Recent and Future Advances in the Chemoenzymatic Synthesis of Homogeneous Glycans for Bacterial Glycoconjugate Vaccine Development
Abstract
1. Introduction
2. Chemoenzymatic Synthesis of Meningococcal Oligosaccharides
2.1. N. meningitidis Serogroups A and X
2.2. Neisseria meningitidis Serogroup C
2.3. Neisseria meningitidis Serogroup W
3. Enzymatic Synthesis of Oligosaccharides from Antibiotic-Resistant Bacteria
3.1. Highest Priority: Mycobacterium tuberculosis
3.2. Critical Priority: Klebsiella pneumoniae
4. Future Directions
4.1. Computational Methods: Molecular Dynamics Simulations of Antigens
4.2. Directed Evolution of Glycosyltransferases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of vaccines; health, economic and social perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Fernández-Tejada, A.; Barjaktarović, Ž.; Bouzalas, I.; Brimnes, J.; Chernysh, S.; Gizurarson, S.; Gursel, I.; Jakopin, Ž.; Lawrenz, M.; et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol. Rev. 2020, 296, 169–190. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate immune adjuvants in subunit vaccines. Pharmaceutics 2020, 12, 965. [Google Scholar] [CrossRef]
- Wang, P. Natural and synthetic saponins as vaccine adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Bonam, S.R.; Bhunia, D.; Muller, S.; Nerella, S.G.; Alvala, M.; Mahabalarao, S.K.H. Novel trisaccharide based phospholipids as immunomodulators. Int. Immunopharmacol. 2019, 74, 105684. [Google Scholar] [CrossRef]
- Reina, J.J.; Díaz, I.; Nieto, P.M.; Campillo, N.E.; Páez, J.A.; Tabarani, G.; Fieschi, F.; Rojo, J. Docking, synthesis, and NMR studies of mannosyl trisaccharide ligands for DC-SIGN lectin. Org. Biomol. Chem. 2008, 6, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.; Cuccui, J.; Wren, B.W. Recent advances in the production of recombinant glycoconjugate vaccines. Npj Vaccines 2019, 4, 16. [Google Scholar] [CrossRef]
- Seeberger, P.H.; Werz, D.B. Synthesis and medical applications of oligosaccharides. Nature 2007, 446, 1046–1051. [Google Scholar] [CrossRef]
- Li, W.; McArthur, J.B.; Chen, X. Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res. 2019, 472, 86–97. [Google Scholar] [CrossRef]
- Stephens, D.S. Conquering the meningococcus. FEMS Microbiol. Rev. 2007, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.C.; Sharyan, A.; Sheikhi Moghaddam, L. Meningococcal vaccines: Current status and emerging strategies. Vaccines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772. [Google Scholar]
- Sharyan, A.; Gonzalez, C.; Ukaegbu, O.; Powell, K.; McCarthy, P.C. Determination of the binding affinities of Neisseria meningitidis serogroup w capsule polymerase with two nucleotide sugar substrates. BMC Res. Notes 2018, 11, 482. [Google Scholar] [CrossRef]
- Romanow, A.; Keys, T.G.; Stummeyer, K.; Freiberger, F.; Henrissat, B.; Gerardy-Schahn, R. Dissection of hexosyl- and sialyltransferase domains in the bifunctional capsule polymerases from Neisseria meningitidis w and y defines a new sialyltransferase family. J. Biol. Chem. 2014, 289, 33945–33957. [Google Scholar] [CrossRef]
- Romanow, A.; Haselhorst, T.; Stummeyer, K.; Claus, H.; Bethe, A.; Mühlenhoff, M.; Vogel, U.; von Itzstein, M.; Gerardy-Schahn, R. Biochemical and biophysical characterization of the sialyl-/hexosyltransferase synthesizing the meningococcal serogroup w135 heteropolysaccharide capsule. J. Biol. Chem. 2013, 288, 11718–11730. [Google Scholar] [CrossRef] [PubMed]
- Muindi, K.M.; McCarthy, P.C.; Wang, T.; Vionnet, J.; Battistel, M.; Jankowska, E.; Vann, W.F. Characterization of the meningococcal serogroup x capsule n-acetylglucosamine-1-phosphotransferase. Glycobiology 2014, 24, 139–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosley, S.L.; Rancy, P.C.; Peterson, D.C.; Vionnet, J.; Saksena, R.; Vann, W.F. Chemoenzymatic synthesis of conjugatable oligosialic acids. Biocatal. Biotransform. 2010, 28, 41–50. [Google Scholar] [CrossRef]
- Ming, S.A.; Cottman-Thomas, E.; Black, N.C.; Chen, Y.; Veeramachineni, V.; Peterson, D.C.; Chen, X.; Tedaldi, L.M.; Wagner, G.K.; Cai, C.; et al. Interaction of Neisseria meningitidis group x n-acetylglucosamine-1-phosphotransferase with its donor substrate. Glycobiology 2018, 28, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.A.; Caro, N.C.; Lanz, N.; Vionnet, J.; Vann, W.F. Effect of acceptor chain length and hydrophobicity on polymerization kinetics of the Neisseria meningitidis group c polysialyltransferase. Biochemistry 2019, 58, 679–686. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Muthana, S.M.; Freedberg, D.I.; Chen, X. Size-controlled chemoenzymatic synthesis of homogeneous oligosaccharides of Neisseria meningitidis w capsular polysaccharide. ACS Catal. 2020, 10, 2791–2798. [Google Scholar] [CrossRef]
- Fiebig, T.; Freiberger, F.; Pinto, V.; Romano, M.R.; Black, A.; Litschko, C.; Bethe, A.; Yashunsky, D.; Adamo, R.; Nikolaev, A.; et al. Molecular cloning and functional characterization of components of the capsule biosynthesis complex of Neisseria meningitidis serogroup a: Toward in vitro vaccine production. J. Biol. Chem. 2014, 289, 19395–19407. [Google Scholar] [CrossRef]
- Fiebig, T.; Berti, F.; Freiberger, F.; Pinto, V.; Claus, H.; Romano, M.R.; Proietti, D.; Brogioni, B.; Stummeyer, K.; Berger, M.; et al. Functional expression of the capsule polymerase of Neisseria meningitidis serogroup x: A new perspective for vaccine development. Glycobiology 2014, 24, 150–158. [Google Scholar] [CrossRef]
- Claus, H.; Stummeyer, K.; Batzilla, J.; Mühlenhoff, M.; Vogel, U. Amino acid 310 determines the donor substrate specificity of serogroup w-135 and y capsule polymerases of Neisseria meningitidis. Mol. Microbiol. 2009, 71, 960–971. [Google Scholar] [CrossRef]
- Fiebig, T.; Litschko, C.; Freiberger, F.; Bethe, A.; Berger, M.; Gerardy-Schahn, R. Efficient solid-phase synthesis of meningococcal capsular oligosaccharides enables simple and fast chemoenzymatic vaccine production. J. Biol. Chem. 2018, 293, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Oldrini, D.; Fiebig, T.; Romano, M.R.; Proietti, D.; Berger, M.; Tontini, M.; De Ricco, R.; Santini, L.; Morelli, L.; Lay, L.; et al. Combined chemical synthesis and tailored enzymatic elongation provide fully synthetic and conjugation-ready Neisseria meningitidis serogroup x vaccine antigens. ACS Chem. Biol. 2018, 13, 984–994. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.C.; Saksena, R.; Peterson, D.C.; Lee, C.H.; An, Y.; Cipollo, J.F.; Vann, W.F. Chemoenzymatic synthesis of immunogenic meningococcal group c polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj. J. 2013, 30, 857–870. [Google Scholar] [CrossRef]
- Li, R.; Kooner, A.S.; Muthana, S.M.; Yuan, Y.; Yu, H.; Chen, X. A chemoenzymatic synthon strategy for synthesizing n-acetyl analogues of o-acetylated N. meningitidis w capsular polysaccharide oligosaccharides. J. Org. Chem. 2020, 85, 16157–16165. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.A.; Potts, C.; Blain, A.E.; Retchless, A.C.; Reese, N.; Swint, S.; Lonsway, D.; Karlsson, M.; Lunquest, K.; Sweitzer, J.J.; et al. Detection of ciprofloxacin-resistant, β-lactamase-producing Neisseria meningitidis serogroup y isolates—United States, 2019–2020. Morb. Mortal. Wkly. Rep. 2020, 69, 735–739. [Google Scholar] [CrossRef]
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The threat of antimicrobial resistance on the human microbiome. Microb. Ecol. 2017, 74, 1001–1008. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Danieli, E.; Proietti, D.; Brogioni, G.; Romano, M.R.; Cappelletti, E.; Tontini, M.; Berti, F.; Lay, L.; Costantino, P.; Adamo, R. Synthesis of staphylococcus aureus type 5 capsular polysaccharide repeating unit using novel l-fucnac and d-fucnac synthons and immunochemical evaluation. Bioorg. Med. Chem. 2012, 20, 6403–6415. [Google Scholar] [CrossRef]
- Hagen, B.; van Dijk, J.H.M.; Zhang, Q.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Synthesis of the Staphylococcus aureus strain m capsular polysaccharide repeating unit. Org. Lett. 2017, 19, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Kaplonek, P.; Khan, N.; Reppe, K.; Schumann, B.; Emmadi, M.; Lisboa, M.P.; Xu, F.F.; Calow, A.D.J.; Parameswarappa, S.G.; Witzenrath, M.; et al. Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc. Natl. Acad. Sci. USA 2018, 115, 13353–13358. [Google Scholar] [CrossRef]
- Verez-Bencomo, V.; Fernández-Santana, V.; Hardy, E.; Toledo, M.E.; Rodríguez, M.C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004, 305, 522–525. [Google Scholar] [CrossRef]
- Hu, Z.; Bongat White, A.F.; Mulard, L.A. Efficient iterative synthesis of o-acetylated tri- to pentadecasaccharides related to the lipopolysaccharide of Shigella flexneri type 3 a through di- and trisaccharide glycosyl donors. Chem. Asian J. 2017, 12, 419–439. [Google Scholar] [CrossRef]
- Mitra, A.; Mukhopadhyay, B. Linear synthesis of the hexasaccharide related to the repeating unit of the o-antigen from Shigella flexneri serotype 1d (i: 7,8). Carbohydr. Res. 2016, 426, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Shiloach, J.; Schneerson, R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei o-specific oligosaccharide-core-protein conjugates. Proc. Natl. Acad. Sci. USA 2009, 106, 7974–7978. [Google Scholar] [CrossRef]
- WHO Global Tuberculosis Report 2020. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020 (accessed on 10 August 2021).
- Moliva, J.I.; Turner, J.; Torrelles, J.B. Prospects in Mycobacterium bovis Bacille Calmette et Guérin (BCG) vaccine diversity and delivery: Why does BCG fail to protect against tuberculosis? Vaccine 2015, 33, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bavaro, T.; Tengattini, S.; Bernardini, R.; Mattei, M.; Annunziata, F.; Cole, R.B.; Zheng, C.; Sollogoub, M.; Tamborini, L.; et al. Chemoenzymatic synthesis of arabinomannan (am) glycoconjugates as potential vaccines for tuberculosis. Eur. J. Med. Chem. 2020, 204, 112578. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Besra, G.S. Synthesis and recycling of the mycobacterial cell envelope. Curr. Opin. Microbiol. 2021, 60, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Britton, J.; Jamison, T.F. The assembly and use of continuous flow systems for chemical synthesis. Nat. Protoc. 2017, 12, 2423–2446. [Google Scholar] [CrossRef]
- Panza, M.; Pistorio, S.G.; Stine, K.J.; Demchenko, A.V. Automated chemical oligosaccharide synthesis: Novel approach to traditional challenges. Chem. Rev. 2018, 118, 8105–8150. [Google Scholar] [CrossRef]
- Gao, Q.; Shen, Z.; Qin, J.; Liu, Y.; Li, M. Antimicrobial resistance and pathogenicity determination of community-acquired hypervirulent Klebsiella pneumoniae. Microb. Drug Resist. 2020, 26, 1195–1200. [Google Scholar] [CrossRef]
- Feldman, M.F.; Bridwell, A.E.M.; Scott, N.E.; Vinogradov, E.; McKee, S.R.; Chavez, S.M.; Twentyman, J.; Stallings, C.L.; Rosen, D.A.; Harding, C.M. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 18655–18663. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Feldman, M.F. Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in e. Coli. Glycobiology 2019, 29, 519–529. [Google Scholar] [CrossRef]
- Ravenscroft, N.; Braun, M.; Schneider, J.; Dreyer, A.M.; Wetter, M.; Haeuptle, M.A.; Kemmler, S.; Steffen, M.; Sirena, D.; Herwig, S.; et al. Characterization and immunogenicity of a Shigella flexneri 2a o-antigen bioconjugate vaccine candidate. Glycobiology 2019, 29, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Owen, S.; Ravenscroft, N.; Kuttel, M.M. Molecular modeling of the Shigella flexneri serogroup 3 and 5 o-antigens and conformational relationships for a vaccine containing serotypes 2a and 3a. Vaccines 2020, 8, 643. [Google Scholar] [CrossRef]
- Kuttel, M.M.; Berti, F.; Ravenscroft, N. Molecular modeling provides insights into the loading of sialic acid-containing antigens onto crm (197): The role of chain flexibility in conjugation efficiency and glycoconjugate architecture. Glycoconj. J. 2021, 38, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Henriques, P.; Iacono, L.D.; Gimeno, A.; Biolchi, A.; Romano, M.R.; Arda, A.; Bernardes, G.J.L.; Jimenez-Barbero, J.; Berti, F.; Rappuoli, R.; et al. Structure of a protective epitope reveals the importance of acetylation of neisseria meningitidis serogroup a capsular polysaccharide. Proc. Natl. Acad. Sci. USA 2020, 117, 29795–29802. [Google Scholar] [CrossRef]
- Zhang, Q.; Gimeno, A.; Santana, D.; Wang, Z.; Valdés-Balbin, Y.; Rodríguez-Noda, L.M.; Hansen, T.; Kong, L.; Shen, M.; Overkleeft, H.S.; et al. Synthetic, zwitterionic sp1 oligosaccharides adopt a helical structure crucial for antibody interaction. ACS Cent. Sci. 2019, 5, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Yang, M.; Ramachandran, G.; Curtis, B.; Shridhar, S.; Laufer, R.S.; Wang, J.Y.; Van Druff, J.; Higginson, E.E.; Hegerle, N.; et al. Development of a glycoconjugate vaccine to prevent invasive Salmonella typhimurium infections in sub-saharan africa. PLoS Negl. Trop. Dis. 2017, 11, e0005493. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Han, Y.; Liu, H.; Chen, H.; Ma, F.; Withers, S.G.; Feng, Y.; Yang, G. Directed evolution of an α1,3-fucosyltransferase using a single-cell ultrahigh-throughput screening method. Sci. Adv. 2019, 5, eaaw8451. [Google Scholar] [CrossRef]
- Janesch, B.; Baumann, L.; Mark, A.; Thompson, N.; Rahmani, S.; Sim, L.; Withers, S.G.; Wakarchuk, W.W. Directed evolution of bacterial polysialyltransferases. Glycobiology 2019, 29, 588–598. [Google Scholar] [CrossRef]
- Aharoni, A.; Thieme, K.; Chiu, C.P.; Buchini, S.; Lairson, L.L.; Chen, H.; Strynadka, N.C.; Wakarchuk, W.W.; Withers, S.G. High-throughput screening methodology for the directed evolution of glycosyltransferases. Nat. Methods 2006, 3, 609–614. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, S.S.; Warren, R.A.; Withers, S.G. Directed evolution of a glycosynthase from Agrobacterium sp. Increases its catalytic activity dramatically and expands its substrate repertoire. J. Biol. Chem. 2004, 279, 42787–42793. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Chen, H.-M.; Rich, J.R.; Goddard-Borger, E.D.; Withers, S.G. Directed evolution of a β-glycosidase from agrobacterium sp. To enhance its glycosynthase activity toward C3-modified donor sugars. Protein Eng. Des. Sel. 2012, 25, 465–472. [Google Scholar] [CrossRef]
- Yang, G.; Rich, J.R.; Gilbert, M.; Wakarchuk, W.W.; Feng, Y.; Withers, S.G. Fluorescence activated cell sorting as a general ultra-high-throughput screening method for directed evolution of glycosyltransferases. J. Am. Chem. Soc. 2010, 132, 10570–10577. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Withers, S.G. Ultrahigh-throughput facs-based screening for directed enzyme evolution. ChemBioChem 2009, 10, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kan, S.B.J.; Lewis, R.D.; Wittmann, B.J.; Arnold, F.H. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc. Natl. Acad. Sci. USA 2019, 116, 8852–8858. [Google Scholar] [CrossRef] [PubMed]
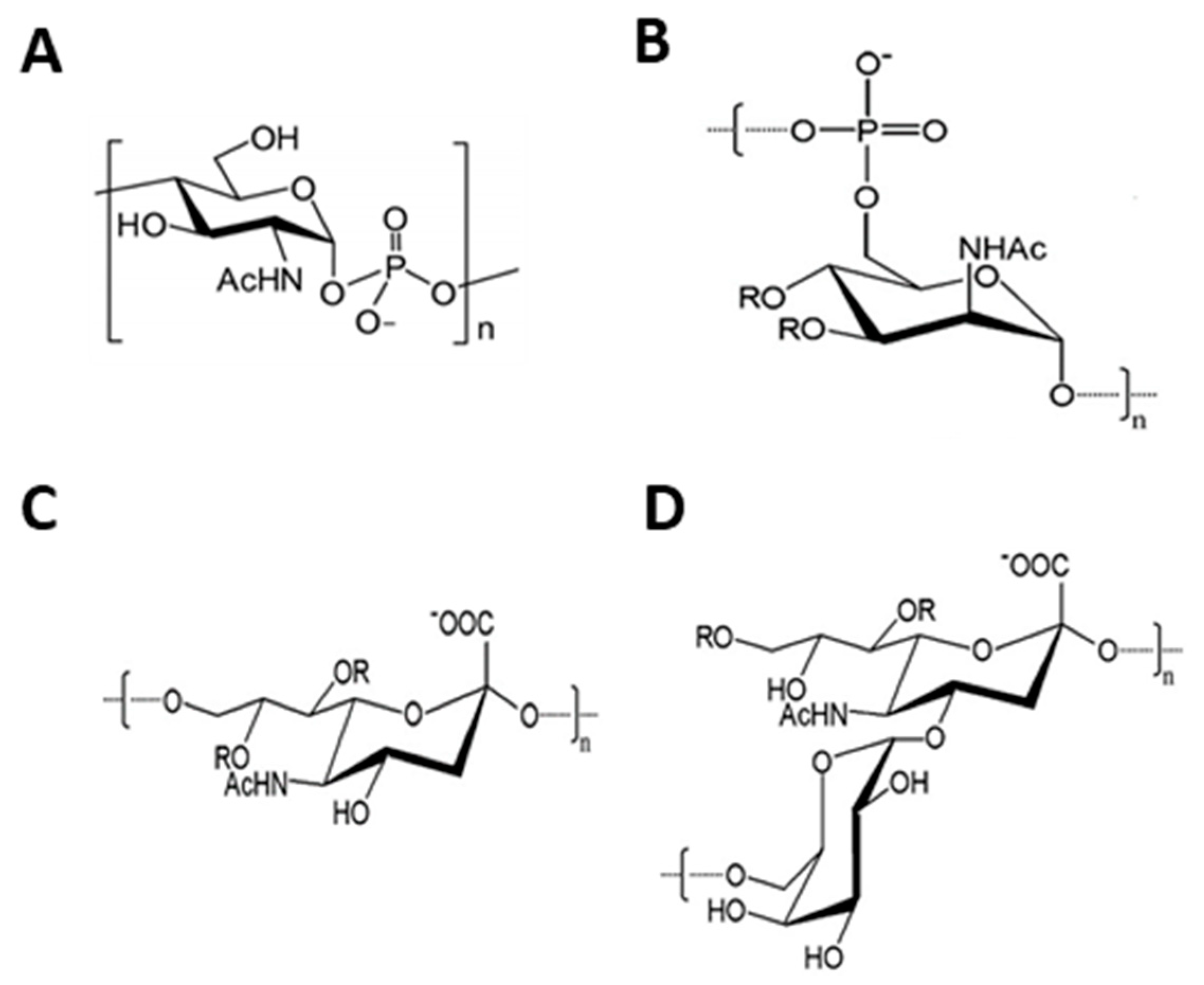

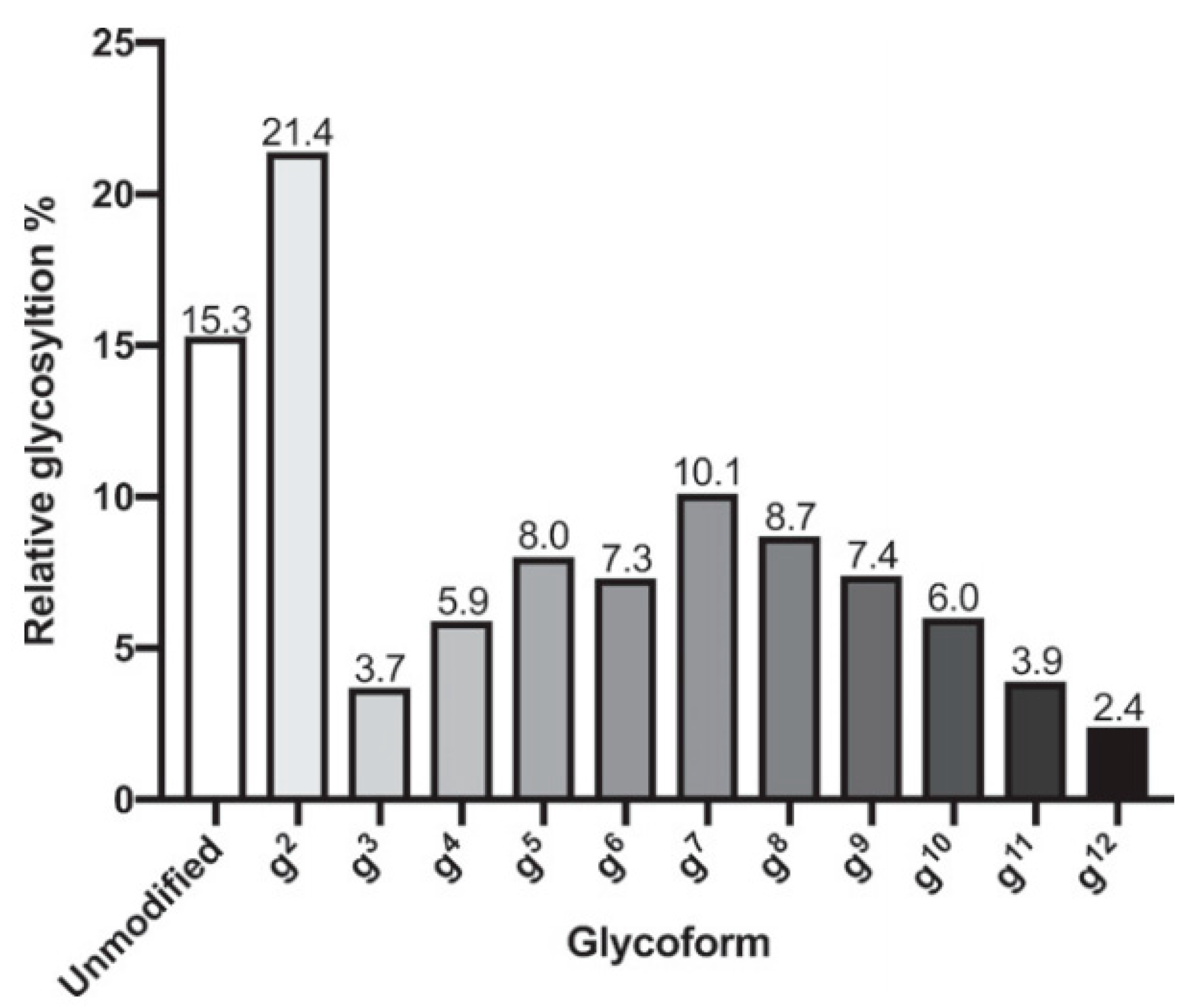
| Chemoenzymatic Synthesis | Chemical Synthesis |
|---|---|
|
|
|
|
| WHO Priority Level | Bacteria | Antibiotic Resistance |
|---|---|---|
| Highest | Mycobacterium tuberculosis | Fluoroquinolone |
| Rifampicin | ||
| Isoniazid | ||
| Critical | Acinetobacter baumannii Pseudomonas aeruginosa Enterobacteriaceae spp. | Carbapenem |
| High | Enterococcus faecium | Vancomycin |
| Staphylococcus aureus | Methicillin, Vancomycin | |
| Helicobacter pylori | Clarithromycin | |
| Campylobacter spp. | Fluoroquinolone | |
| Salmonellae | Fluoroquinolone | |
| Neisseria gonorrhoeae | Cephalosporin, Fluoroquinolone | |
| Medium | Streptococcus pneumoniae | Penicillin |
| Haemophilus influenzae | Ampicillin | |
| Shigella spp. | Fluoroquinolone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegbite, A.; McCarthy, P.C. Recent and Future Advances in the Chemoenzymatic Synthesis of Homogeneous Glycans for Bacterial Glycoconjugate Vaccine Development. Vaccines 2021, 9, 1021. https://doi.org/10.3390/vaccines9091021
Adegbite A, McCarthy PC. Recent and Future Advances in the Chemoenzymatic Synthesis of Homogeneous Glycans for Bacterial Glycoconjugate Vaccine Development. Vaccines. 2021; 9(9):1021. https://doi.org/10.3390/vaccines9091021
Chicago/Turabian StyleAdegbite, Ayobami, and Pumtiwitt C. McCarthy. 2021. "Recent and Future Advances in the Chemoenzymatic Synthesis of Homogeneous Glycans for Bacterial Glycoconjugate Vaccine Development" Vaccines 9, no. 9: 1021. https://doi.org/10.3390/vaccines9091021
APA StyleAdegbite, A., & McCarthy, P. C. (2021). Recent and Future Advances in the Chemoenzymatic Synthesis of Homogeneous Glycans for Bacterial Glycoconjugate Vaccine Development. Vaccines, 9(9), 1021. https://doi.org/10.3390/vaccines9091021






