Is There a New Road to Spinal Cord Injury Rehabilitation? A Case Report about the Effects of Driving a Go-Kart on Muscle Spasticity
Abstract
:1. Introduction
2. Case Presentation
2.1. Patient’s Information
2.2. Materials and Methods
2.2.1. Go-Kart Driving Session
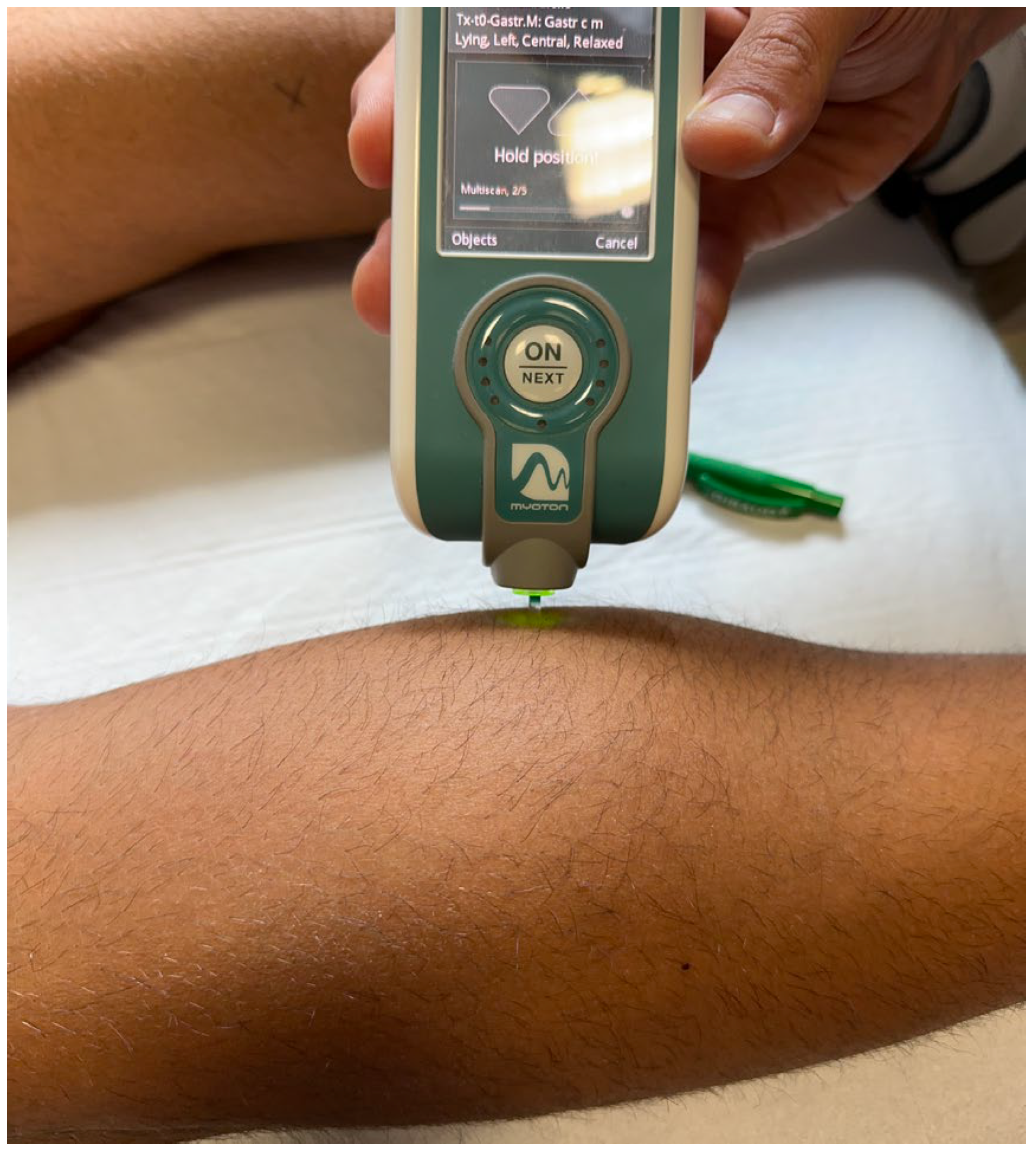
2.2.2. Spasticity Evaluation
3. Results
4. Discussion
4.1. WBV Hypothesis
4.2. Neuroendocrine Hypothesis
4.3. Limitations and Ideas for the Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jazayeri, S.B.; Beygi, S.; Shokraneh, F.; Hagen, E.M.; Rahimi-Movaghar, V. Incidence of traumatic spinal cord injury worldwide: A systematic review. Eur. Spine J. 2015, 24, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Nas, K.; Levent, Y.; Volkan, Ş.; Abdulkadir, A.; Kadriye, Ö. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Hohmann, G.W. Psychological aspects of treatment and rehabilitation of the spinal cord injured person. Clin. Orthop. Relat. Res. 1975, 112, 81–88. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Mariwalla, N.R.; Horn, E.M.; Tubbs, R.S.; Theodore, N. Absence of MRI soft tissue abnormalities in severe spinal cord injury in children: Case-based update. Childs Nerv. Syst. 2011, 27, 1369–1373. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Rahimi-Movaghar, V.; Sayyah, M.K.; Akbari, H.; Khorramirouz, R.; Rasouli, M.R.; Moradi-Lakeh, M.; Shokraneh, F.; Vaccaro, A.R. Epidemiology of traumatic spinal cord injury in developing countries: A systematic review. Neuroepidemiology 2013, 41, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.S.; Boateng, E.A.; Amponsah, A.K.; Kyei-Dompim, J.; Laari, T.T. Experiences of family caregivers of people with spinal cord injury at the neurosurgical units of the Komfo Anokye Teaching Hospital, Ghana. PLoS ONE 2023, 18, e0284436. [Google Scholar] [CrossRef]
- Maynard, F.M.; Karunas, R.S.; Waring, W.P., 3rd. Epidemiology of spasticity following traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 1990, 71, 566–569. [Google Scholar]
- Burke, D.; Ashby, P. Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? J. Neurol. Sci. 1972, 15, 321–326. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakravarty, A. Spasticity mechanisms—For the clinician. Front. Neurol. 2010, 1, 149. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Goodkin, D.E. Drugs used to treat spasticity. Drugs 2000, 59, 487–495. [Google Scholar] [CrossRef]
- McKay, W.B.; Sweatman, W.M.; Field-Fote, E.C. The experience of spasticity after spinal cord injury: Perceived characteristics and impact on daily life. Spinal Cord. 2018, 56, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Bensmail, D.; Yelni, A. Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews. Ann. Phys. Rehabil. Med. 2019, 62, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bovend’Eerdt, T.J.; Newman, M.; Barker, K.; Dawes, H.; Minelli, C.; Wade, D.T. The effects of stretching in spasticity: A systematic review. Arch. Phys. Med. Rehabil. 2008, 89, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, A.; Giovannini, S.; Finamore, P.; Loreti, C.; Vannetti, F.; Coraci, D.; Incalzi, R.A.; Zuccal, G.; Macchi, C.; Padua, L. Mugello Study Working Group. Muscle strength is related to mental and physical quality of life in the oldest old. Arch. Gerontol. Geriatr. 2020, 89, 104109. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; vanWegen, E.; van Peppen, R.; van der Wess, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Megna, M.; Marvulli, R.; Farì, G.; Gallo, G.; Dicuonzo, F.; Fiore, P.; Ianieri, G. Pain and Muscles Properties Modifications After Botulinum Toxin Type A (BTX-A) and Radial Extracorporeal Shock Wave (rESWT) Combined Treatment. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1127–1133. [Google Scholar] [PubMed]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Zhang, J.; Zhao, Y.; Shone, F.; Li, Z.; Frangi, A.F.; Xie, S.Q.; Zhang, Z.Q. Physics-Informed Deep Learning for Musculoskeletal Modeling: Predicting Muscle Forces and Joint Kinematics From Surface EMG. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 31, 484–493. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xiao, W.; Zhang, Z. Non-iterative and Fast Deep Learning: Multilayer Extreme Learning Machines. J. Frankl. Inst. 2020, 357, 8925–8955. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J. Neurosci. Methods. 1997, 74, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Jones, G. Psychological well-being in wheelchair sport participants and nonparticipants. Adapt. Phys. Act. Q. 1994, 11, 404–415. [Google Scholar] [CrossRef]
- McCullagh, R.; Fitzgerald, A.P.; Murphy, R.P.; Cooke, G. Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: A pilot study. Clin. Rehabil. 2007, 22, 206–2014. [Google Scholar] [CrossRef] [PubMed]
- Farì, G.; Megna, M.; Ranieri, M.; Agostini, F.; Ricci, V.; Bianchi, F.P.; Rizzo, L.; Farì, E.; Tognolo, L.; Bonavolontà, V.; et al. Could the Improvement of Supraspinatus Muscle Activity Speed up Shoulder Pain Rehabilitation Outcomes in Wheelchair Basketball Players? Int. J. Environ. Res. Public Health 2022, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour-Dehkordi, A.; Jivad, N. Comparison of regular aerobic and yoga on the quality of life in patients with multiple sclerosis. Med. J. Islam. Repub. Iran. 2014, 6, 28–141. [Google Scholar]
- Velikonja, O.; Čurić, K.; Ožura, A.; Jazbec, S.Š. Influence of sports climbing and yoga on spasticity, cognitive function, mood and fatigue in patients with multiple sclerosis. Clin. Neurol. Neurosurg. 2010, 112, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T. Measurement in neurological rehabilitation. Curr. Opin. Neurol. Neurosurg. 1992, 5, 682–686. [Google Scholar]
- Meseguer-Henarejos, A.B.; Sánchez-Meca, J.; López-Pina, J.A.; Carles-Hernández, R. Inter- and intra-rater reliability of the Modified Ashworth Scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Notarnicola, A.; Covelli, I.; Maccagnano, G.; Marvulli, R.; Mastromauro, L.; Ianieri, G.; Boodhoo, S.; Turitto, A.; Petruzzella, L.; Farì, G.; et al. Extracorporeal shockwave therapy on muscle tissue: The effects on healthy athletes. J. Biol. Regul. Homeost. Agents 2018, 32, 185–193. [Google Scholar]
- Lo, W.L.A.; Zhao, J.L.; Li, L.; Mao, Y.R.; Huang, D.F. Relative and Absolute Interrater Reliabilities of a Hand-Held Myotonometer to Quantify Mechanical Muscle Properties in Patients with Acute Stroke in an Inpatient Ward. BioMed Res. Int. 2017, 4294028. [Google Scholar] [CrossRef]
- Mullix, J.; Warner, M.; Stokes, M. Testing muscle tone and mechanical properties of rectus femoris and biceps femoris using a novel hand held MyotonPRO device: Relative ratios and reliability. Work. Pap. Health Sci. 2012, 1, 1–8. [Google Scholar]
- Nguyen, A.P.; Detrembleur, C.; Fisette, P.; Selves, C.; Mahaudens, P. MyotonPro Is a Valid Device for Assessing Wrist Biomechanical Stiffness in Healthy Young Adults. Front. Sports Act. Living 2022, 4, 797975. [Google Scholar] [CrossRef] [PubMed]
- Estes, S.; Iddings, J.A.; Ray, S.; Kirk- Sanchez, N.J.; Field-Fote, E.C. Comparison of Single-Session Dose Response Effects of Whole Body Vibration on Spasticity and Walking Speed in Persons with Spinal Cord Injury. Neurotherapeutics 2018, 15, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Alp, A.; Efe, B.; Adalı, M.; Bilgiç, A.; Demir Türe, S.; Coşkun, Ş.; Karabulut, M.; Ertem, U.; Günay, S.M. The Impact of Whole Body Vibration Therapy on Spasticity and Disability of the Patients with Poststroke Hemiplegia. Rehabil. Res. Pract. 2018, 2, 8637573. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Godaux, E. Mechanism of the vibration paradox: Excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J. Physiol. 1987, 285, 197–207. [Google Scholar] [CrossRef]
- Ritzmann, R.; Kramer, A.; Gruber, M.; Gollhofer, A.; Taube, W. EMG activity during whole body vibration: Motion artifacts or stretch reflexes? Eur. J. Appl. Physiol. 2010, 110, 143–151. [Google Scholar] [CrossRef]
- Kim, Y.; Youm, Y.; Wu, M.; Schmit, B.D. Modulation of flexor reflexes by static and dynamic hip proprioceptors in chronic human spinal cord injury. J. Clin. Neurosci. 2007, 14, 1078–1088. [Google Scholar] [CrossRef]
- Garatachea, N.; Jiménez, A.; Bresciani, G.; Marino, N.A.; Gonzalez-Gallego, J.; De Paz, J.A. The effects of movement velocity during squatting on energy expenditure and substrate utilization in whole-body vibration. J. Strength. Cond. Res. 2007, 21, 594–598. [Google Scholar]
- Hadi, S.C.; Delparte, J.J.; Hitzig, S.L.; Craven, B.C. Subjective experiences of men with and without spinal cord injury: Tolerability of the Juvent and WAVE whole body vibration plates. PM&R 2012, 4, 954–962. [Google Scholar]
- Herrero, A.J.; Menendez, H.; Gil, L.; Martìn, J.; Martìn, T.; Garcìa-Lòpez, D.; Gil-Agudo, A.; Marìn, P.J. Effects of wholebody vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord. 2011, 49, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ness, L.L.; Field-Fote, E.C. Whole-body vibration improves walking function in individuals with spinal cord injury: A pilot study. Gait Posture 2009, 30, 436–440. [Google Scholar] [CrossRef]
- Huang, M.; Liao, L.R.; Pang, M.Y. Effects of whole body vibration on muscle spasticity for people with central nervous system disorders: A systematic review. Clin. Rehabil. 2015, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schyns, F.; Paul, L.; Finlay, K.; Ferfuson, C.; Noble, E. Vibration therapy in multiple sclerosis: A pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin. Rehabil. 2009, 23, 771–781. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Whole-Body Vibration on Motor Impairments in Patients With Neurological Disorders: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Masani, K.; Alizadeh-Meghrazi, M.; Popovic, M.R.; Craven, B.C. Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci. Lett. 2010, 482, 66–70. [Google Scholar] [CrossRef]
- Shields, R.K. Muscular, skeletal, and neural adaptations following spinal cord injury. J. Orthop. Sports Phys. Ther. 2002, 32, 65–74. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sawatzky, B. Effects of vibration on spasticity in individuals with spinal cord injury: A scoping systematic review. Am. J. Phys. Med. Rehabil. 2014, 93, 995–1007. [Google Scholar] [CrossRef]
- Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. Int. J. Mol. Sci. 2021, 22, 11632. [Google Scholar] [CrossRef]
- Engfred, K.; Kjær, M.; Secher, N.H.; Friedman, D.B.; Hanel, B.; Nielsen, O.J.; Bach, F.W.; Galbo, H.; Levine, B.D. Hypoxia and training-induced adaptation of hormonal responses to exercise in humans. Eur. J. Appl. Physiol. 1994, 68, 303–309. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Frank, C.M.; Fobker, M.; Lechtermann, A.; Krueger, K.; Frome, A.; Korsukewitz, C. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef]
- Rosa, J.P.P.; Silva, A.; Rodrigues, D.F.; Menslin, R.; Araújo, L.T.; Vital, R.; Tufik, S.; Stieler, E.; de Mello, M.T. Association Between Hormonal Status, Stress, Recovery, and Motivation of Paralympic Swimmers. Res. Q. Exerc. Sport. 2020, 91, 652–661. [Google Scholar] [CrossRef]
- Pryce, G.; Baker, D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br. J. Pharmacol. 2007, 150, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen, A.C.; Staats, P.G.M.; Gorter, R.W.; Uitdehaag, B.M.J.; Polman, C.H. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002, 58, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Laezza, C.; Malfitano, A.M. From anecdotal evidence of cannabinoids in multiple sclerosis to emerging new therapeutical approaches. Mult. Scler. 2007, 13, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Araque, A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef]
- Maddock, R.J.; Casazza, G.A.; Buonocore, M.h.; Tanase, C. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): A dynamic 1H-MRS study. Neuroimage 2011, 57, 1324–1330. [Google Scholar] [CrossRef]
- Sjölund, B.H. Pain and rehabilitation after spinal cord injury: The case of sensory spasticity? Brain Res. Brain Res. Rev. 2002, 40, 250–256. [Google Scholar] [CrossRef]



| Muscle | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Right long adductor | 3 | 1+ | 2 | 3 |
| Left long adductor | 3 | 1+ | 1+ | 3 |
| Right femoral biceps | 3 | 1+ | 1+ | 3 |
| Left femoral biceps | 3 | 1+ | 1+ | 2 |
| Right gastrocnemius | 2 | 1+ | 2 | 2 |
| Left gastrocnemius | 2 | 1 | 1+ | 2 |
| Muscle | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Right long adductor | 16.8 Hz | 10.1 Hz | 14.5 Hz | 16 Hz |
| Left long adductor | 17.2 Hz | 8.6 Hz | 13.8 Hz | 16.8 Hz |
| Right femoral biceps | 16.8 Hz | 9.8 Hz | 13 Hz | 15.1 Hz |
| Left femoral biceps | 11.7 Hz | 7.3 Hz | 10.3 Hz | 11.2 Hz |
| Right medial gastrocnemius | 10.7 Hz | 6.5 Hz | 8.8 Hz | 10 Hz |
| Left medial gastrocnemius | 10 Hz | 6.1 Hz | 8.5 Hz | 9.7 Hz |
| Right lateral gastrocnemius | 24.6 Hz | 14 Hz | 20.4 Hz | 23 Hz |
| Left lateral gastrocnemius | 25.1 Hz | 15 Hz | 20.8 Hz | 24.2 Hz |
| Muscle | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Right long adductor | 403 N/m | 226 N/m | 300 N/m | 382 N/m |
| Left long adductor | 347 N/m | 205 N/m | 268 N/m | 293 N/m |
| Right femoral biceps | 223 N/m | 160 N/m | 181 N/m | 199 N/m |
| Left femoral biceps | 342 N/m | 202 N/m | 256 N/m | 322 N/m |
| Right medial gastrocnemius | 317 N/m | 273 N/m | 288 N/m | 300 N/m |
| Left medial gastrocnemius | 344 N/m | 304 N/m | 392 N/m | 333 N/m |
| Right lateral gastrocnemius | 397 N/m | 300 N/m | 362 N/m | 378 N/m |
| Left lateral gastrocnemius | 395 N/m | 286 N/m | 300 N/m | 384 N/m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farì, G.; Ranieri, M.; Marvulli, R.; Dell’Anna, L.; Fai, A.; Tognolo, L.; Bernetti, A.; Caforio, L.; Megna, M.; Losavio, E. Is There a New Road to Spinal Cord Injury Rehabilitation? A Case Report about the Effects of Driving a Go-Kart on Muscle Spasticity. Diseases 2023, 11, 107. https://doi.org/10.3390/diseases11030107
Farì G, Ranieri M, Marvulli R, Dell’Anna L, Fai A, Tognolo L, Bernetti A, Caforio L, Megna M, Losavio E. Is There a New Road to Spinal Cord Injury Rehabilitation? A Case Report about the Effects of Driving a Go-Kart on Muscle Spasticity. Diseases. 2023; 11(3):107. https://doi.org/10.3390/diseases11030107
Chicago/Turabian StyleFarì, Giacomo, Maurizio Ranieri, Riccardo Marvulli, Laura Dell’Anna, Annatonia Fai, Lucrezia Tognolo, Andrea Bernetti, Laura Caforio, Marisa Megna, and Ernesto Losavio. 2023. "Is There a New Road to Spinal Cord Injury Rehabilitation? A Case Report about the Effects of Driving a Go-Kart on Muscle Spasticity" Diseases 11, no. 3: 107. https://doi.org/10.3390/diseases11030107
APA StyleFarì, G., Ranieri, M., Marvulli, R., Dell’Anna, L., Fai, A., Tognolo, L., Bernetti, A., Caforio, L., Megna, M., & Losavio, E. (2023). Is There a New Road to Spinal Cord Injury Rehabilitation? A Case Report about the Effects of Driving a Go-Kart on Muscle Spasticity. Diseases, 11(3), 107. https://doi.org/10.3390/diseases11030107









