuminum cyminum L. | - | + | - |
| Foeniculum vulgare Mill. | + | + | - |
| Petroselinum crispum (Mill.) Fuss | + | + | - |
| Pimpinella anisum L. | - | + | + |
| Ridolfia segetum (L.) Moris | + | - | - |
| Caralluma europaea (Guss.) N.E.Br. | + | + | - |
| Nerium oleander L. | + | + | - |
| Chamaerops humilis L. | - | + | - |
| Phoenix dactylifera L. | - | - | + |
| Asparagus albus L. | + | - | - |
| Achillea odorata L. | + | - | - |
| Achillea santolinoides Lag. | - | + | - |
| Artemisia absinthium L. | + | + | - |
| Artemisia campestris L. | - | + | - |
| Artemisia herba-alba Asso | + | + | + |
| Artemisia mesatlantica Maire | - | + | - |
| Chamaemelum mixtum (L.) Alloni | - | + | - |
| Chamaemelum nobile (L.) All. | + | + | - |
| Chrysanthemum coronarium L. | + | - | - |
| Cladanthus arabicus (L.) Cass. | + | - | - |
| Cynara cardunculus L. | + | + | - |
| Cynara cardunculus subsp. scolymus (L.) | + | + | - |
| Dittrichia viscosa (L.) Greuter | + | - | - |
| Lactuca sativa L. | - | + | - |
| Matricaria chamomilla L. | - | - | + |
| Pallenis spinosa (L.) Cass. | + | - | - |
| Saussurea costus (Falc.) Lipschitz | - | + | - |
| Scolymus hispanicus L. | - | + | - |
| Sonchus asper (L.) Hill | - | + | - |
| Sonchus tenerrimus L. | - | + | - |
| Silybum marianum L. | - | + | - |
| Tanacetum vulgare L. | + | - | - |
| Berberis vulgaris subsp. Australis (Boiss.) Heywood | - | + | - |
| Anastatica hierochuntica L. | - | + | + |
| Brassica oleracea L. | - | + | + |
| Brassica rapa L. | + | - | - |
| Eruca vesicaria (L.) Cav. | + | - | - |
| Lepidium sativum L. | + | + | + |
| Raphanus raphanistrum subsp. sativus (L.) | + | + | + |
| Boswellia sacra Flueck. | + | + | - |
| Opuntia ficus indica (L.) Mill. | - | + | - |
| Capparis spinosa L. | + | + | - |
| Cistus laurifolius L. | + | - | - |
| Cistus ladanifer L. | + | - | - |
| Atriplex halimus L. | - | + | - |
| Chenopodium ambrosioides L., | + | + | - |
| Ipomoea batatas (L.) | - | + | - |
| Bryonia dioica Jacq. | - | + | - |
| Citrullus colocynthis (L.) Schrad. | + | + | - |
| Citrullus vulgaris Schard. | + | + | - |
| Cucumis sativus L. | - | + | - |
| Cucurbita maxima Duchesne | + | - | - |
| Cucurbita pepo L. | - | + | - |
| Juniperus phoenicea L. | + | + | - |
| Juniperus oxycedrus L. | + | + | - |
| Tetraclinis articulata (Vahl) Mast. | + | + | - |
| Cyperus longus L. | + | - | - |
| Cyperus rotundus L. | - | + | - |
| Equisetum ramosissimum Desf | + | - | - |
| Arbutus unedo L. | + | - | - |
| Euphorbia officinarum subsp.echinus | - | + | - |
| Euphorbia officinarum L. | + | + | - |
| Euphorbia peplis L. | - | - | + |
| Euphorbia resinifera O. Berg | + | + | - |
| Mercurialis annua L. | + | + | - |
| Quercus suber L. | - | + | - |
| Quercus ilex L. | + | - | - |
| Centaurium spicatum (L.) Fritsch | + | - | - |
| Pelargonium roseum Willd. | + | - | - |
| Crocus sativus L. | - | + | + |
| Juglans regia L. | + | + | - |
| Ajuga iva (L.) Schreb. | - | - | + |
| Ballota hirsuta Benth | + | - | - |
| Calamintha officinalis Moench. | + | - | - |
| Calamintha alpina L | - | + | - |
| Lavandula angustifolia Mill | - | + | - |
| Lavandula dentata L. | - | + | - |
| Lavandula maroccana Murb. | + | - | - |
| Lavandula multifida L. | + | + | - |
| Lavandula stoechas L. | - | - | + |
| Marrubium vulgare L. | + | + | + |
| Mentha pulegium L. | + | + | - |
| Melissa officinalis L. | + | - | - |
| Mentha spicata L. | + | + | - |
| Mentha suaveolens Ehrh. | + | + | - |
| Ocimum basilicum L. | - | - | + |
| Origanum compactum Benth. | - | + | - |
| Origanum elongatum (Bonnet) | + | + | - |
| Origanum majorana L. | + | + | - |
| Origanum vulgare L | + | + | - |
| Rosmarinus officinalis L. | + | + | + |
| Salvia officinalis L. | + | + | - |
| Teucrium polium L. | + | + | - |
| Thymus broussonetii Boiss. | + | - | - |
| Thymus maroccanus Ball. | + | + | - |
| Thymus satureioides Coss. | + | + | - |
| Thymus vulgaris L. | - | + | - |
| Cinnamomum cassia (L.) J. Presl | + | - | - |
| Cinnamomum verum J. Presl | + | + | - |
| Laurus nobilis L. | - | + | - |
| Persea americana Mill. | + | + | - |
| Acacia gummifera Willd. | - | + | - |
| Acacia nilotica (L.) Delile | - | + | - |
| Acacia senegal (L.) Willd. | - | + | + |
| Acacia tortilis (Forssk.) Hayne | - | + | - |
| Acacia albida Delile | - | + | - |
| Anagyris foetida L. | - | + | - |
| Arachis hypogaea L. | - | + | - |
| Cassia absus L. | + | - | - |
| Cassia fistula L. | - | + | - |
| Ceratonia siliqua L. | + | + | - |
| Cicer arietinum L. | + | - | - |
| Glycine max (L.) Merr. | + | - | - |
| Glycyrrhiza glabra L | + | + | - |
| Lupinus albus L. | + | + | - |
| Lupinus angustifolius L. | - | + | - |
| Lupinus luteus L. | - | + | - |
| Medicago sativa L. | + | - | - |
| Ononis natrix L. | - | + | - |
| Phaseolus aureus Roxb. | - | + | - |
| Phaseolus vulgaris L. | + | + | - |
| Retama monosperma (L.) Boiss. | - | + | - |
| Retama raetam (Forssk.) Webb | - | + | - |
| Trigonella foenum-graecum L. | + | + | + |
| Vicia faba L. | + | - | - |
| Vigna radiata (L.) R. Wilczek | - | + | - |
| Linum usitatissimum L. | + | + | - |
| Punica granatum L. | - | + | - |
| Abelmoschus esculentus (L.) Moench | + | + | - |
| Hibiscus sabdariffa L. | + | - | - |
| Ficus carica L. | + | + | - |
| Ficus dottata Gasp. | - | - | + |
| Morus alba L. | - | + | - |
| Morus nigra L. | - | + | - |
| Myristica fragrans Houtt. | - | + | - |
| Eucalyptus camaldulensis Dehnh. | - | + | - |
| Eucalyptus globulus Labill. | + | - | - |
| Eugenia caryophyllata Thunb | + | - | - |
| Jasminum fruticans L. | + | + | - |
| Myrtus communis L. | - | + | - |
| Syzygium aromaticum L. | + | + | - |
| Peganum harmala L. | - | + | - |
| Olea europaea L. | + | + | + |
| Olea europaea subsp. maroccana | + | + | - |
| O. europea L. subsp. europaea var. sylvestris | + | + | - |
| O. oleaster Hoffm. & Link. | + | - | - |
| Fumaria officinalis L. | + | - | - |
| Plantago ovata Forssk. | - | + | - |
| Sesamum indicum L. | + | + | + |
| Globularia alypum L. | + | - | - |
| Avena sativa L. | + | + | - |
| Avena sterilis L. | + | - | - |
| Castellia tuberculosa Moris | + | - | - |
| Hordeum vulgare L. | + | + | - |
| Lolium perenne L. | - | - | + |
| Lolium rigidum Gaudin | - | + | - |
| Panicum miliaceum L. | - | + | - |
| Pennisetum glaucum L. | - | + | - |
| Phalaris canariensis L. | - | + | - |
| Sorghum bicolor L. | - | + | - |
| Triticum durum Desf. | + | + | - |
| Triticum aestivum L. | - | + | - |
| Triticum turgidum L. | - | + | - |
| Portulaca oleracea L. | + | - | - |
| Nigella Sativa L. | + | + | - |
| Ziziphus lotus L. | - | + | - |
| Ziziphus jujube Mill | - | + | - |
| Chaenomeles sinensis Dum.Cours. | - | + | - |
| Eriobotrya japonica Thunb. | - | + | - |
| Malus communis L. | + | - | - |
| Prunus armeniaca L. | + | - | - |
| Prunus dulcis Mill. | - | + | - |
| Rubus fruticosus var. vulgaris | - | + | - |
| Rubus fruticosus var. ulmifolius, (Schott) | - | - | + |
| Rubia tinctorum L. | - | + | - |
| Coffea arabica L. | + | + | - |
| Citrus medica var. limon L. | + | + | + |
| Citrus paradisi Macfad. | + | + | - |
| Citrus sinensis L. | - | + | - |
| Citrus aurantium L. | + | + | - |
| Ruta graveolens L. | + | - | - |
| Ruta chalepensis L. | - | + | - |
| Ruta montana L. | + | + | - |
| Salvadora persica L. | - | + | - |
| Viscum album L. | - | + | - |
| Argania spinosa L. | + | + | - |
| Illicium verum Hook.f. | - | + | - |
| Capsicum annuum L. | - | + | - |
| Lycopersicon esculentum Mill. | + | + | - |
| Solanum melongena L. | + | + | - |
| Withania frutescens L. | + | - | - |
| Taxus baccata L. | + | - | - |
| Camellia sinensis L. | + | + | - |
| Thymelaea hirsuta L. | + | + | - |
| Thymelaea tartonraira L. | - | + | - |
| Thymelaea virgata Desf. | + | - | - |
| Aquilaria malaccensis Lam | + | + | - |
| Urtica urens L. | + | - | - |
| Nardostachys jatamansi D. Don | + | - | - |
| Aloysia citriodora Palau | - | + | + |
| Verbena officinalis L. | + | - | - |
| Vitis vinifera L. | - | + | - |
| Aloe succotrina Lam. | + | - | + |
| Asphodelus microcarpus Salzm. & Viv. | - | - | + |
| Asphodelus tenuifolius Cav. | - | - | + |
| Zingiber officinale Roscoe. | + | + | - |
| Curcuma longa L. | - | + | - |
| Tetraena gaetula Emb. & Maire | - | - | + |
| Zygophyllum gaetulum Emb. &Maire | + | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutaj, H. A Comprehensive Review of Moroccan Medicinal Plants for Diabetes Management. Diseases 2024, 12, 246. https://doi.org/10.3390/diseases12100246
Boutaj H. A Comprehensive Review of Moroccan Medicinal Plants for Diabetes Management. Diseases. 2024; 12(10):246. https://doi.org/10.3390/diseases12100246
Chicago/Turabian StyleBoutaj, Hanane. 2024. "A Comprehensive Review of Moroccan Medicinal Plants for Diabetes Management" Diseases 12, no. 10: 246. https://doi.org/10.3390/diseases12100246
APA StyleBoutaj, H. (2024). A Comprehensive Review of Moroccan Medicinal Plants for Diabetes Management. Diseases, 12(10), 246. https://doi.org/10.3390/diseases12100246






