Mathematical Modelling of Tuberculosis Outbreak in an East African Country Incorporating Vaccination and Treatment
Abstract
1. Introduction
2. Model Formulation
3. Preliminaries
4. Non-Negativity and Boundedness of the Model Solution
5. Basic Reproduction Number Dynamics and Stability Analysis
5.1. Basic Reproduction Number
- ; this means that the infection will be able to start spreading in the population
- ; this means that the infection will not be able to start spreading in the population.
5.2. Global Stability
6. Existence and Uniqueness Analysis for Caputo Fractional Tuberculosis Outbreak Model
7. Quantitative Analysis
7.1. Data Fitting
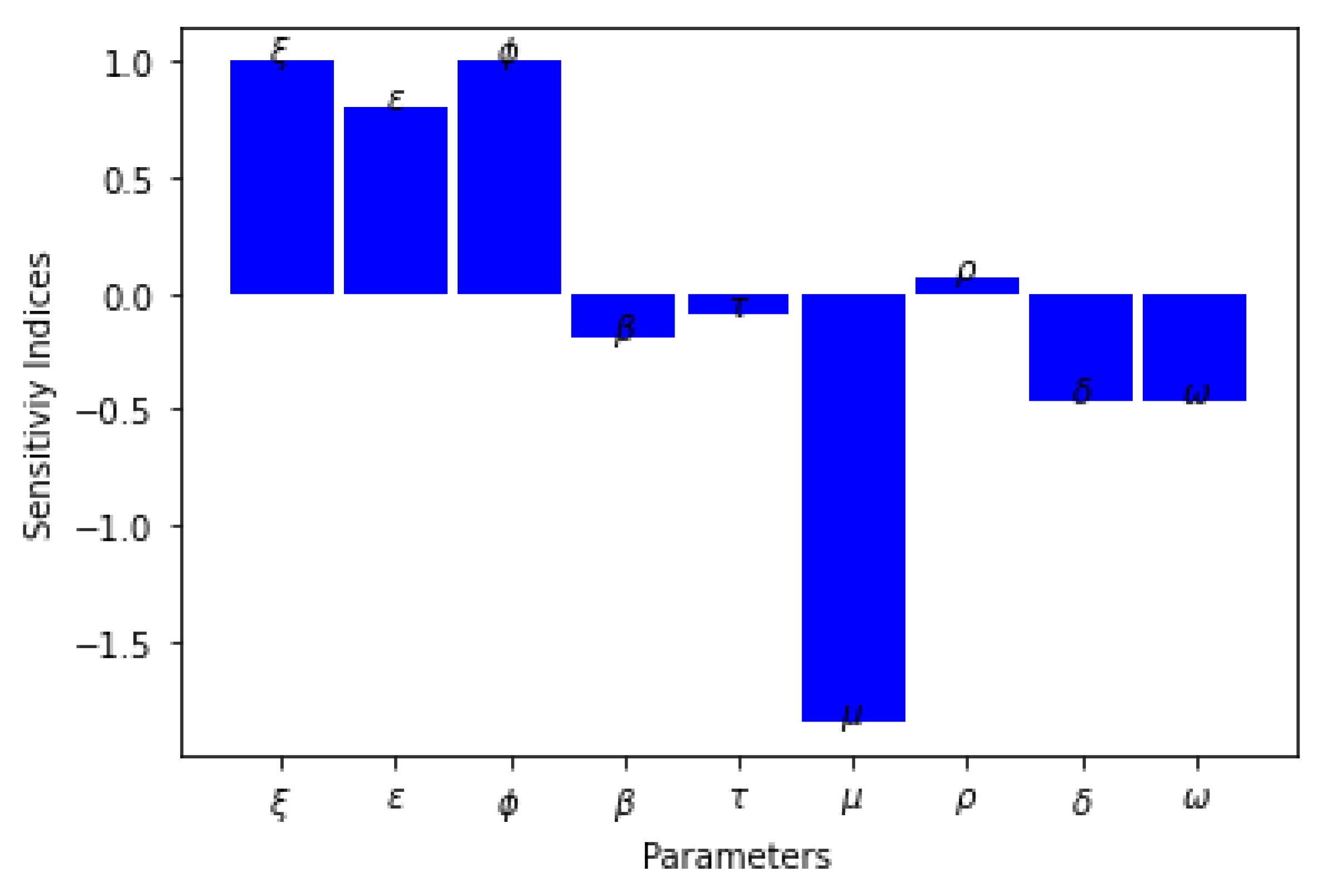
7.2. Sensitivity Analysis
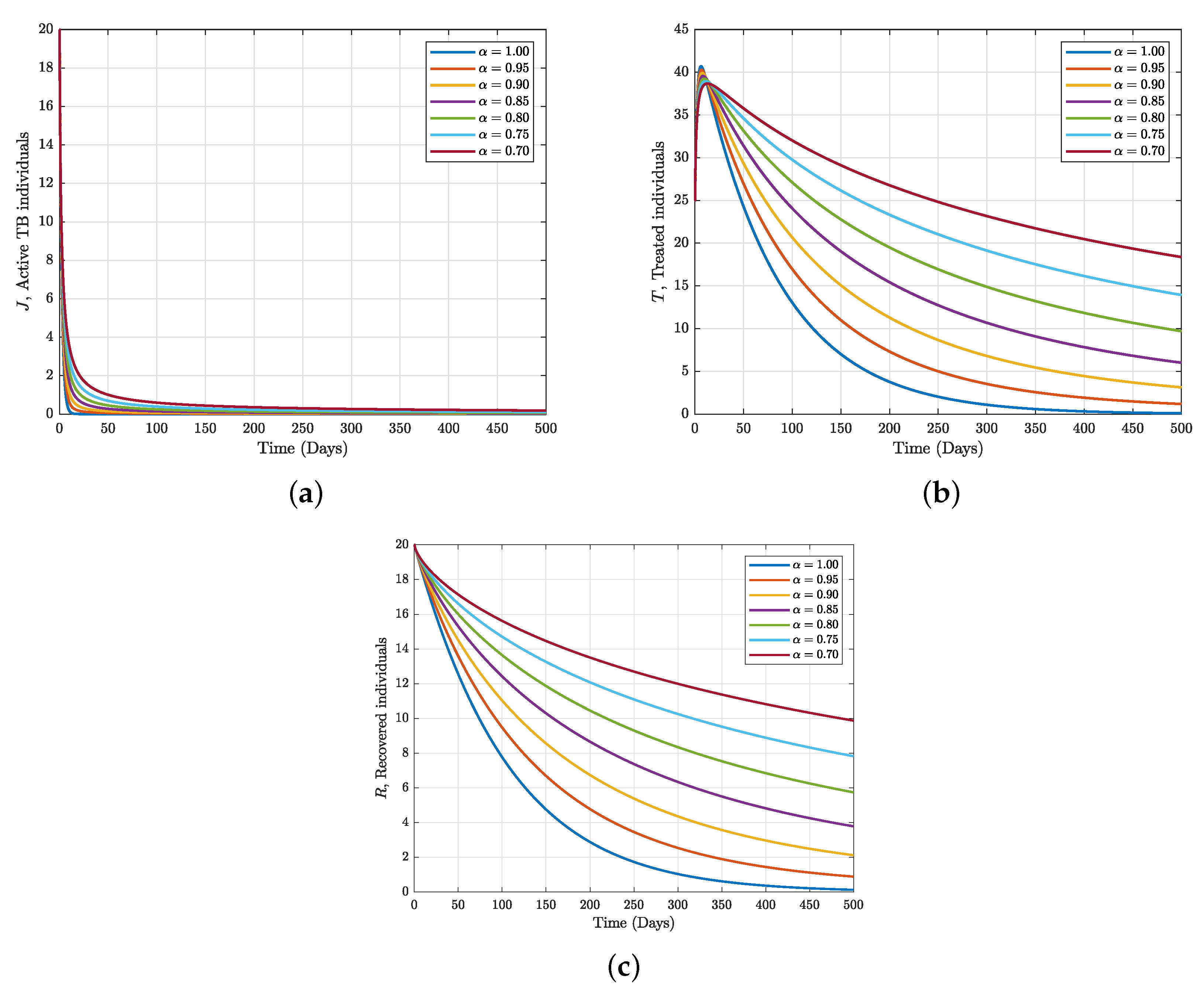
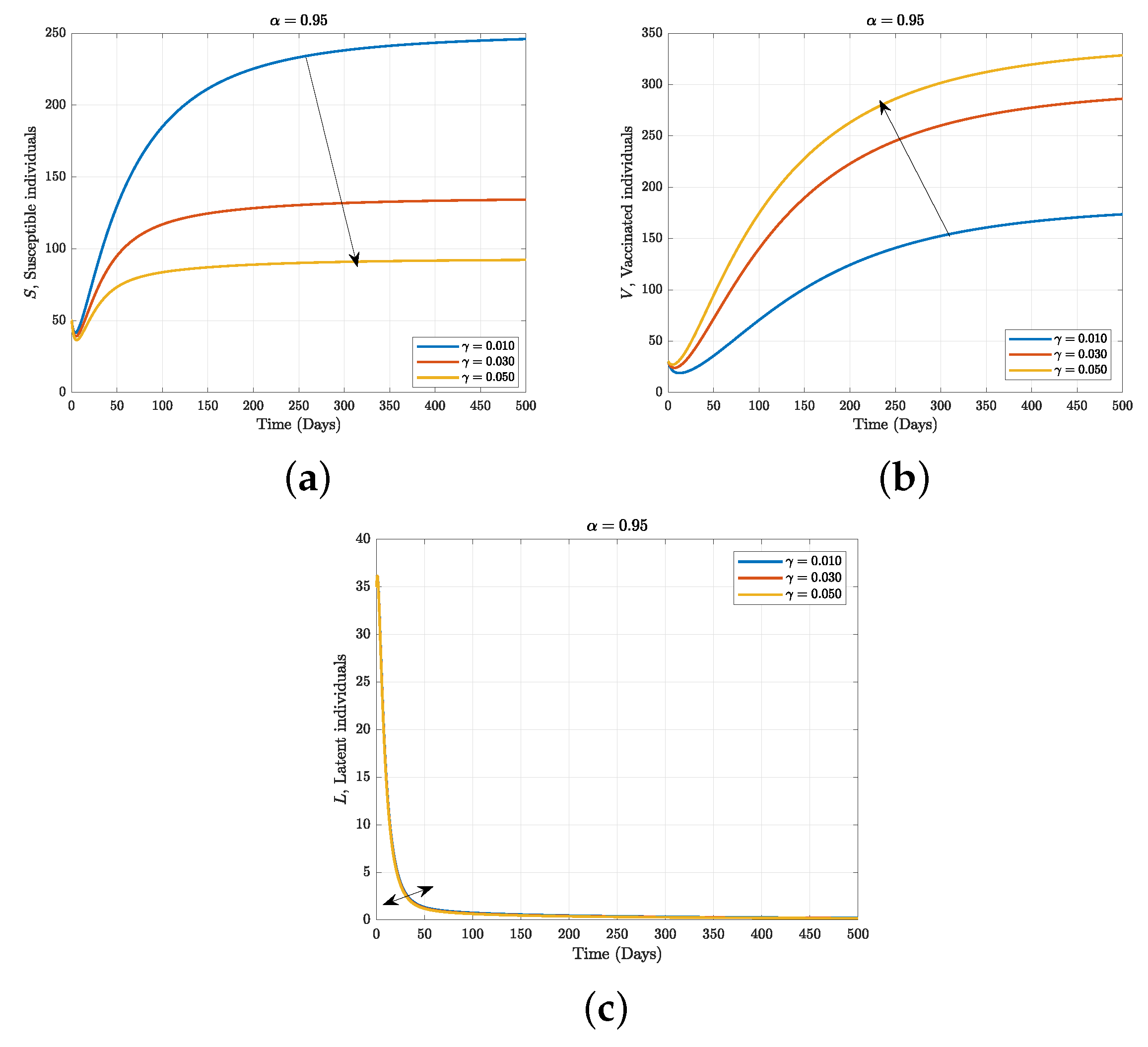
7.3. Numerical Simulation
8. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuberculosis Model, a Case Study of Tigania West, Kenya. Available online: https://www.researchgate.net/publication/308631904 (accessed on 2 January 2023).
- Molla, K.A.; Reta, M.A.; Ayene, Y.Y. Prevalence of multi drug-resistant tuberculosis in East Africa: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0270272. [Google Scholar] [CrossRef] [PubMed]
- Gichuki, J.; Mategula, D. Characterisation of tuberculosis mortality in informal settlements in Nairobi, Kenya: Analysis of data between 2002 and 2016. BMC Infect. Dis. 2021, 21, 718. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis Regional Factsheet. Available online: https://files.aho.afro.who.int/afahobckpcontainer/production/files/iAHO_TB_regional_Factsheet.pdf (accessed on 2 January 2023).
- Mnyambwa, N.P.; Philbert, D.; Kimaro, G.; Wandiga, S.; Kirenga, B.; Mmbaga, B.T.; Ngadaya, E. Gaps related to screening and diagnosis of tuberculosis in care cascade in selected health facilities in East Africa countries: A retrospective study. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 25, 100278. [Google Scholar] [CrossRef]
- Porous Border Blamed for TB Cases in Kenya and Ethiopia. Available online: https://www.theeastafrican.co.ke/tea/science-health/porous-border-blamed-for-tb-cases-in-kenya-and-ethiopia-3764090 (accessed on 2 January 2023).
- Tuberculosis in Kenya. Available online: https://www.tbonline.info/media/uploads/documents/guidelines-on-management-of-leprosy-and-tuberculosis-in-kenya- (accessed on 2 January 2023).
- Gakii, G.; Malonza, D. Mathematical Modeling of TB—HIV Co Infection, Case Study of Tigania West Sub County, Kenya. J. Adv. Math. Comput. Sci. 2018, 27, 1–18. [Google Scholar]
- Milligan, G.N.; Barrett, A.D. Vaccinology: An Essential Guide; Wiley Blackwell: Chichester, West Sussex, UK, 2015; p. 310. [Google Scholar]
- Fine, P.; Eames, K.; Heymann, D.L. Herd Immunity’: A Rough Guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; ur Mati, R.; Ahmad, S.; Baleanu, D.; Anjam, Y.N. A new fractional infectious disease model under the non-singular Mittag–Leffler derivative. Waves Random Complex Media 2022, 1–27. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Sayed, A.M.A.; El-Saka, H.A. Equilibrium points, stability and numerical solutions of fractional-order predator prey and rabies models. J. Math. Anal. Appl. 2007, 325, 542–553. [Google Scholar] [CrossRef]
- Ojo, M.M.; Peter, O.J.; Goufo, E.F.D.; Panigoro, H.S.; Oguntolu, F.A. Mathematical model for control of tuberculosis epidemiology. J. Appl. Math. Comput. 2022, 69, 1865–2085. [Google Scholar] [CrossRef]
- Adewale, S.O.; Podder, C.N.; Gumel, A.B. Mathematical analysis of a TB transmission model with DOTS. Can. Appl. Math. Q. 2009, 17, 1–36. [Google Scholar]
- Gomes, M.G.M.; Aguas, R.; Lopes, J.S.; Nunes, M.C.; Rebelo, C.; Rodrigues, P.; Struchiner, C.J. How host heterogeneity governs tuberculosis reinfection? Proc. Roy. Soc. B-Biol. Sci. 2012, 279, 2473–2478. [Google Scholar] [CrossRef]
- Sterne, J.; Rodrigues, L.; Guedes, I. Does the efficacy of BCG decline with time since vaccination. Int. J. Tuberc. Lung Dis. 2022, 2, 200–207. [Google Scholar]
- GHO|By Category|BCG—Immunization Coverage Estimates by Country. Retrieved 25 March 2023. 2023. Available online: https://apps.who.int/gho/data/view.main.80500?lang=en (accessed on 2 April 2023).
- Kasereka Kabunga, S.; Doungmo Goufo, E.F.; Ho Tuong, V. Analysis and simulation of a mathematical model of tuberculosis transmission in Democratic Republic of the Congo. Adv. Differ. Equ. 2020, 2020, 642. [Google Scholar] [CrossRef]
- Okuonghae, D.; Ikhimwin, B.O. Dynamics of a Mathematical Model for Tuberculosis with Variability in Susceptibility and Disease Progressions Due to Difference in Awareness Level. Front Microbiol. 2016, 2016, 1530. [Google Scholar] [CrossRef]
- Nayeem, J.; Sultana, I. Mathematical Analysis of the Transmission Dynamics of Tuberculosis. Am. J. Comput. Math. 2019, 9, 158–173. [Google Scholar] [CrossRef]
- Mekonen, K.G.; Balcha, S.F.; Obsu, L.L.; Hassen, A. Mathematical Modeling and Analysis of TB and COVID-19 Coinfection. J. Appl. Math. 2022, 2022, 2449710. [Google Scholar] [CrossRef]
- Inayaturohmat, F.; Anggriani, N.; Supriatna, A.K. A mathematical model of tuberculosis and COVID-19 coinfection with the effect of isolation and treatment. Front. Appl. Math. 2022, 8, 2297–4687. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.-Y.; Ryu, H.; Kwon, J.-W. Age-Specific Mathematical Model for Tuberculosis Transmission Dynamics in South Korea. Mathematics 2021, 9, 804. [Google Scholar] [CrossRef]
- Addai, E.; Adeniji, A.; Peter, O.J.; Agbaje, J.O.; Oshinubi, K. Dynamics of Age-Structure Smoking Models with Government Intervention Coverage under Fractal-fractional-order derivatives. Fractal Fract. 2023, 7, 370. [Google Scholar] [CrossRef]
- Almeida, R.A. Caputo fractional derivative of a function with respect to another function. Communic Nonline Sci. Nume. Simul. 2017, 44, 460–481. [Google Scholar] [CrossRef]
- Khalil, R.; Horani, M.A.; Yousef, A.; Sababheh, M. A new definition of fractional derivative. J. Comput. Appl. Math. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Scott, A.C. Encyclopedia of Nonlinear Science; Routledge, Taylor and Francis Group: New York, NY, USA, 2005. [Google Scholar]
- Sousa, J.; de Oliveira, E.C. A new truncated M-fractional derivative type unifying some fractional derivative types with classical properties. Int. J. Anal. Appl. 2018, 16, 83–96. [Google Scholar]
- Jumarie, G. Modified Riemann Liouville derivative and fractional Taylor series of no-differentiable functions further results. Comput. Math. Appl. 2006, 51, 1367–1376. [Google Scholar]
- Caputo, M.; Fabrizio, M. A new definition of fractional derivative without singular kernel. Prog. Fract. Differ. Appl. 2015, 1, 73–85. [Google Scholar]
- Atangana, A.; Baleanu, D. New fractional derivative without non-local and non-singular kernel: Theory and application to heat transfer model. Therm. Sci. 2016, 20, 763–769. [Google Scholar] [CrossRef]
- Zhang, L.; Addai, E.; Ackora-Prah, J.; Dissou Arthur, Y.; Asamoah, J.K.K. Fractional-Order Ebola-Malaria Coinfection Model with a Focus on Detection and Treatment Rate. Comput. Math. Methods Med. 2022, 2022, 6502598. [Google Scholar] [CrossRef]
- Ngungu, M.; Addai, E.; Adeniji, A.; Adam, U.M.; Oshinubi, K. Mathematical epidemiological modeling and analysis of monkeypox dynamism with nonpharmaceutical intervention using real data from United Kingdom. Front. Public Health 2023, 11, 1101436. [Google Scholar] [CrossRef]
- Addai, E.; Zhang, L.; Preko, A.K.; Asamoah, J.K.K. Fractional order epidemiological model of SARS-CoV-2 dynamism involving Alzheimer’s disease. Healthc. Anal. 2022, 2, 2100114. [Google Scholar] [CrossRef]
- Asamoah, J.K.K.; Okyere, E.; Yankson, E.; Opoku, A.A.; Adom-Konadu, A.; Acheampong, E.; Dissou Arthur, Y. Non-fractional and fractional mathematical analysis and simulations for Q fever. Chaos Solitons Fractals 2022, 156, 111821. [Google Scholar] [CrossRef]
- Baba, I.A. Existence and uniqueness of a fractional order tuberculosis model. Eur. Phys. J. Plus 2019, 134, 489. [Google Scholar] [CrossRef]
- Higazy, M.; Alyami, M.A. New Caputo–Fabrizio fractional order SEIASqEqHR model for COVID-19 epidemic transmission with genetic algorithm based control strategy. Alex. Eng. J. 2020, 59, 4719–4736. [Google Scholar] [CrossRef]
- Owolabi, K.M.; Atangana, A. Mathematical modelling and analysis of fractional epidemic models using derivative with exponential kernel. In Fractional Calculus in Medical and Health Science; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–128. [Google Scholar]
- Djida, J.D.; Atangana, A. More generalized groundwater model with space-time Caputo Fabrizio fractional differentiation. Numer. Methods Partial Differ. Equ. 2017, 33, 1616–1627. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, D.; Qurashi, M.A.; Baleanu, D. A new fractional model for giving up smoking dynamics. Adv. Differ. Equ. 2017, 2017, 88. [Google Scholar] [CrossRef]
- Ain, Q.T.; Anjum, N.; Din, A.; Zeb, A.; Djilali, S.; Khan, Z.A. On the analysis of Caputo fractional order dynamics of Middle East Lungs Coronavirus (MERS-CoV) model. Alex. Eng. J. 2022, 61, 5123–5131. [Google Scholar] [CrossRef]
- Mustapha, U.T.; Qureshi, S.; Yusuf, A.; Hincal, E. Fractional modeling for the spread of Hookworm infection under Caputo operator. Chaos Solitons Fractals 2020, 137, 109878. [Google Scholar] [CrossRef]
- Ahmed, I.; Baba, I.A.; Yusuf, A.; Kumam, P.; Kumam, W. Analysis of Caputo fractional-order model for COVID-19 with lockdown. Adv. Differ. Equ. 2020, 2020, 394. [Google Scholar] [CrossRef]
- Mahatekar, Y.; Scindia, P.S.; Kumar, P. A new numerical method to solve fractional differential equations in terms of Caputo–Fabrizio derivatives. Phys. Scr. 2023, 98, 024001. [Google Scholar] [CrossRef]




| Variables | Description |
|---|---|
| Susceptible individuals | |
| Vaccinated individuals | |
| Latent individuals | |
| Active TB individuals | |
| Treated individuals | |
| Recovered individuals | |
| Parameters | Description |
| Recruitment rate of individuals in susceptible classes | |
| Vaccine rate | |
| Vaccine wane rate | |
| Contact rate | |
| Efficacy of the vaccine | |
| Natural death rate | |
| Diseases induced death rate | |
| Progression rate from latent to active TB | |
| Treatment failure rate | |
| Movement rate of individuals in the treated class | |
| Recovery rate of treated individuals | |
| Rate of treatment for active TB individuals |
| Parameters | Values | References | Units |
|---|---|---|---|
| 5 | [13] | number of persons | |
| 0.1 | [16] | persons vaccinated/N | |
| 0.067, 0.1 | [13] | persons loss of immunity/N | |
| 0.6501 | fitted | 1/days 1/persons | |
| 1.6583 | fitted | 1/days 1/persons | |
| 1/67.7 | fitted | 1/days | |
| 0.1 | [13] | 1/days | |
| 0.00375 | [13] | 1/days 1/persons | |
| 0–1 | [14,15] | 1/days 1/persons | |
| 0–1 | assumed | 1/days 1/persons | |
| 0.01 | [13] | 1/days | |
| 0.1 | [13] | 1/days |
| Parameters | Sensitivity Index |
|---|---|
| 1 | |
| 0.7975 | |
| 1 | |
| 0.0729 | |
| −1.8502 | |
| −0.0889 | |
| −0.1977 | |
| −0.4656 | |
| −0.4656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshinubi, K.; Peter, O.J.; Addai, E.; Mwizerwa, E.; Babasola, O.; Nwabufo, I.V.; Sane, I.; Adam, U.M.; Adeniji, A.; Agbaje, J.O. Mathematical Modelling of Tuberculosis Outbreak in an East African Country Incorporating Vaccination and Treatment. Computation 2023, 11, 143. https://doi.org/10.3390/computation11070143
Oshinubi K, Peter OJ, Addai E, Mwizerwa E, Babasola O, Nwabufo IV, Sane I, Adam UM, Adeniji A, Agbaje JO. Mathematical Modelling of Tuberculosis Outbreak in an East African Country Incorporating Vaccination and Treatment. Computation. 2023; 11(7):143. https://doi.org/10.3390/computation11070143
Chicago/Turabian StyleOshinubi, Kayode, Olumuyiwa James Peter, Emmanuel Addai, Enock Mwizerwa, Oluwatosin Babasola, Ifeoma Veronica Nwabufo, Ibrahima Sane, Umar Muhammad Adam, Adejimi Adeniji, and Janet O. Agbaje. 2023. "Mathematical Modelling of Tuberculosis Outbreak in an East African Country Incorporating Vaccination and Treatment" Computation 11, no. 7: 143. https://doi.org/10.3390/computation11070143
APA StyleOshinubi, K., Peter, O. J., Addai, E., Mwizerwa, E., Babasola, O., Nwabufo, I. V., Sane, I., Adam, U. M., Adeniji, A., & Agbaje, J. O. (2023). Mathematical Modelling of Tuberculosis Outbreak in an East African Country Incorporating Vaccination and Treatment. Computation, 11(7), 143. https://doi.org/10.3390/computation11070143










